ABSTRACT
The aim of this study was to evaluate the metabolic, inflammatory, and hepatic aspects, as well as the milk yield in heifers submitted to protocol for induction of lactation compared to primiparous cows. Sixty Holstein heifers were selected and enrolled into two groups: Control (n= 30), pregnant heifers and Induction heifers (n= 30), non-pregnant femeales, submitted to a lactation induction protocol. Blood samples were collected at: pre-lactation period (weeks -3, -2 and -1) and post-lactation period (weeks 1, 2 and 3), aiming to evaluate glucose, non-esterified fatty acids, paraoxonase-1, albumin, ALT, GGT and cortisol. The protocol efficiently induced lactation in all the heifers, which produced 74.54% of the total production of milk from primiparous cows. In the pre-lactation period, induced animals presented higher concentrations of non-esterified fatty acids than the Control heifers, and the opposite was observed in the post lactation period. In both moments albumin and ALT were lower in the Induction group, and paraoxonase-1 activity and GGT concentrations were higher, compared to the Control. Thus, lactation induction protocol is efficient to initiate milk production in dairy heifers with no considerable changes in energetic, metabolic and hepatic profile when compared to heifers in physiological lactation.
Keywords:
energetic status; hepatic profile; hormonal protocol; inflammatory
RESUMO
O objetivo deste estudo foi avaliar os perfis metabólico, inflamatório, hepático e a produção de leite de novilhas induzidas à lactação comparadas a primíparas. Sessenta novilhas da raça Holandês foram selecionadas e alocadas em grupos: controle (n=30), novilhas prenhas, e indução (n=30), novilhas vazias submetidas a um protocolo de indução de lactação. As amostras de sangue foram coletadas nas semanas -3, -2 e -1 (pré-lactação) e nas semanas 1, 2 e 3 (pós-início de lactação) para avaliação de glicose, ácidos graxos não esterificados, paraoxonase-1, albumina, ALT, GGT e cortisol. O protocolo induziu eficientemente a lactação em todas as novilhas, que produziram 74,54% da produção total de leite do controle. No período pré-lactação, o grupo indução apresentou maiores concentrações de ácidos graxos não esterificados que o controle, e o oposto foi observado pós-lactação. Em ambos os momentos, albumina e ALT foram menores no grupo indução, e a atividade da paraoxonase-1 e as concentrações de GGT foram maiores, em comparação ao controle. Assim, o protocolo de indução de lactação foi eficiente para iniciar a produção de leite em novilhas induzidas, além de terem sido observadas alterações nos perfis energético, metabólico e hepático em comparação a novilhas em lactação fisiológica.
Palavras-chave:
estado energético; perfil hepático; protocolo hormonal; inflamatório
INTRODUCTION
In the last decades, genetic selection of dairy cows for high milk yield has negatively affected fertility, increasing the culling rates of animals due to reproductive failure. Infertility is considered one of the main reasons for culling and is among the costliest factors in dairy farms, involving direct costs associated to rearing or purchasing the animals, and indirect costs related to maintenance of unproductive animals in the system (Pritchard et al., 2013PRITCHARD, T.; COFFEY, M.; MRODE, R. et al. Genetic parameters for production, health, fertility and longevity traits in dairy cows. Animal, v.7, p.34-46, 2013.).
A strategy to minimize the problems related to the culling of females in productive age, enabling these cows with high genetic merit for milk production to remain in the farm, is the use of protocols of artificial induction of lactation (AIL), which allows lactations to be obtained in the absence of gestation (Freitas et al., 2010FREITAS, P.R.C.; COELHO, S.G.; RABELO, E. et al. Indução artificial de lactação em bovinos. Rev. Bras. Zootec., v.39, p.2268-2272, 2010.). The conventional hormonal protocol lasts for approximately 21 days (Freitas et al., 2010), with daily administrations of hormones (estradiol, progesterone, prostaglandin and corticoids), which make it possible to simulate the endocrine profile observed in the last days of cow’s gestation.
