ABSTRACT
Due to the doubts and questions about the inflammatory reaction caused by chemical castration, this study aimed to use infrared thermography to detect, evaluate and monitor the inflammatory reaction caused by the intratesticular injection of calcium chloride (CaCl2) 20% with lidocaine 1%. For this, thermographic measurements were taken before (M0), 10 minutes (M1), 1 and 6 hours (M2 and M3), for 7 consecutive days (M4 to M10), at 15 (M11), 30 (M12) and 60 (M13) days after intratesticular injection. Additionally, changes to testicular tissue and effects over spermatogenesis were evaluated by andrological exam before (M0) and 60 days (M13) after intratesticular injection. All cats were orchiectomized at M13, and testicles were submitted to histological analysis. CaCl2 (20%) with lidocaine (1%) administration produced testicular tissue damage and interfered with the spermatogenesis in 70% of treated cats without exacerbating the inflammatory reaction or impairing the cat’s welfare. It was concluded that thermographic evaluation is a useful, efficient, easy and quick method to diagnose and monitor cat testicular inflammatory reactions.
Keywords:
chemical castration; electroejaculation; feline; semen analysis; thermography; testicular degeneration
RESUMO
Devido a constantes dúvidas e questionamentos sobre a reação inflamatória ocasionada pela castração química, este estudo objetivou o uso da termografia infravermelha para detectar, avaliar e monitorar a reação inflamatória causada pela injeção intratesticular de cloreto de cálcio (CaCl 2 ) 20% associada à lidocaína 1%. Para isso, medidas termográficas foram aferidas antes (M0), 10 minutos (M1), uma e seis horas (M2 e M3), por sete dias consecutivos (M4 a M10), aos 15 (M11), 30 (M12), e 60 (M13) dias após injeção intratesticular, nos grupos tratado e controle. Todos os gatos foram orquiectomizados no M13, e os testículos submetidos à análise histológica. A injeção CaCl 2 a 20% associada com lidocaína a 1% produziu lesão testicular e interferiu na espermatogênese de 70% dos gatos tratados, sem exacerbar a reação inflamatória ou prejudicar o bem-estar do animal. Foi concluído que a avaliação termográfica é uma ferramenta útil, eficiente, rápida e fácil para o diagnóstico e o monitoramento da reação inflamatória em gatos.
Palavras-chave:
castração química; felinos; termograma; eletroejaculação; análise semin
INTRODUCTION
Due to stray population control needs and welfare maintenance, alternatives to surgical castration have been studied, which are less costly, have fewer adverse effects and save time for veterinary practitioners. Available worldwide, beyond orchiectomy, there are alternatives to male contraception, including hormonal, immunological and chemical methods (Moldave; Rhodes, 2013MOLDAVE, K.; RHODES, L. Contraception and fertility control in dogs and cats. (Eds.) Alliance for contraception in cats & dogs. ACC&D, 2013. Available in: <http://www.acc-d.org/docs/default-source/Resource-Library-Docs/accd-e-book.pdf?sfvrsn=0>. Accessed in: November 22nd, 2015
http://www.acc-d.org/docs/default-source...
).
Chemical compounds, used as a chemical castration method when injected into the testis, can cause an inflammatory reaction that may progress to testicular degeneration, and it is one of the causes of infertility in males (Nascimento and Santos, 2003NASCIMENTO, E.F.; SANTOS, R.L. Patologia da bolsa escrotal e do testículo. In: _____. (Eds.). Patologia da reprodução dos animais domésticos. Rio de Janeiro: Guanabara Koogan, 2003. p.91-104.). Calcium chloride (CaCl2) has being tested in bovines (Koger, 1978KOGER, L.M. Calcium chloride castration. Modern Vet. Prac., 1978. 119-121p. ), rats (Jana et al., 2002JANA, K.; SAMANTA, P.K.; GHOSH, D. Dose-dependent response to an intratesticular injection of calcium chloride for induction of chemosterilization in adult albino rats. Vet. Res. Commun., v.26, p.651-673, 2002.; Jana and Samanta, 2006), goats (Jana et al., 2005), dogs (Jana and Samanta, 2007) and cats (Jana and Samanta, 2011) with varying concentrations and dosages. Although it can cause infertility, the interruption of androgenizes is questionable (Jana and samanta, 2011; Leoci et al., 2014aLEOCI, R.; AIUDI, G.; SILVESTRE, F.; LISSNER, E.A.; LACALANDRA, G.M. Alcohol diluent provides the optimal formulation for calcium chloride non-surgical sterilization in dogs. Acta Vet. Scand., v.56, p.62, 2014a.; Leoci et al., 2014b).
