ABSTRACT
The aim of this study was to evaluate the frequency of isolation of agents causing subclinical mastitis in a herd and to estimate production losses associated with SCC> 200,000cells/mL. Three 7-day interval microbiological cultures were performed in all lactating animals from the same farm that was evaluated from June to July. To evaluate the negative and positive isolation frequencies between the lactation phases, a Chi-square test was performed. Simple linear regressions were performed to evaluate the lactation curve of animals grouped by pathogens isolated from negative cows in the microbiological culture and with SCC <200,000cells/mL. To estimate the production losses between the groups, regression coefficients were used. Results found in this experiment were: Culture-negative cows with SCC ≥ 200,000cells/mL, cows testing positive in milk culture, with SCC <200,000cells/mL and cows testing positive in milk culture, with SCC ≥ 200,000cells/mL. Milk production was -3.5; -0.5 and -4.27kg, respectively, when compared to culture-negative cows with SCC <200,000cells/mL. Cows infected with yeast cells, Coagulase-negative staphylococci (CNS), Staphylococcus aureus and environmental streptococci produced -3.42; -0.5; -0.168 and -2.5kg of milk when compared to culture-negative cows with SCC <200,000cells/mL. SCC indicates an inflammatory reaction in the mammary gland and it is directly associated with milk production losses and with presence of microorganisms in the mammary gland.
Keywords:
subclinical mastitis; environmental; contagious; milk quality
RESUMO
O objetivo deste estudo foi avaliar a frequência de isolamento de agentes causadores de mastite subclínica em um rebanho e estimar as perdas de produção associadas com CCS>200.000 cél./mL. Três cultivos microbiológicos intervalados por sete dias foram realizados em todos os animais em lactação da propriedade avaliada, no período de junho a julho. Para avaliar as frequências de isolamento negativo e positivo entre as fases da lactação, foi realizado um teste de qui-quadrado. Foram realizadas regressões lineares simples para avaliar a curva de lactação dos animais agrupados por patógenos isolados em relação a vacas negativas na cultura microbiológica e com CCS < 200.000 cél./mL. Os coeficientes das regressões foram utilizados para estimar as perdas de produção entre os grupos. Vacas com resultado negativo na microbiologia, mas com CCS ≥ 200.000 cél./mL, positivas na microbiologia com CCS < 200.000 cél./mL e positivas com CCS ≥ 200.000 cél./mL, produziram por dia, respectivamente, -3,5; -0,5 e -4,27kg de leite em relação às vacas negativas com CCS < 200.000 cél./mL. Vacas infectadas com células leveduriformes, Staphylococcus coagulase negativa, Staphylococcus aureus e Streptococcus ambientais produziram, respectivamente, -3,42; -0,5; -0,168 e -2,5kg de leite, comparadas a vacas negativas com CCS < 200.000 cél./mL. A CCS, indicativa de reação inflamatória, encontra-se diretamente associada às perdas de produção de leite, assim como a presença do microrganismo na glândula mamária.
Palavras-chave:
mastite subclínica; ambiente; contagioso; qualidade do leite
INTRODUCTION
Mastitis is the most expensive disease that affects dairy herds around the world (Jashari et al., 2016JASHARI, R.; PIEPERS, S.; VLIEGHER, S. Evaluation of the composite milk somatic cell count as a predictor of intramammary infection in dairy cattle. J. Dairy Sci., v.99, p.9271-9286, 2016.). A persistent inflammatory response to an intramammary infection is characterized as Mastitis, which can be categorized as clinical or subclinical. Mastitis also affects animal welfare and causes economic losses due to the reduction of milk production, compromised milk quality, premature culling, treatment and veterinary costs and milk waste because of the antibiotic treatment (Taponen et al., 2017TAPONEN, S.; LISKI, E.; HEIKKILÄ, A.M.; PYÖRÄLÄ, S. Factors associated with intramammary infection in dairy cows caused by coagulase-negative staphylococci, Staphylococcus aureus, Streptococcus uberis, Streptococcus dysgalactiae, Corynebacterium bovis, or Escherichia coli. J. Dairy Sci., v.100, p.493-503, 2017.). Milk somatic cells come majorly from the immune system, as part of the natural defense mechanism. The increase in the somatic cell count (SCC) is, therefore, a reflex of an inflammatory response to an infection or another injury of the mammary gland (Schukken et al., 2003SCHUKKEN, Y.H.; WILSON, D.J.; WELCOME, F. et al. Monitoring udder health and milk quality using somatic cell counts. Vet. Res., v.34, p.579-596, 2003.). The milk SCC is an important tool for the subclinical mastitis diagnose and is internationally accepted as an evaluation criteria of the cow mammary gland health (Cicconi-Hogan et al., 2013).
Many SCC parameters are used aiming to classify the mammary gland health. Counts equal or superior to 200.000cells/mL are widely accepted as an indicator of animals with subclinical mastitis (Rhoda e Pantoja, 2012RHODA, D.A.; PANTOJA, J.C.F. Using mastitis records and somatic cell count data. Vet. Clin. N. Am. Food Anim. Pract., v.28, p.347-361, 2012.; Jashari et al., 2016JASHARI, R.; PIEPERS, S.; VLIEGHER, S. Evaluation of the composite milk somatic cell count as a predictor of intramammary infection in dairy cattle. J. Dairy Sci., v.99, p.9271-9286, 2016.). Four - quarter milk samples had a sensitivity and specificity of 73% and 89%, respectively, which is considered a reliable index (Dohoo e Leslie., 1991DOHOO, I.R.; LESLIE, K.E. Evaluation of changes in somatic cell counts as indicators of new intramammary infections. Prev. Vet. Med., v.10, p.225-237, 1991.).