Most of the previous studies evaluated the productive and reproductive aspects after the AIL protocol and report satisfactory results, i.e. from 85 to 100% of cows initiating lactation, as well as around 70 to 78% of the milk production expected for cows in a physiologic lactation. An improvement in fertility has also been reported, with pregnancy rates varying from 41.4% to 71% during the induced lactation (Mellado et al., 2006MELLADO, M.; NAZARRE, E.; OLIVARES, L. et al. Milk production and reproductive performance of cows induced into lactation and treated with bovine somatotropin. Anim. Sci., v.82, p.555-559, 2006.; Freitas et al., 2010FREITAS, P.R.C.; COELHO, S.G.; RABELO, E. et al. Indução artificial de lactação em bovinos. Rev. Bras. Zootec., v.39, p.2268-2272, 2010.), although the mechanisms involved are completely unknown. In the same regard, limited information regarding the metabolism and endocrinology of the animals submitted to AIL are available.
The physiological adaptations that occur in periparturient dairy cows during the transition from a pregnant and non-lactant to non-pregnant and lactant status is well established. Peripartum is a critical period, envolving physiological events and homeorhetic adaptations characterized by decreased energy intake and lipomobilization during early lactation, which are commonly negatively aftected by transition period diseases. Besides that, the cows also experience immunosuppression, aggravated by dramatic changes in circulating progesterone, estrogen and cortisol concentrations (Leblanc, 2012LEBLANC, S.J. Interactions of metabolism, inflammation, and reproductive tract health in the postpartum period in dairy cattle. Reprod. Domest. Anim., v.47, p.18-30, 2012.). Since lactation artificially induced cows to not experience most of the dramatic changes involved with parturition, the objective of this study was to evaluate the energetic and hepatic metabolism, inflammatory status, as well as milk production in heifers submitted to an induction protocol of lactation in comparison to primiparous cows.
MATERIALS AND METHODS
All procedures involving animals in the study were approved by the University of Pelotas Animal Care and Use Committee and by the University of Pelotas Research Committee (CEEA 8716-2016).
Sixty Holstein heifers averaged 32±0.6 months of age, with a body condition score between 3-3.5 (Rennó et al., 2011RENNÓ, F.P.; BARLETTA, R.V.; JUNIOR, J.E.F. et al. Escore de condição corporal e sua relação com a produtividade, saúde e bem estar de vacas em lactação. In: SIMPÓSIO INTERNACIONAL DE BOVINOCULTURA LEITEIRA, 3., 2011, Viçosa. Anais... Viçosa: [s.n.], 2011. p.335-370. (Resumo).) were selected and allocated into two groups: Control Group (n= 30), pregnant heifers, evaluated from 21 days prepartum to 224 days in milk (DIM); and Induction Group (n= 30), non-pregnant heifers, submitted to artificial induction of lactation protocol. From the 1st to 8th day, 30 mg of estradiol benzoate (Sincrodiol® Ourofino Saúde Animal, São Paulo, Brasil) were administered daily, with 300mg of progesterone (Sincrogest® Ourofino Saúde Animal, São Paulo, Brasil). From the 9th until 14th, animals received only daily doses of 20mg EB. On the 16th day, 0.56mg of sodium cloprostenol (Sincrocio® Ourofino Saúde Animal, São Paulo, Brasil) was administered and injections of 40mg of dexamethasone sodium (Cortiflan® Ourofino Saúde Animal, São Paulo, Brasil) phosphate were administered daily from day 19 to 21. On days 1, 8, 15 and 22, the animals received a dose of 500mg of bovine somatotropin bovine (BST) (Lactotropin® Elanco Saúde Animal, São Paulo, Brasil). On the 22nd day, milking began, heifers and cows being milked twice a day until 224 DIM. Table 1 shows the diet composition of daily rations for both groups in the late 21 days before lactation and during lactation.
Nutrient composition of daily rations for heifers of Control Group and Induction Group 21 days before lactation and during lactation
In both groups, weekly blood collections were performed, from 21 days before the beginning of lactation (weeks: -3, -2 and -1) until 21 days in milking (weeks: 1, 2 and 3). Blood was collected from the coccygeal arteriovenous complex by the vacutainer system, into two tubes without anticoagulant for posterior evaluation of the energetic, inflammatory and hormonal parameters and one tube with sodium fluoride for evaluation of the glucose levels. Immediately after blood collection, the samples were centrifuged, processed and frozen (-20ºC), in microtubes until further analysis.