Thermography is a diagnostic imaging method that measures the infrared radiation directly emitted by the skin, and it can be used to diagnose inflammatory lesions of the underlying tissue (Meira et al., 2014MEIRA, L.; KRUEGER, E.; NEVES, E.; NOHAMA, P.; SOUZA, M. Termografia na área biomédica. Pan Am. J. Med. Thermol., v.1, p.31-41, 2014.). Thermography assists in locating and graduating tissue involvement as well as providing a quantitative analysis of the measured data (Pereira, 2012PEREIRA, V.H. A termografia como auxílio diagnóstico na medicina. Paraná: Universidade Tuiuti do Paraná, 2012.). Due to technological advances, technique sensitivity, simplicity, objectivity, quickness, safety for the patient and operator, and excellent cost benefit, this diagnostic method is receiving attention in the biomedical field (Pereira, 2012). For humans, it has been used as an adjuvant for breast neoplasia diagnosis, vascularization disorders, and sports medicine (Fernández-Cuevas et al., 2015). In veterinary medicine, infrared thermography is mainly used to diagnose equine soft tissue injuries (Çetinkaya and Demirutku, 2012ÇETINKAYA, M.A.; DEMIRUTKU, A. Thermography in the assessment of equine lameness. Turk. J. Vet. Anim. Sci., v.36, p.43-48, 2012.), bovine thermal reproductive stress indicators (Menegassi et al., 2014MENEGASSI, S.R.O.; BARCELLOS, J.O.J.; DIAS, E.A. et al. Scrotal infrared digital thermography as a predictor of seasonal effects on sperm traits in Braford bulls. Int. J. Biometeorol., p.357-364, 2014.), and we hypothesize to be an instantly effective method to diagnose inflammatory reactions of the cat testis after sclerosing substance injection for chemical castration.
Faced with the need to obtain a chemical castrating substance that does not interfere with animal welfare, the aim of this study was to detect, evaluate and monitor the inflammatory reaction caused by the intratesticular injection of 20% CaCl2 with 1% lidocaine by infrared thermography, as well as the effects on spermatogenesis according to andrological exam and testis histology.
MATERIALS AND METHODS
Animals were managed in accordance to the Brazilian National Council for Control of Animal Experimentation (CONCEA) and approved by the Ethics Committee on the use of Animals (CEUA) at the Universidade Estadual de Londrina, under CEUA order number 29850.2013.38. Twelve mixed breed stray tomcats, weighing 3.8±0.7kg, were used with permission from the guardians. The ages of the cats were unknown, but they were all adult because they already had penile spines.
The cats were randomly divided into two groups: NaCl (n= 6), treated with 0.9% NaCl, and CaCl2 (n= 6) treated with 20% CaCl2 with 1% lidocaine. After anesthesia, infrared thermography, testicular biometry, penile spicule observation, seminal collection/evaluation and intratesticular injection either with 0.25mL 0.9% NaCl or 20% CaCl2 with 1% lidocaine, were performed. After sixty days, the cats were anesthetized again, for testicular biometry, penile spicule observation, seminal collection/evaluation and orchiectomy.
The CaCl2 solution was prepared diluting 0.022mg of CaCl2 in 0.5mL of sterile water, and later, added 0.5mL of lidocaine 2% (Lidovet®, Lidocaine hydrochloride 2% Bravet, Brazil). Its injections were conducted with a 1mL syringe and a 27 - gauge ½ needle, which was gently and directly introduced on the caudal aspect of each testis, 0.5cm from the epididymal tail, through the medial region of the organ. The substance was slowly deposited in the cranial portion of the testis. After the injection, all cats were housed in a cattery for 7 days with free access to premium food and water for clinical and thermographic evaluation.
The anesthetic protocol was established with intramuscular 12mg/Kg of ketamine (Dopalen®, Ceva, Brazil) associated with 30µg/Kg of medetomidine (Dormitor®, Medetomidine, Pfizer, France). When necessary, additional doses were given with one-third of the initial ketamine dose (iv). The anesthesia was reversed with 1mg/Kg of yohimbine (Drogavet - preparation pharmacy, Brazil). Meloxicam (0,2mg/Kg, IM, Maxican®, meloxicam, Ourofino, Brazil) was performed in the anesthetized animals prior to the procedures. The vital parameters, such as the heart and respiratory rates, mucosal staining, capillary refill time and pulse intensity were monitored throughout the procedures, which lasted 40 to 60 minutes, however, intratesticular injection takes less than 40 seconds to be performed.