Independently of the pathogen causing the mammary gland infection, the parenchyma lesion will occur due to the inflammatory response (Corl et al., 2010CORL, C.M.; ROBINSON, H.R.; CONTRERAS, G.A. et al. Ethyl pyruvate diminishes the endotoxin-induced inflammatory response of bovine mammary endothelial cells. J. Dairy Sci., v.93, p.5188-5199, 2010. ), which leads to milk production loss during all lactation. Intramammary infections decrease the integrity of the blood-milk barrier, causing the opening of the tight junctions (Burton e Erskine, 2003BURTON, J.L.; ERSKINE, R.J. Immunity and mastitis. Some new ideas for an old disease. Vet. Clin. N. Am. Food Anim. Pract., v.19, p.1-45, 2003.). This integrity loss of the blood-milk barrier might be pathogen specific: Wellnitz et al. (2013WELLNITZ, O.; ARNOLD, E.T.; LEHMANN, M.; BRUCKMAIER, R.M. Short communication: differential immunoglobulin transfer during mastitis challenge by pathogen-specific components. J. Dairy Sci., v.96, p.1681-1684, 2013.) evaluated mastitis induced by LPS derived from E. coli or induced by lipotheicoic acid (LTA) of the S. aureus and found that the mastitis caused by E. coli results in a higher transfer of blood components, including IgG2, to milk, even with similar SCC. This difference may influence the course of mastitis and cure, as also determine differences in milk production loss during the inflammatory process. According to Silanikove et al. (2011SILANIKOVE, N.; RAUCH-COHEN, A.; SHAPIRO, F. et al. Lipopolysaccharide challenge of the mammary gland in bovine induced a transient glandular shift to anaerobic metabolism. J. Dairy Sci., v.94, p.4468-4475, 2011.), the decrease in milk production might also be associated to the different cell metabolism during the inflammatory process, where there is a deviation of the metabolic resources, normally used to synthesize milk, to support the immunological system.
We hypothesized that different microorganisms and the inflammatory reaction intensity could generate distinct milk production losses. The aim of this study was to determine the isolation frequency of mastitis causing-microorganisms in different lactation stages, and to evaluate possible production losses due to the presence or not of microorganisms, associated or not to subclinical mastitis (SCC>200.000cells/mL).
MATERIAL AND METHODS
The Animal Ethics Committee of the Federal University of Minas Gerais (protocol n. 117/2011) approved the protocols for this study.
The study was conducted in a dairy farm located in the center-west region of the Minas Gerais state. The region has an altitude tropical climate with hot summers, annual average temperature of 21.8˚C, minimal temperature of 7˚C in winter and maximum of 35˚C during summer, with a relative humidity of 74.8% and annual precipitation of 1657mm, according to the farm historical data. The farm was chosen because of the typical semi-intensive system of production. There are not many studies evaluating the profile of pathogens in this system.
Three 7-day interval microbiological cultures were performed in all lactating animals from the farm evaluated. Samples were collected between June and July, during the dry season. The herd was composed by crossbred animals of different genetic compositions, originated from Holstein x Gir crossing. The milk production daily average during the experimental period was 16.8kg.
One hundred eighty three lactating cows were evaluated during the study period, 32.2% primiparous and 67.8% multiparous. Animals were confined in paddocks with 15m2 to 20m2 per cow, with access to the feed bunk, artificial shades, and trees. Animals were kept all the time in this area and divided in groups according to milk production. Diets were balanced with sugar cane and concentrate in order to attend the nutritional requirements, according to the NRC (2001) and cow were fed with a total mixed ration twice a day. Milking was performed daily at 0500am and at 1600pm. The milking equipment was a middle line with eight automatic extraction units, from Intermac (Intermac, Porto Alegre, RS, Brasil). The average somatic cell count from the milk tank was 477.000cells/mL during the evaluated period.
To perform the milk microbiological analyses, the animals went throughout pre-milking procedures, which consisted of test for clinical mastitis detection, teats disinfection with the premilking disinfectant based on chlorine (2%) and dried with disposable paper toils. Milk sampling was performed immediately before the coupling of the milking units. Each teat end was scrubbed vigorously with cotton soaked in an ethanol solution (70%) and air-dried. After that, similar amounts of milk were collected from each quarter. Approximately 50mL sample was set in sterile tubes and frozen at -20˚C until microbiology culture was performed.
The microbiological culture procedure, isolation and identification of microorganisms were performed according to Oliver et al. (2004bOLIVER, S.P.; LEWIS, M.J.; GILLESPIE, B.E. et al. Microbiological procedures for the diagnosis of bovine udder infection and determination of milk quality. 4.ed. Verona: National Mastitis Council, 2004b. p.47.). A culture was considered positive when three or more colonies were detected, except for Staphylococcus aureus or Streptococcus agalactiae, where a positive-culture was that one with one or more colonies detected (Blagitz et al., 2015BLAGITZ, M.G.; SOUZA, F.N.; BATISTA, C.F. et al. Flow cytometry analysis: interdependence of healthy and infected udder quarters. J. Dairy Sci., v.98, p.2401-2408, 2015.). Samples that showed growths of more than three bacterial species in the microbiological analysis were considered contaminated and were excluded.