Serum of Induction (n= 30) and Control (n= 30) heifers were analyzed at weeks -3, -2, -1, 1, 2 and 3 for paraoxonase-1 (PON1) activity, non-esterified fatty acids (NEFA), albumin, ALT, GGT and glucose. The PON1 activity was analyzed by spectrophotometry according to methodology described by Browne et al. (2007BROWNE, R.W.; KOURY, S.T.; MARION, S. et al. Accuracy and biological variation of human serum paraoxonase 1 activity and polymorphism (Q192R) by kinetic enzyme assay. Clin. Chemist., v.53, p.310-317, 2007.). Samples for the other metabolites were analyzed in the automatic biochemical analyzer Labmax Plenno (Labtest Diagnóstica ®, Minas Gerais, Brazil), by spectrophotometry, programmed for biochemical and immunochemical tests with specific reagents for each analysis.
Subgroups from induced (n= 5) and control (n= 5) heifers were randomly selected for cortisol dosages evaluating at -3, -2, -1, 1, 2 and 3 weeks. These analyses were performed by chemiluminescence using the Access2 Immunoassay System (Beckman Coulter®, California, EUA) with specific kit (Accces Cortisol, Beckman Coulter®, California, EUA).
Milk production evaluation was carried out twice a day, checking the individual milk yield with the digital reader. Lactation was monitored weekly until the 28th week and then monthly, until the 8th month for milk yield comparison between groups. Since the animals were in the same milking herd, both groups were subject to the same routine herd management protocols regarding milking, breeding, and health care.
Biochemical and hormonal data were analyzed as repeated measures in a completely randomized design, considering the main effects of group (Control and Induced) over time (e.g., week -3 to 0 and 1 to 3 related to the beginning of lactation) and with cow as a random effect. Data were analyzed by repeated measurements of ANOVA (NCSS 2004 and PASS 2005 Number Cruncher Statistical Systems. Kaysville, Utah) using the following statistical model:
where: Yijklis the dependent continuous variable, μ is the overall mean, Mjis fixed effect of group (j = control vs. induction), Tkis the fixed effect of time (6 weeks), MTjkis the interaction between group and time, cl(bi) is the random effect of cow, and eijklis the random residual error.
Milk production was analyzed by ANOVA considering the main effects of group (Control and Induced), week and their first order interaction until 224 DIM.
RESULTS AND DISCUSSION
All the heifers submitted to AIL responded to the protocol, i.e. reached more than 12kg/day at some point during lactation. From the 1st to the 32nd week of lactation, induced heifers produced 74.54% of the total production of milk from primiparous cows, being observed an average daily milk production of 19.17kg and 25.48kg, respectively (P< 0.05). Similar results were observed in previous studies in which induced Holstein cows produced 65 to 78% of the total milk yield produced by calved cows (Mellado et al., 2006MELLADO, M.; NAZARRE, E.; OLIVARES, L. et al. Milk production and reproductive performance of cows induced into lactation and treated with bovine somatotropin. Anim. Sci., v.82, p.555-559, 2006.; Freitas et al., 2010FREITAS, P.R.C.; COELHO, S.G.; RABELO, E. et al. Indução artificial de lactação em bovinos. Rev. Bras. Zootec., v.39, p.2268-2272, 2010.).
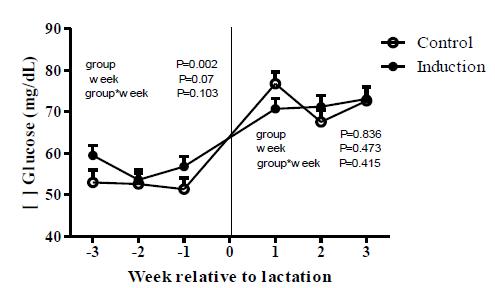
Glucose levels were different (P< 0.01) between groups at pre-lactation moment (Figure 1), when induced heifers had higher levels (56.7±0.9mg/dL) than Control animals (52.3±1mg/dL). No significant effects (P> 0.05) of week and week*group interaction were observed. During the transition period, glucose acts as an important energetic nutrient and approximately 85% of the total blood glucose is directed to the mammary gland (Bell and Bauman, 1997BELL, A.W.; BAUMAN, D.E. Adaptations of glucose metabolism during pregnancy and lactation. J. Mamm. Gland Biol Neoplasia, v.2, p.265-278, 1997.). The increase observed after the beginning of lactation could be caused by the increase in glucagon and glucocorticoids in both groups that promote the glycogen mobilization from the liver to support lactation (Grummer, 1995GRUMMER, R.R. Impact of changes in organic nutrient metabolism on feeding the transition dairy cow impact of changes in organic nutrient metabolism feeding the transition dairy cowl. J. Anim. Sci., v.73, p.2820-2833, 1995.).