Thermographic assessments of the testicular area were performed with a thermographic camera (Flir® model T440, Boston, MA, USA) with the animals in the dorsal recumbent position, after being physically restrained, except at M0 and M13 moments, when the measurements were taken after being pharmacologically restrained. The measurements were taken before (M0), 10min (M1), one (M2) and six (M3) hours after intratesticular injection, during seven consecutive days (M4-10), at 15 (M11), 30 (M12) and 60 days (M13) of intratesticular injection. To standardize the images, a distance of 60cm between the thermographic camera and testicles was maintained. From M4 to M10, measurements were taken in the morning, between 9 and 10 hours in the acclimatized cattery (23°C). M11 and M12 measurements were conducted at the guardian house during different times of day. Flir quick report Software ® (2009) was used to calculate testicular temperature average.
Rectal temperature was also measured during all anesthetic procedures and at the thermography measurements. The length (L), width (W) and depth (D) of each testis were measured in cm before (M0) and 60 days (M13) after intratesticular injection, using stainless digital calipers (Kobalt®, model 53247, North Wilkesboro, North Carolina, USA). The formula used to calculate the testicular volume (TV) was proposed by Tebet (2004TEBET, J.M. Efeitos da criopreservação sobre a célula espermática em três espécies de felinos : o gato-do-mato-pequeno (Leopardus tigrinus - Schreber, 1775), a jaguatirica (Leopardus pardalis- Linnaeus, 1758 ) e o gato doméstico (Felis catus). 2004. 116f. Tese (Doutorado em Medicina Veterinária) - Universidade Estadual Paulista, Faculdade de Medicina Veterinária e Zootecnia, Botucatu, SP.) as follows: 3/4 x π x C/2 x L/2 x D/2. π = 3.14. To calculate total testicular volume (TTV) the right and left TV were added and the results were presented in cm3.
Semen collection was done by electroejaculation (EEJ) at M0 and M13. The protocol used here was modified from the one described by Howard et al. (1990HOWARD, J.G.; BROWN, J.L.; BUSH, M.; WILDT, D.E. Teratospermic and normospermic domestic cats: ejaculate traits, pituitary-gonadal hormones, and improvement of spermatozoal motility and morphology after swim-up processing. J. Androl., v.11, p.204-215, 1990.) (Martins, M.I.M. personal communication). In this case, each stimulus was 10 seconds in duration, 80 stimuli were performed varying between 2 and 4 Volts divided into three series with 5-minute intervals for each series. The seminal analysis was performed immediately after collection at M0 and M13. The concentration and kinetic parameters were analyzed by CASA (Computer Assisted Sperm Analyzer, Hamilton-Thorne IVOS, Beverly, MA, USA) using the setup for feline semen. Five µl of semen was laid up in a Cell- VU slide (Millennium Sciences Inc., New York, USA), placed on the pre-warmed stage (35°C). Six randomly selected fields were analyzed with 30 frames acquired per field, at a frame rate of 60Hz. Sperm with straightness ≥80%, VAP ≥70mm/s and ≥50mm/s progressively motile were considered. The cutoff VAP was 30mm/s and VSL 20mm/s. Morphological changes in stained semen smears (Pope et al., 1991POPE, C.E.; ZHANG, M.; DRESSER, L.B. A simple staining method for evoluating acrosomal statue of cat sperm. J. Zoo Wildl. Med., v.22, p.87-95, 1991.) counting of 100 cells, under an optical microscope at 1000x magnification and classified into major and minor defects.
The kinetic evaluation path velocity - VAP; curvilinear velocity - VCL, linear velocity - VSL; amplitude of lateral displacement of the head - ALH; linearity - LIN; and straightness - STR, were determined. Additionally, the oscillation index (WOB) = (VAP/VCL)*100, sperm velocity index (SVI) = (VCL*0.87) + (VSL*0.76) + (VAP*0.90) + (ALH*0.92) and sperm motility index (SMI) = (VSL*0.59) + (VAP*0.37) + (LIN*0.95) + (STR*0.89) + (0.83*WOB) were calculated according to Núñez-Martínez et al. (2006).