Milk samples for SCC and composition analyses, individualized per cow and composed by the pool of mammary quarters, were collected using collectors (Waikato, DeLaval, Estocolmo, Suécia), set in plastic tubes containing bronopol conservatives (2-bromo-2-nitro-1,3-propandiol) and maintained under refrigeration (4˚C). Milk samples were analyzed for milk fat and total protein by infrared analyses (Bentley 2000 Bentley Instruments Inc., Chaska, MN) (IDF, 2000). The SCC was determined using the flow cytometry method (Somacount 300, Bentley Instruments Inc., Chaska, MN).
The data base was constructed with the SCC, milk production corrected for fat, days in milk (DIM) information, besides the microbiological data obtained from the cultures of all lactating cows, during the experimental period. From 549 cultures, 323 microbiological cultures were evaluated, 171 negative and 152 positives. 226 cultures were excluded from analysis, once in the sampling day, milk production was not recorded and milk was not collected for SCC. In addition, positive culture database excluded 28 samples with SCC< 50.000. However, Hagnestam-Nielsen et al. (2009) showed in their study that these SCC values do not suit an infected animal, which can be considered an error in the analysis. For statistical reasons, primiparous and multiparous data were analyzed together so there was not a reduction in the experimental n.
Cows that had positive result for the microbiological culture were divided according to the SCC in two different groups: <200.000cells/mL and > 200.000cells/mL, and compared to cows with negative microbiological results and SCC < 200.00cells/mL. The same way, cows with negative cultures with SCC> 200.000cells/mL were compared to cows with negative cultures and SCC < 200.000cells/mL.
A Chi-square test (α=5%) was performed to evaluate the frequency of animals grouped by pathogens isolated from negative cows in the microbiological culture and of animals grouped by pathogens isolated from positive cows in the microbiological culture between the phases of lactation. Simple linear regressions study by PROC REG from SAS (Statistical…, 2002) were performed to evaluate the lactation curve of animals grouped by pathogens isolated from negative cows in the microbiological culture and with SCC <200,000cells/mL. Regression coefficients were used to monthly estimate milk production loss, production loss of cows with negative cultures and SCC> 200.000cells/mL, and milk production loss of cows grouped by isolated pathogen (yeast cells, CNS, S. aureus and environmental streptococci), in relation to negative cows and SCC of <200.000cells/mL.
RESULTS AND DISCUSSION
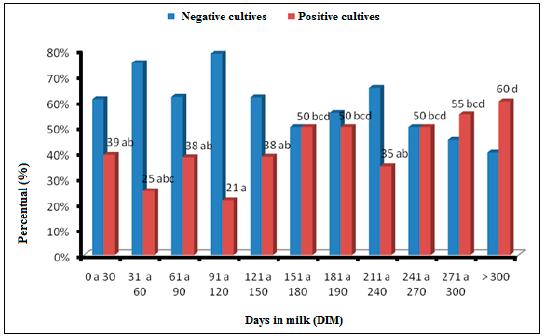
Among the samples analyzed (n= 323), 169 milk samples tested showed subclinical mastitis. Thirty nine percent of animals until 30DIM (days in milk) presented a positive microbiological culture. There was an oscillation in the isolation frequency of mastitis-causing microorganisms until 150DIM, with a minimum of 21% (91 to 120DIM) and maximum of 39% (until 30DIM). For animals with more than 151DIM there was an increase in pathogens isolation, with about 50% of infected animals. There was a decrease in the isolation values from 211 to 240DIM and an increase during 271 to 300DIM (55%) and > 301 (60%) (Figure 1).
Percentage of negative and positive cultures during herd lactation, obtained through microbiological culture. Distinct lower-case letters between phases and days in milk, differ by the Qui-square test (P< 0.05).
The values found in the first 100 days in milk concur with many studies in the international literature, which say that new infection rates, especially for environmental pathogens, is higher during the cow dry period and in the first months of lactation (Bradley and Green, 2004BRADLEY, A.J.; GREEN, M.J. The importance of the nonlactating period in the epidemiology of intramammary infection and strategies for prevention. Vet. Clin. N. Am. Food Anim. Pract., v.20, p.547-568, 2004.; Green et al., 2005). The increase in the microorganism isolation in animals with higher DIM was expected in facilities with high prevalence of contagious microorganisms, according to Fox and Gay (1993FOX, L.K.; GAY, J.M. Contagious mastitis. Vet. Clin. N. Am. Food Anim. Pract., v.9, p.475-487, 1993.), the milking increases the chances of intramammary infection dissemination and as days in milk increases, higher is the chance of the animal to be infected. Notwithstanding, as demonstrated in Figure 2 and 3, there was no isolation of Streptococcus agalactiae and the herd S. aureus isolation frequency in all lactation phases, was low, reaching 10% in animals between 121 and 150DIM, and representing only 5.5% of all cultures. This information is important especially because of the low prevalence of major pathogens in the farm. However, other risk factors determine the high intramammary infection rates in all lactation phases, especially from 150DIM.
Prevalence distribution of each microorganism according to days in milk (YC: yeast cells, CNS: Coagulase-negative staphylococci, SAU: Sthapylococcus aureus and Estrep: environmental Streptococcus) of the microbiological cultures.