After injection, all cats were housed in an institutional cattery for 7 days, with free access to premium food and water, for clinical and thermographic evaluation. The room temperature was maintained at approximately 23°C for optimal comfort and to minimize interference with the testicular thermographic measures. Because the cats were not accustomed to being handled, captivity or people, a Feliway® diffuser (Ceva, Paulínea, Brazil) was kept on all the times, which contains a synthetic analog of cat facial pheromones that has been indicated to provide a sense of security and tranquility to animals (Pereira et al., 2015PEREIRA, J.S.; FRAGOSO, S.; BECK, A. et al. Improving the feline veterinary consultation: the usefulness of Feliway spray in reducing cats’ stress. J.Feline Med. Surg., v.18, p.959-964, 2015.). The handling, feeding and clinical evaluation was performed by the same person to avoid the stress of a non familiar person, which could interfere with the clinical evaluation.
Before (M0) the intratesticular injection and at 6 hours (M5), for 7 consecutive days (M4-10) at 15 (M11), 30 (M12) and 60 days (M13), general attitude, ability to interact with the environment, appetite, scrotal skin, and cat testes were observed/palpated. Even without scoring, behavioral items were evaluated as suggested by the validated pain scale in cats according to Brondani et al. (2013BRONDANI, J.T.; MAMA, K.R.; LUNA, S.P.L. et al. Validation of the English version of the UNESP-Botucatu multidimensional composite pain scale for assessing postoperative pain in cats. BMC Vet. Res., v.9, p.143, 2013.). Sixty days after NaCl and CaCl2 testicular injection, all animals underwent orchiectomy. All animals received 30mg/kg cefazolin - iv during surgery as well as 25mg/kg dipyrone, orally, every 8 hours for 3 days. The bandage was done once daily for 7 days with chlorhexidine spray.
After orchiectomy, testicles were longitudinally sliced and fixed in 10% buffered formalin solution, dehydrated in graded alcohols and embedded in paraffin for histological analysis. Sections of 5μm thickness were stained with haematoxylin and eosin (HE), and mounted with coverslips. Histological changes were evaluated using a tissue score where the following criteria were considered: degeneration, necrosis, calcification, fibrosis and inflammatory infiltrate. The histological score was based on whether lesions affected seminiferous tubules and Sertoli cells, interstitial tissue and Leydig cells for each testicle separately. The histological score considered lesion extension and its intensity from 0 to 3; score 0 for normal tissues (unchanged); score 1 for mild changes (less than 20% of affected testicular tissue); score 2 for moderate changes (20% to 50%); and score 3 for severe changes (more than 50%).
The Shapiro-Wilk normality test was applied to all variables. To compare thermography measures, TTV, sperm concentration and kinetic parameters before (M0) and after injection (M13), T test were used for samples with homogeneous distribution and Wilcoxon for non-homogeneous samples. ANOVA was also used to compare the temperature at the 14 different timepoints between groups (NaCl vs CaCl2). Five percent of significance level was applied by R software version 3.2.2 (2015). The results from the histologic evaluation were described according to the frequency of findings and scores.
RESULTS
Testicular temperature mean and standard error of each moment for the NaCl and CaCl2 group are displayed in Figure 1. The comparison of the inflammatory reaction between NaCl and CaCl2 each time did not reveal a significant difference, demonstrating that the clinical manifestation of the inflammatory reaction caused by the application of NaCl and CaCl2 in cat testes had the same intensity. When comparing M0 and other moments, the NaCl group showed significant differences at M1 (29.11°C) and M13 (28.55°C) and the CaCl2 group showed differences at M11 (33.17ºC) and M12 (33.28°C) (P< 0.05). The rectal temperature was within the normal average for the species in all thermographic measures.
Average temperature, in Celsius, and standard error of 12 cat scrotal areas (NaCl and CaCl2 groups) measured by infrared thermography, before and after intratesticular sclerosing agent injection.
The TTV average and reduction percentages in both groups are summarized in Table 1. The NaCl group had 6.19% reduction versus 19.18% in the CaCl2 group. There was no significant difference in the TTV size and reduction percentages between the two groups. Testes that presented a reduction in size greater than 40% had firmer consistency.
Total feline testicular volume (in cm3) observed before and 60 days after 0.9% NaCl (n= 6) and 20% CaCl2 with 1% lidocaine (n= 6) intratesticular injection and reduction percentage, Londrina, Pr, Brazil, march - 2017
One animal in the NaCl group had total motility (TM)= 5% and progressive motility (PM)= 2% at M13. Cats in the NaCl group showed 38% morphological sperm defects, which later increased to 48%. NaCl promoted sperm motility reduction in 4/6 animals (below 45%).The concentration parameters, kinetic rates, morphology percentage and kinetic indexes showed no significant difference, except beat cross frequency (BCF) in the NaCl group (P≤ 0.05).
CaCl2 group morphological sperm defects were 37% at M0 and 57% at M13. The 20% CaCl2 with 1% lidocaine treatment promoted oligospermia in 1/6 animals and necrospermia in 3/6 (TM= 0%) animals. Although there was no statistical significance, the motility average decreased in both groups overall. Sperm morphological changes were found before and after the testicular injection. Coiled tail and acrosome defects were intensified in the NaCl group, as well as intermediate pieces and acrosome defects in the CaCl2 group. All results are described in Table 2.
Two to three hours from anesthesia reversal, the cats were already eating and drinking water. Testicular palpation was performed for seven consecutive days, and all cats (100%) seemed to be calm and tolerant of this procedure. An increased testicular volume and consistency in the first seven days was noted, which gradually decreased until the 15th day following injection. The testes of the cats in the NaCl group returned their normal fibroelastic consistency after the 15th day. No group showed scrotal dermatitis. Four cats in the CaCl2 group had penile spine testosterone-dependent size reduction.
During orchiectomy, it was noted that all testicles in the CaCl2 group and 4/12 testicles in the NaCl group were adhered to the vaginalis tunica. Microscopic evaluation identified different degrees and severity of injuries to the testicular tissue. Two testes in each group presented Leydig cell hyperplasia. The absolute frequencies of the testicular lesions are summarized in Table 3. The total score for the NaCl group was 30 and for the CaCl2 group was 70. In the NaCl group, histological lesions were limited to tubular degeneration, while in the CaCl2 group necrosis, inflammatory infiltrate and fibrosis were also observed.
DISCUSSION
The inflammatory reaction occurrence in chemical castration has been questioned regarding the interference on animal welfare. With this in mind, this study aimed to use infrared thermography to detect, evaluate and monitor testicular (Figure 2) scrotal superficial temperature variation that indicates inflammatory reaction on underlying tissue, after intratesticular injection of 20% CaCl2 associated with 1% lidocaine. Additionally, the effectiveness of this substance was evaluated as an alternative to surgical sterilization in cats.
Thermal image of cat testicles (white circle) at M0 (A), M1 (B) and M3 (C) moments by a thermographer warm color palette and the same moments, (D), (E), and (F), respectively according to the software medical palette that can be used to locate the regions with higher and lower temperature intensities and can be used to map the alterations.
To the authors’ knowledge, there is no previous study using infrared thermography to evaluate scrotal temperature in cats or to monitor the inflammatory reaction caused by CaCl2 with lidocaine testicular injection in cats. The first impasse of this study was related to the methodology for measuring feline scrotal temperature by the infrared thermography. There is no literature describing it, and it was necessary to establish a standard protocol for measurement modeling. Based on human research, it is known that many factors can interfere with thermographic measurements (Fernández-Cuevas et al., 2015) such as environmental, individual (species, metabolism, age, and physical efforts) and technical factors (related to the equipment used and methodology analysis) (Fernández-Cuevas et al. 2015), and these should be avoided as much as possible.
To reduce environmental interference, and standardize the protocol, the cats were kept in a cattery with temperature maintained at 23°C from M0 to M10. This is an adaptation from Ring & Ammer (2000RING, E.; AMMER, K. The technique of infrared imaging in medicine. Thermol. Int., v.10, p.7-14, 2000.) and Fernández-Cuevas et al. (2015) studies in humans, that recommend environmental temperatures between 22 and 24ºC. To avoid metabolism interference, the measures were taken at the same time (9-10 AM), every day, and to avoid capture physical effort, they were kept in cages.
Environmental and metabolic interference is observed with the increase in scrotal temperature measurements at M11 and M12 in both groups, with significance in the CaCl2 group. Those measurements were performed at the guardians’ residence because the cattery did not have acclimatization and cats were hard to capture. Also, on the day of those measurements, environmental temperatures reached 31.6°C (Source: IAPAR 2015- IAPAR - Instituto Agronômico do Paraná). As it is possible to see, another environmental interference with the scrotal temperature drop at M13. These measurements were taken at the operation room where the temperature was approximately 20°C.
Scrotal temperature drop at M1 is related to the substance temperature injection, which were stocked at room temperature (23°C); in other words, it was approximately 7.8°C below cats testicular average temperature (30.2°C) when injected into the testes. This phenomenon is observed in two other two, and is not related to animal hypothermia (Pereira, 2009PEREIRA, L.F. Avaliação do cloreto de cálcio por via intratesticular como método de esterilização química em bovinos. São Paulo: Universidade de Franca, 2009. ; Torteli, 2014 - unpublished data). Although those interferences occurred, none of them prejudiced inflammatory reaction detection and monitoring during the 10-day period (M0, M2-10).