Frequency (%) of each microorganism according to days in milk (YC: yeast cells, CNS: Coagulase-negative staphylococci, SAU: Sthapylococcus aureus and Estrep: environmental Streptococcus).
The environmental Streptococcus isolation was uniform independently of lactation stage, isolated in average 11% of animals, except during 151 to 180DIM and from 181 to 210 days in milk, during which there was an isolation of 22% and 38%, representing frequencies of 1.7% and 2.3% respectively. The use of mastitis control procedures including teat disinfection, antibiotic therapy and culling of chronically infected animals, lead to a progress in the control of mastitis caused by contagious microorganisms in herds around the world (Hillerton and Berry, 2005HILLERTON, J.E.; BERRY, E.A. Treating mastitis in the cow-a tradition or an archaism. J. Appl. Microbiol., v.98, p.1250-1255, 2005.; Zadoks and Fitzpatrick, 2009ZADOKS, R.N.; FITZPATRICK, J.L. Changing trends in mastitis. Ir. Vet. J., v.62, p.S59, 2009.). On the other hand, these procedures are less efficient against environmental Streptococcus species and gram-negative bacteria (Oliver et al., 2004aOLIVER, S.P.; ALMEIDA, R.A.; GILLESPIE, B.E. Extended ceftiofur therapy for treatment of experimentally-induced Streptococcus uberis mastitis in lactating dairy cattle. J. Dairy Sci., v.87, p.3322-3329, 2004a.). Studies showed that a decrease in the contagious microorganism’s prevalence in a herd increases the proportion of intramammary infections caused by environmental pathogens (Zadoks and Fitzpatrick, 2009). This might be related to a reduction in the competition to colonize the mammary gland and a smaller amount of defense cells in the infection site when it occurs (Suriyasathaporn et al., 2000SURIYASATHAPORN, W.; SCHUKKEN, Y.H.; NIELEN, M.; BRAND, A. Low somatic cell count: a risk factor for subsequent clinical mastitis in a dairy herd. J. Dairy Sci., v.83, p.1248-1255, 2000.).
Among environmental Streptococcus, the S. uberis seems to be the most prevalent (Jayarao et al., 1999JAYARAO, B.M.; GILLESPIE, B.E.; LEWIS, M.J. Epidemiology of Streptococcus uberis intramammary infections in a dairy herd. Zentralbl. Veterinärmed. B., v.46, p.433-442, 1999.), so it is important to understand its epidemiology to comprehend its risk factors. S. uberis is considered and environmental pathogen and its primary reserve and new infection source is the environment (Radostitis et al., 2008). Leelahapongsathon et al. (2016LEELAHAPONGSATHON, K.; SCHUKKEN, Y.H.; PINYOPUMMINTR, T.; SURIYASATHAPORN, W. Comparison of transmission dynamics between Streptococcus uberis and Streptococcus agalactiae intramammary infections. J. Dairy Sci., v.99, p.1418-1426, 2016.) reported high transmission rates for S. uberis when compared to S. agalactiae transmission, but with low infection persistency and high spontaneous cure rate. Abureema et al. (2014ABUREEMA, S.; SMOOKER, P.; MALMO, J.; DEIGHTON, M. Molecular epidemiology of recurrent clinical mastitis due to Streptococcus uberis: evidence of both an environmental source and recurring infection with the same strain. J. Dairy Sci., v.97, p.285-290, 2014.) worked with strain identification by electrophoresis and found strains with the same molecular pattern in samples collected from two clinical mastitis cases with 8 weeks interval, demonstrating this microorganism persistency in the mammary gland. Despite the evidences of cow-to-cow transmission, the biggest reservoir is the environment, what led to the hypothesis that one of the factors that led to a percentage of animals infected with environmental Streptococcus was the higher environmental challenge. Corroborating this we found that for YC the higher prevalence occurred during the period between 121 to 150 days and above 241DIM. The lowest prevalence was 0.3% (from 61 to 120DIM) and the highest (4%) in cows above 300DIM.
Yeast are unicellular ubiquitous organisms and considered opportunists pathogens of the mammary gland (Santos and Marin, 2005SANTOS, R.C.; MARIN, J.M. Isolation of Candida spp. from mastitic bovine milk in Brazil. Mycopathologia, v.159, p.251-253, 2005.), what justifies the absence of a specific infection behavior in different lactation phases. Akdouche et al. (2014AKDOUCHE, L.; AISSI, M.; ZENIA, S.; SAADI, A. Importance of yeasts in the mammary infection of the cattle in the region of Sidi M'Hamed Ben Ali, Wilaya of Relizane, Algeria. Vet. Sci. Technol., v.5, p.1, 2014.) reported that the mastitis caused by fungus might be responsible for 1.76% of all mastitis cases, clinical and subclinical, but this number may be underestimated. Aalbæk et al. (1994AALBÆK, B.E.N.T.; STENDERUP, J.Ø.R.G.E.N. et al. Mycotic and algal bovine mastitis in Denmark. Apmis, v.102, p.451-456, 1994.) showed that fungus are isolated in at least 7% of routine samples collected in the north and central Europe. In the present work the isolation frequency in different times of lactation varied from less than 0.5% (from 61 to 90DIM) to 4% (above 300DIM), being isolated in 13% of all evaluated samples. Scaccabarozzi et al. (2011SCACCABAROZZI, L.; LOCATELLI, C.; PISONI, G. et al. Short communication: epidemiology and genotyping of Candida rugosa strains responsible for persistent intramammary infections in dairy cows. J. Dairy Sci., v.94, p.4574-4577, 2011.) hypothesized that the teat skin contamination with fungus such as C. rugose can occur via feces and can spread through milking by the contamination of pre and postmilking disinfectant recipients, for example. Because the animals were housed in paddocks without vegetation cover, in which there was organic matter accumulation, we can hypothesize that the high environmental contamination might have increased the risk factor, justifying a high isolation in the evaluated facility.
The CNS was more isolated until 120DIM, after that the positive isolation frequency decreased until 190 days and increased again until the end of lactation. The higher percentage of infected cows was 2.8% and occurred in animals above 300DIM (Figure 2), representing, during this period, 28.3% of positive milk samples for this organism (Figure 3). Studies pointed toward differences in infection susceptibility among primiparous and multiparous cows, as primiparous are infected before or right after calving and multiparous are more susceptible in further lactation stages (Pyörӓlӓ and Taponen, 2009PYÖRÄLÄ, S.; TAPONEN, S. Coagulase-negative staphylococci-emerging mastitis pathogens. Vet. Microbiol., v.134, p.3-8, 2009.). In the present work, primiparous and multiparous were evaluated together, which may justify the behavior found. The infection pattern of CNS is not yet fully understood, once they are a heterogeneous group and each specie must be studied separately (De Visscher et al., 2015). Fry et al. (2014FRY, P.R.; MIDDLETON, J.R.; DUFOUR, S. et al. Association of coagulase-negative staphylococcal species, mammary quarter milk somatic cell count, and persistence of intramammary infection in dairy cattle. J. Dairy Sci., v.97, p.4876-4885, 2014.) isolated and characterized CNS species from subclinical mastitis cases in Canadian herds, and found different CNS species, some more prevalent, as S. xylosus, S. chromogenes and S. simulans, associated to persistent infections and causing high SCC. From 20 species, nine were associated with persistent infections and 11 with softer infections. This difference between species might cause this variation in the dynamic of infection during lactation, causing short infections and high spontaneous cure rates, and persistent subclinical infections, that can be transmitted between animals, since some species are more adapted to the host than others.
The data of the present work demonstrated that the mastitis-causing microorganisms pattern was the same in different lactation stages, with predominance of environmental microorganisms. 15.3% of negative cultures were from animals with SCC above 200.000cells/mL, that might have been originated from animals that eliminated the microorganism causing mastitis, but that were still facing an inflammatory process. Once the samples were sent frozen to the laboratory, we can hypothesize that part of these culture-negative milk samples, with SCC > 200.000cells/mL could be derived from cows infected by gram-negative microorganisms. We hypothesized that since the freezing and the storage time may reduce the chances of microorganisms, such as Escherichia coli isolation (Schukken et al., 1989SCHUKKEN, Y.H.; SMIT, J.A.H.; GROMMERS, F.J. et al. Effect of freezing on bacteriologic culturing of mastitis milk samples. J. Dairy Sci., v.72, p.1900-1906, 1989.), what could reinforce the environmental mastitis epidemiological behavior in the farm.
In facilities such as the evaluated here, a possible new infection curve can occur, which the cows in the first months of lactation continue to be susceptible to, but with high new infection rates in the second half of lactation, with an increase in the SCC as a reflex during this phase. These findings might be related to a high reinfection rates due to the high environmental challenge or to the presence of S. uberis and CNS strains with higher infection persistency, but more studies must be made in other herds under the same conditions in order to confirm these hypotheses.
The animals with negative microbiological culture and SCC below 200.000cells/mL presented higher milk yield during lactation compared to cows negative and positive with SCC above 200.000cells/mL. Although animals with positive culture, with SCC beneath 200.000cells/mL did not have a reduction in production comparatively to the control group (Table 1). Despite the cows in this group had the mammary gland infected by pathogens, the production loss when comparing negative and positive cows with SCC > 200.000cells/mL, to negative cows with SCC < 200.000cells/mL, was small and non-significant (0.5kg vs 3.5 and 4.27kg, respectively). The absence of significance in production loss between these two groups might be related to the lower inflammatory status of these animals, indicated by the somatic cell count. The milk production loss may be a result from the direct effects of the microorganism in the mammary gland or resulting from damages caused by the immune response in the gland secretory tissue (Detilleux et al., 2015DETILLEUX, J.; KASTELIC, J.P.; BARKEMA, H.W. et al. Mediation analysis to estimate direct and indirect milk losses due to clinical mastitis in dairy cattle. Prev. Vet. Med., v.118, p.449-456, 2015.).