As shown by the comparative thermographic analysis between the groups, the inflammatory reaction had equal intensity in all thermographic measures. The increased temperature in both groups from M2 to M10 is a consequence of the inflammatory reaction and may also be caused by mechanical injury from the needle introduction and capsular distension. There is an increase in temperature in M2 in both groups, with peaks at M3. This might reveal, that in this study, due to the type of experimental design, the maximum inflammatory reaction was observed 6 hours after intratesticular injection. Tissue response to chemical substances occurs after the initial injury, reaches a peak at 2 to 4 hours, and is restored within 6 to 8 hours (Ringler et al. 2000RINGLER, D. J. Inflamação e reparo. In: JONES, T C; HUNT, R D; KING, N W Patologia Veterinária. 6th ed. Sao Paulo: Ed. Manole; 2000. p. 133. ). It is believed that the temperature during the upcoming measured moments (M4-10) fluctuated due to individual temperature variation but did not exceed the temperature measured at M3.
Immediately after intratesticular injection, all cats in the NaCl and CaCl2 groups had increased testicular consistency due to the volume injected. At this point, pain related to capsular distention could occur. However, the cats were under anesthesia at the injection time. The use of an anti-inflammatory agent (meloxicam) as a pre-medication contributed to control the testicular inflammatory reaction and testicular pain in the first 24 hours. Unlikely to the zinc gluconate intratesticular injection study in cats, in which the authors report discomfort after injection and analgesic rescue was necessary (Oliveira et al., 2012OLIVEIRA, E.C.S.; MULLER, P.M.; SILVA, F.L.M. et al. Oral administration of an anti-inflammatory does not compromise the efficacy of intra-testicular injection of zinc gluconate as a contraceptive for dogs. Anim. Reprod. Sci., v.132, p.207-212, 2012.).
It was possible to observe that CaCl2 salt enhances testicular tissue destruction, without magnifying local inflammatory reaction, when compared with NaCl injection, as evidenced by histological scores. The individual testis score demonstrated that even using the same substance volume and concentration, the tissue response mechanism is independent and may vary due to the involvement of sequentially local mediator responses (Ringler, 2000RINGLER, D. J. Inflamação e reparo. In: JONES, T C; HUNT, R D; KING, N W Patologia Veterinária. 6th ed. Sao Paulo: Ed. Manole; 2000. p. 133. ). Additionally, the penile spine size reduction in four animals in the CaCl2 group may be related to the reduction in testosterone production.
The decrease in spermatic quality observed in this study is consistent with the studies published by Jana and Samanta (2011JANA, K.; SAMANTA, P.K. Clinical evaluation of non-surgical sterilization of male cats with single intra-testicular injection of calcium chloride. BMC Vet. Res., v.7, p.39, 2011.). There is a concern regarding the fertility disruption after CaCl2 intratesticular injection because it can be temporary, with a possibility of regaining fertility one year after this treatment due to the return of testicular activity.
Among the evaluated seminal parameters, there was a wide variation in the sperm concentration between the two groups at M0. Seasonality, animal breed (inbreeding) (Jana and Samanta, 2011JANA, K.; SAMANTA, P.K. Clinical evaluation of non-surgical sterilization of male cats with single intra-testicular injection of calcium chloride. BMC Vet. Res., v.7, p.39, 2011.; Leoci et al., 2014bLEOCI, R.; AIUDI, G.; SILVESTRE, F. et al. A dose-finding, long-term study on the use of calcium chloride in saline solution as a method of nonsurgical sterilization in dogs: evaluation of the most effective concentration with the lowest risk. Acta Vet. Scand., v.56, p.63, 2014b.) and nutrition (Axnér and Linde-Forsberg, 2007AXNÉR, E.; LINDE FORSBERG, C. Sperm morphology in the domestic cat, and its relation with fertility: a retrospective study. Reprod. Dom. Anim., v.42, p.282-291, 2007.) influence the ejaculate quality. Because they were stray animals, there was no knowledge regarding their age/maturity, health history, diet or stressor events. During the initial part of the study, the cats were maintained in a quiet environment with enrichment and without street stressors. Consequently, their physical activity was reduced, and they were given high quality pet food. Ninety percent of the cats gained weight. The varying conditions for each individual and sample volume variation in the cats contributed substantially to seminal evaluation differences between groups.