Regression coefficient of daily milk production (kg) and estimative of daily production loss (kg) on the transformed SCC (log10), based on monthly tests, milk production and SCC of negative cows on microbiology (CCS ≥ 200.000cells/mL), positive cows (CCS < 200.000cells/mL), positive cows (≥ 200.000cells/mL) in relation to negative cows on microbiology (CCS < 200.000cells/mL; n= 92), average and median of SCC (x 103)
Is interesting to notice that the cows with negative microbiological tests, but with SCC > 200.000cells/mL, produced less milk than cows under 200.000cells/mL (Figure 4). The neutrophils (the predominant type of cell in milk of infected cows) can damage the mammary tissue by activating prematurely during migration, liberating toxic products during the death of bacteria, removal of debris and infected or damaged host cells, or because of failure in the acute inflammatory responses resolution (Zhao and Lacasse, 2008ZHAO, X.; LACASSE, P. Mammary tissue damage during bovine mastitis: causes and control. J. Anim. Sci., v.86, p.57-65, 2008.). The indirect effects of intramammary infections caused by the inflammatory response were previously described as more significant than the ones caused by the microorganisms itself (Detilleux et al., 2013DETILLEUX, J.; THERON, L.; DUPREZ, J.N. et al. Structural equation models to estimate risk of infection and tolerance to bovine mastitis. Genet. Select. Evol., v.45, p.6, 2013.; 2015). Each microorganism seems to affect the blood-milk barrier in different way, causing different inflammatory responses (Wellnitz et al., 2016WELLNITZ, O.; ZBINDEN, C.; HUANG, X.; BRUCKMAIER, R.M. Short communication: differential loss of bovine mammary epithelial barrier integrity in response to lipopolysaccharide and lipoteichoic acid. J. Dairy Sci., v.99, p.4851-4856, 2016.). Higher immune responses that could result in higher somatic cell count might have more significant deleterious effects, which explains the results found in the groups with SCC > 200.000cells/mL and not in animals that had isolated microorganisms in the microbiological culture, but that presented SCC < 200.000cells/mL.
Lactation curve of negative cows in culture with SCC below and above 200.000cells/mL, and of positive cows in culture with SCC below and above 200.000cells/mL (primiparous and multiparous data were analyzed together).
There was an effect of the microorganism presence in the mammary gland and of the SCC> 200.00cells/mL in negative cows on the daily milk production, illustrated by the regression coefficients on Table 2. The lower regression coefficients and, as a consequence, the higher production loss estimates were found for animals from which samples had yeast cells isolation, negative cows (SCC> 200.000cells/mL) and environmental streptococcus, of -3.33, -2.57 and -2.29kg, respectively. On the other hand, for CNS and S. aureus, the regression coefficients were smaller and without statistical difference in relation to the negative cows with SCC < 200.000cells/mL. These results corroborate the determinant factor for SCC in the production loss results. The microorganisms which caused more losses were the ones that more elevated the SCC, what suggests a higher inflammatory process, with more lesions in the mammary parenchyma.
Regression Coefficients of daily milk production (kg) and estimative of daily production loss (kg) on the transformed SCC (log10), based on monthly tests of milk production and SCC of negative cows (SCC ≥ 200.000cells/mL), and of positive cultures for YC, CNS, Stap and Strep versus negative cows (SCC < 200.000cells/mL, n: 92 e SCC average: 63 x 103), average and median of SCC (x 103)
The infections caused by microorganisms adapted to the mammary gland, such as S. aureus, tend to have less intense inflammatory answers because these microorganisms bypass the host’s immune system (Bannerman, 2009BANNERMAN, D.D. Pathogen-dependent induction of cytokines and other soluble inflammatory mediators during intramammary infection of dairy cows. J. Anim. Sci., v.87, p.10-25, 2009.). The environmental streptococcus as S. uberis are characterized by causing a delayed and less dramatic immune response than gram-negative microorganisms, such as E. coli, but more intense than S. aureus (Schukken et al., 2011SCHUKKEN, Y. H.; GÜNTHER, J.; FITZPATRICK, J. et al. Host-response patterns of intramammary infections in dairy cows. Vet. Immunol. Immunopathol., v.144, p.270-289, 2011.).
Tomazi et al. (2015TOMAZI, T.; GONÇALVES, J.L.; BARREIRO, J.R. et al. Bovine subclinical intramammary infection caused by coagulase-negative staphylococci increases somatic cell count but has no effect on milk yield or composition. J. Dairy Sci., v.98, p.3071-3078, 2015.) found a geometric average for SCC of 278.512cells/mL in animals with quarters infected by CNS, what shows that despite the recent studies are pointing that some CNS species, especially the ones adapted to the host, have a similar ability to bind to mammary cells to S. aureus, what increases the SCC (Supré et al., 2011SUPRÉ, K.; HAESEBROUCK, F.; ZADOKS, R.N. et al. Some coagulase-negative Staphylococcus species affect udder health more than others. J. Dairy Sci., v.94, p.2329-2340, 2011.), the majority still causes potentially less damage to secretory tissues (Pyörӓlӓ and Taponen, 2009PYÖRÄLÄ, S.; TAPONEN, S. Coagulase-negative staphylococci-emerging mastitis pathogens. Vet. Microbiol., v.134, p.3-8, 2009.) and consequently less production loss (Tomazi et al., 2015). Although few works exist evaluating fungus infections, Scaccabarozzi et al. (2011SCACCABAROZZI, L.; LOCATELLI, C.; PISONI, G. et al. Short communication: epidemiology and genotyping of Candida rugosa strains responsible for persistent intramammary infections in dairy cows. J. Dairy Sci., v.94, p.4574-4577, 2011.) found an average SCC of 2.033 x 103cells/mL, in animals that yeast cells were isolated, showing a high inflammatory response to the invasion by this microorganism, what justifies the higher losses found.
CONCLUSIONS
In the herd and under the conditions in which the present study was conducted there are no significant differences related to the infection caused by the studied pathogens and the lactation phase. The SCC, an indicative of the inflammatory reaction, is directly associated to milk production losses, beyond the presence of microorganisms in the mammary quarter, possibly being responsible for more significant losses in milk production then the microorganism isolation itself. Herds with mammary gland infections caused by environmental pathogens are characterized for high and oscillating SCC. High new infection rates occur not only in the beginning of lactation, but also in the middle and in the end of it.
REFERENCES
- AALBÆK, B.E.N.T.; STENDERUP, J.Ø.R.G.E.N. et al. Mycotic and algal bovine mastitis in Denmark. Apmis, v.102, p.451-456, 1994.
- ABUREEMA, S.; SMOOKER, P.; MALMO, J.; DEIGHTON, M. Molecular epidemiology of recurrent clinical mastitis due to Streptococcus uberis: evidence of both an environmental source and recurring infection with the same strain. J. Dairy Sci., v.97, p.285-290, 2014.
- AKDOUCHE, L.; AISSI, M.; ZENIA, S.; SAADI, A. Importance of yeasts in the mammary infection of the cattle in the region of Sidi M'Hamed Ben Ali, Wilaya of Relizane, Algeria. Vet. Sci. Technol., v.5, p.1, 2014.
- BANNERMAN, D.D. Pathogen-dependent induction of cytokines and other soluble inflammatory mediators during intramammary infection of dairy cows. J. Anim. Sci., v.87, p.10-25, 2009.
- BLAGITZ, M.G.; SOUZA, F.N.; BATISTA, C.F. et al. Flow cytometry analysis: interdependence of healthy and infected udder quarters. J. Dairy Sci., v.98, p.2401-2408, 2015.
- BRADLEY, A.J.; GREEN, M.J. The importance of the nonlactating period in the epidemiology of intramammary infection and strategies for prevention. Vet. Clin. N. Am. Food Anim. Pract., v.20, p.547-568, 2004.
- BURTON, J.L.; ERSKINE, R.J. Immunity and mastitis. Some new ideas for an old disease. Vet. Clin. N. Am. Food Anim. Pract., v.19, p.1-45, 2003.
- CICCONI-HOGAN, K.M.; GAMROTH, M.; RICHERT, R. et al. Associations of risk factors with somatic cell count in bulk tank milk on organic and conventional dairy farms in the United States. J. Dairy Sci., v.96, p.3689-3702, 2013
- CORL, C.M.; ROBINSON, H.R.; CONTRERAS, G.A. et al. Ethyl pyruvate diminishes the endotoxin-induced inflammatory response of bovine mammary endothelial cells. J. Dairy Sci., v.93, p.5188-5199, 2010.
- DE VISSCHER, A.; PIEPERS, S.; SUPRÉ, K. Short communication: species group-specific predictors at the cow and quarter level for intramammary infection with coagulase-negative staphylococci in dairy cattle throughout lactation. J. Dairy Sci., v.98, p.5448-5453, 2015.
- DETILLEUX, J.; KASTELIC, J.P.; BARKEMA, H.W. et al. Mediation analysis to estimate direct and indirect milk losses due to clinical mastitis in dairy cattle. Prev. Vet. Med., v.118, p.449-456, 2015.
- DETILLEUX, J.; THERON, L.; DUPREZ, J.N. et al. Structural equation models to estimate risk of infection and tolerance to bovine mastitis. Genet. Select. Evol., v.45, p.6, 2013.
- DOHOO, I.R.; LESLIE, K.E. Evaluation of changes in somatic cell counts as indicators of new intramammary infections. Prev. Vet. Med., v.10, p.225-237, 1991.
- FOX, L.K.; GAY, J.M. Contagious mastitis. Vet. Clin. N. Am. Food Anim. Pract., v.9, p.475-487, 1993.
- FRY, P.R.; MIDDLETON, J.R.; DUFOUR, S. et al. Association of coagulase-negative staphylococcal species, mammary quarter milk somatic cell count, and persistence of intramammary infection in dairy cattle. J. Dairy Sci., v.97, p.4876-4885, 2014.
- GREEN, M.J.; GREEN, L.E.; BRADLEY, A.J. et al. Prevalence and associations between bacterial isolates from dry mammary glands of dairy cows. Vet. Rec., v.156, p.71-77, 2005.
- HAGNESTAM-NIELSEN, C.; EMANUELSON, U.; BERQLUND, B. et al. Relationship between somatic cell count and milk yield in different stages of lactation. J. Dairy Sci., v.92, p.3124-3133, 2009.
- HILLERTON, J.E.; BERRY, E.A. Treating mastitis in the cow-a tradition or an archaism. J. Appl. Microbiol., v.98, p.1250-1255, 2005.
- IDF - INTERNATIONAL Dairy Federation 141C - Determination of milkfat, protein and lactose content - Guidance on the operation of mid-infrared instruments. Brussels, Belgium, 2000. 15p.
- JASHARI, R.; PIEPERS, S.; VLIEGHER, S. Evaluation of the composite milk somatic cell count as a predictor of intramammary infection in dairy cattle. J. Dairy Sci., v.99, p.9271-9286, 2016.
- JAYARAO, B.M.; GILLESPIE, B.E.; LEWIS, M.J. Epidemiology of Streptococcus uberis intramammary infections in a dairy herd. Zentralbl. Veterinärmed. B., v.46, p.433-442, 1999.