It is believed that 20% CaCl2 is safe for use as a chemical castration agent in cats because it causes minimal local inflammatory reaction. The cats did not show systemic clinical signs or cutaneous scrotal dermatitis. No other behavioral changes related to pain were noted.
CONCLUSION
The results of this study have demonstrated that testicular area thermography is an efficient method for evaluating the scrotal temperature in cats. This method efficiently and qualitatively captures temperature changes; additionally, it is precise, safe and comfortable in routine veterinarian practice. The combination of 20% CaCl2 with 1% lidocaine causes local inflammation, but it does not compromise the cat’s welfare, and it has impaired spermatogenesis in 70% of the treated cats.
ACKNOWLEDGMENTS
The authors are very grateful to the Coordination for the Improvement of Higher Education Personnel (CAPES) by scholarship to Paranzini, C.S., North Parana University (UNOPAR), Arapongas, Brazil, for the production of the 20% CaCl2 with 1% lidocaine solution, Special Cats - premium cat food and Zoetis for the support. None of the authors of this paper has a financial or personal relationship with people or organizations that could inappropriately influence or bias the content of this paper.
REFERENCES
- AXNÉR, E.; LINDE FORSBERG, C. Sperm morphology in the domestic cat, and its relation with fertility: a retrospective study. Reprod. Dom. Anim., v.42, p.282-291, 2007.
- BRONDANI, J.T.; MAMA, K.R.; LUNA, S.P.L. et al. Validation of the English version of the UNESP-Botucatu multidimensional composite pain scale for assessing postoperative pain in cats. BMC Vet. Res., v.9, p.143, 2013.
- ÇETINKAYA, M.A.; DEMIRUTKU, A. Thermography in the assessment of equine lameness. Turk. J. Vet. Anim. Sci., v.36, p.43-48, 2012.
- FERNÁNDEZ-CUEVAS, I.; MARINS, J.C.B.; LASTRAS, J.A. et al. Classification of factors influencing the use of infrared thermography in humans : a review. Infrared Phys. Technol., v.71, p.28-55, 2015.
- HOWARD, J.G.; BROWN, J.L.; BUSH, M.; WILDT, D.E. Teratospermic and normospermic domestic cats: ejaculate traits, pituitary-gonadal hormones, and improvement of spermatozoal motility and morphology after swim-up processing. J. Androl., v.11, p.204-215, 1990.
- JANA, K.; SAMANTA, P.K. Evaluation of single intratesticular injection of calcium chloride for nonsurgical sterilization in adult albino rats. Contraception, v.73, p.289-300, 2006.
- JANA, K.; SAMANTA, P.K. Sterilization of male stray dogs with a single intratesticular injection of calcium chloride: a dose-dependent study. Contraception, v.75, p.390-400, 2007.
- JANA, K.; SAMANTA, P.K. Clinical evaluation of non-surgical sterilization of male cats with single intra-testicular injection of calcium chloride. BMC Vet. Res., v.7, p.39, 2011.
- JANA, K.; SAMANTA, P.K.; GHOSH, D. Dose-dependent response to an intratesticular injection of calcium chloride for induction of chemosterilization in adult albino rats. Vet. Res. Commun., v.26, p.651-673, 2002.
- JANA, K.; SAMANTA, P.K.; GHOSH, D. Evaluation of single intratesticular injection of calcium chloride for nonsurgical sterilization of male Black Bengal goats (Capra hircus): a dose-dependent study. Anim. Reprod. Sci., v.86, p.89-108, 2005.
- KOGER, L.M. Calcium chloride castration. Modern Vet. Prac., 1978. 119-121p.
- LEOCI, R.; AIUDI, G.; SILVESTRE, F.; LISSNER, E.A.; LACALANDRA, G.M. Alcohol diluent provides the optimal formulation for calcium chloride non-surgical sterilization in dogs. Acta Vet. Scand., v.56, p.62, 2014a.
- LEOCI, R.; AIUDI, G.; SILVESTRE, F. et al. A dose-finding, long-term study on the use of calcium chloride in saline solution as a method of nonsurgical sterilization in dogs: evaluation of the most effective concentration with the lowest risk. Acta Vet. Scand., v.56, p.63, 2014b.
- MEIRA, L.; KRUEGER, E.; NEVES, E.; NOHAMA, P.; SOUZA, M. Termografia na área biomédica. Pan Am. J. Med. Thermol., v.1, p.31-41, 2014.
- MENEGASSI, S.R.O.; BARCELLOS, J.O.J.; DIAS, E.A. et al. Scrotal infrared digital thermography as a predictor of seasonal effects on sperm traits in Braford bulls. Int. J. Biometeorol., p.357-364, 2014.