- LEELAHAPONGSATHON, K.; SCHUKKEN, Y.H.; PINYOPUMMINTR, T.; SURIYASATHAPORN, W. Comparison of transmission dynamics between Streptococcus uberis and Streptococcus agalactiae intramammary infections. J. Dairy Sci., v.99, p.1418-1426, 2016.
- NRC - NATIONAL reserch council-NRC. Nutrient requirements of dairy cattle. 7. ed. rev. Washington, D.C.: NATIONAL ACADEMY PRESS, 2001. 381p.
- OLIVER, S.P.; ALMEIDA, R.A.; GILLESPIE, B.E. Extended ceftiofur therapy for treatment of experimentally-induced Streptococcus uberis mastitis in lactating dairy cattle. J. Dairy Sci., v.87, p.3322-3329, 2004a.
- OLIVER, S.P.; LEWIS, M.J.; GILLESPIE, B.E. et al. Microbiological procedures for the diagnosis of bovine udder infection and determination of milk quality. 4.ed. Verona: National Mastitis Council, 2004b. p.47.
- PYÖRÄLÄ, S.; TAPONEN, S. Coagulase-negative staphylococci-emerging mastitis pathogens. Vet. Microbiol., v.134, p.3-8, 2009.
- RADOSTITS, O.M.; GAY, CC.; HINCHCLIFF, KW.; CONSTABLE, PD. Veterinary medicine; a textbook of the diseases of cattle, horses, sheep, pigs and goats. Philadelphia: WB Sauders, 2008.
- RHODA, D.A.; PANTOJA, J.C.F. Using mastitis records and somatic cell count data. Vet. Clin. N. Am. Food Anim. Pract., v.28, p.347-361, 2012.
- SANTOS, R.C.; MARIN, J.M. Isolation of Candida spp. from mastitic bovine milk in Brazil. Mycopathologia, v.159, p.251-253, 2005.
- SCACCABAROZZI, L.; LOCATELLI, C.; PISONI, G. et al. Short communication: epidemiology and genotyping of Candida rugosa strains responsible for persistent intramammary infections in dairy cows. J. Dairy Sci., v.94, p.4574-4577, 2011.
- SCHUKKEN, Y. H.; GÜNTHER, J.; FITZPATRICK, J. et al. Host-response patterns of intramammary infections in dairy cows. Vet. Immunol. Immunopathol., v.144, p.270-289, 2011.
- SCHUKKEN, Y.H.; SMIT, J.A.H.; GROMMERS, F.J. et al. Effect of freezing on bacteriologic culturing of mastitis milk samples. J. Dairy Sci., v.72, p.1900-1906, 1989.
- SCHUKKEN, Y.H.; WILSON, D.J.; WELCOME, F. et al. Monitoring udder health and milk quality using somatic cell counts. Vet. Res., v.34, p.579-596, 2003.
- SILANIKOVE, N.; RAUCH-COHEN, A.; SHAPIRO, F. et al. Lipopolysaccharide challenge of the mammary gland in bovine induced a transient glandular shift to anaerobic metabolism. J. Dairy Sci., v.94, p.4468-4475, 2011.
- STATISTICAL analysis system. Version 8. Cary: SAS Institute, 2002.
- SUPRÉ, K.; HAESEBROUCK, F.; ZADOKS, R.N. et al. Some coagulase-negative Staphylococcus species affect udder health more than others. J. Dairy Sci., v.94, p.2329-2340, 2011.
- SURIYASATHAPORN, W.; SCHUKKEN, Y.H.; NIELEN, M.; BRAND, A. Low somatic cell count: a risk factor for subsequent clinical mastitis in a dairy herd. J. Dairy Sci., v.83, p.1248-1255, 2000.
- TAPONEN, S.; LISKI, E.; HEIKKILÄ, A.M.; PYÖRÄLÄ, S. Factors associated with intramammary infection in dairy cows caused by coagulase-negative staphylococci, Staphylococcus aureus, Streptococcus uberis, Streptococcus dysgalactiae, Corynebacterium bovis, or Escherichia coli. J. Dairy Sci., v.100, p.493-503, 2017.
- TOMAZI, T.; GONÇALVES, J.L.; BARREIRO, J.R. et al. Bovine subclinical intramammary infection caused by coagulase-negative staphylococci increases somatic cell count but has no effect on milk yield or composition. J. Dairy Sci., v.98, p.3071-3078, 2015.
- WELLNITZ, O.; ARNOLD, E.T.; LEHMANN, M.; BRUCKMAIER, R.M. Short communication: differential immunoglobulin transfer during mastitis challenge by pathogen-specific components. J. Dairy Sci., v.96, p.1681-1684, 2013.
- WELLNITZ, O.; ZBINDEN, C.; HUANG, X.; BRUCKMAIER, R.M. Short communication: differential loss of bovine mammary epithelial barrier integrity in response to lipopolysaccharide and lipoteichoic acid. J. Dairy Sci., v.99, p.4851-4856, 2016.
- ZADOKS, R.N.; FITZPATRICK, J.L. Changing trends in mastitis. Ir. Vet. J., v.62, p.S59, 2009.
- ZHAO, X.; LACASSE, P. Mammary tissue damage during bovine mastitis: causes and control. J. Anim. Sci., v.86, p.57-65, 2008.
Publication Dates
-
Publication in this collection
06 June 2019 -
Date of issue
Mar-Apr 2019
History
-
Received
25 July 2017 -
Accepted
14 Feb 2018