- MOLDAVE, K.; RHODES, L. Contraception and fertility control in dogs and cats. (Eds.) Alliance for contraception in cats & dogs. ACC&D, 2013. Available in: <http://www.acc-d.org/docs/default-source/Resource-Library-Docs/accd-e-book.pdf?sfvrsn=0>. Accessed in: November 22nd, 2015
» http://www.acc-d.org/docs/default-source/Resource-Library-Docs/accd-e-book.pdf?sfvrsn=0 - NASCIMENTO, E.F.; SANTOS, R.L. Patologia da bolsa escrotal e do testículo. In: _____. (Eds.). Patologia da reprodução dos animais domésticos. Rio de Janeiro: Guanabara Koogan, 2003. p.91-104.
- NÚÑEZ-MARTÍNEZ, I.; MORAN, J.M.; PEÑA, F.J. A three-step statistical procedure to identify sperm kinematic subpopulations in canine ejaculates: changes after cryopreservation. Reprod. Domest. Animal., v.41, p.408-415, 2006.
- OLIVEIRA, E.C.S.; MULLER, P.M.; SILVA, F.L.M. et al. Oral administration of an anti-inflammatory does not compromise the efficacy of intra-testicular injection of zinc gluconate as a contraceptive for dogs. Anim. Reprod. Sci., v.132, p.207-212, 2012.
- PEREIRA, J.S.; FRAGOSO, S.; BECK, A. et al. Improving the feline veterinary consultation: the usefulness of Feliway spray in reducing cats’ stress. J.Feline Med. Surg., v.18, p.959-964, 2015.
- PEREIRA, L.F. Avaliação do cloreto de cálcio por via intratesticular como método de esterilização química em bovinos. São Paulo: Universidade de Franca, 2009.
- PEREIRA, V.H. A termografia como auxílio diagnóstico na medicina. Paraná: Universidade Tuiuti do Paraná, 2012.
- POPE, C.E.; ZHANG, M.; DRESSER, L.B. A simple staining method for evoluating acrosomal statue of cat sperm. J. Zoo Wildl. Med., v.22, p.87-95, 1991.
- RING, E.; AMMER, K. The technique of infrared imaging in medicine. Thermol. Int., v.10, p.7-14, 2000.
- RINGLER, D. J. Inflamação e reparo. In: JONES, T C; HUNT, R D; KING, N W Patologia Veterinária. 6th ed. Sao Paulo: Ed. Manole; 2000. p. 133.
- TEBET, J.M. Efeitos da criopreservação sobre a célula espermática em três espécies de felinos : o gato-do-mato-pequeno (Leopardus tigrinus - Schreber, 1775), a jaguatirica (Leopardus pardalis- Linnaeus, 1758 ) e o gato doméstico (Felis catus). 2004. 116f. Tese (Doutorado em Medicina Veterinária) - Universidade Estadual Paulista, Faculdade de Medicina Veterinária e Zootecnia, Botucatu, SP.
Publication Dates
-
Publication in this collection
14 June 2019 -
Date of issue
May-Jun 2019
History
-
Received
16 Apr 2018 -
Accepted
07 Dec 2018


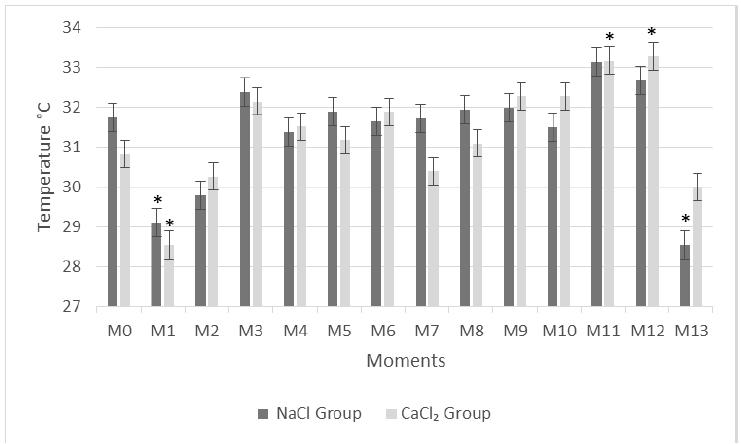 *Statistical significance evaluated between the initial control time (M0) for the same group (P< 0.05).
*Statistical significance evaluated between the initial control time (M0) for the same group (P< 0.05).