Concentrations of glucose for control and induced heifers during pre-lactation (weeks -3, -2 and -1) and post-lactation (weeks 1, 2 and 3).
The NEFA concentrations were different (P< 0.001) between groups both pre- and post-lactation (Figure 2). Induced heifers had a peak of lipomobilization one week before the beggining of lactation, which could be related to acute energy demands of the mammary gland and other tissues involved in lactation, as well as the intense daily routine of induction protocol. In Control heifers, NEFA concentration increased progressively during both pre- and post-lactation as an adaptation of tissues to the increasing milk production.
Concentrations of non-esterified fatty acids (NEFA) for control (
 ) and induced (
) and induced ( ) heifers during pre-lactation (weeks -3, -2, -1) and post -lactation (weeks 1, 2 and 3).
) heifers during pre-lactation (weeks -3, -2, -1) and post -lactation (weeks 1, 2 and 3).
During peripartum, the neuroendocrine system interacts in different ways to maintain homeostasis and hormones such as insulin, somatotropin, cortisol and estrogen have important roles in lipolysis regulation (Bauman and Vernon, 1993BAUMAN, D.E.; VERNON, R.G. Effects of exogenous bovine somatotropin on lactatation. Annu. Rev. Nutr., v.13, p.437-461, 1993.; Bell and Bauman, 1997BELL, A.W.; BAUMAN, D.E. Adaptations of glucose metabolism during pregnancy and lactation. J. Mamm. Gland Biol Neoplasia, v.2, p.265-278, 1997.). Thus, the endocrine regulation causes an intense mobilization of adipose tissue, providing the nutrients required for maintenance and milk production and, consequently, increasing circulating NEFA. Besides that, the greater serum NEFA concentrations during the pre-lactation (moment of the protocol) for Induction group, can be associated to BST administration, whereas, according to some researches, BST has been shown to inhibit lipogenesis and stimulate activity in adipose tissues (Hart et al., 1984HART, I.C.; CHADWICK, P.M.; BOONE, T.C. et al. A comparison of the growth-promoting, lipolytic, diabetogenic and immunological properties of pituitary and recombinant-DNA-derived bovine growth hormone (somatotropin). Biochem. J., v.224, p.93-100, 1984.; Lanna et al., 1995LANNA, D.P.; HOUSEKNECHT, K.L.; HARRIS, D.M. et al. Effect of somatotropin treatment on lipogenesis, lipolysis, and related cellular mechanisms in adipose tissue of lactating cows. J. Dairy Sci., v.78, p.1703-1712, 1995.; Aboin et al., 2013ABOIN, A.C.; COOKE, R.F.; VIEIRA, F.V.R. et al. Effects of bovine somatotropin injection on serum concentrations of progesterone in non-lactating dairy cows. Livest. Sci., v.154, p.240-245, 2013.). The severity of lipomobilization is positively associated to immunosuppressive conditions and disease risk during the transition period (Leblanc, 2012LEBLANC, S.J. Interactions of metabolism, inflammation, and reproductive tract health in the postpartum period in dairy cattle. Reprod. Domest. Anim., v.47, p.18-30, 2012.).
The acute phase proteins (APP) have been used as important inflammatory biomarkers in dairy cows during the transition period (Huzzey et al., 2011HUZZEY, J.M.; NYDAM, D.V.; GRANT, R.J. et al. Associations of prepartum plasma cortisol, haptoglobin, fecal cortisol metabolites, and nonesterified fatty acids with postpartum health status in Holstein dairy cows. J. Dairy Sci., v.94, p.5878-5889, 2011.). In this study, induced heifers had higher (P< 0.05) PON1 activity compared to control in both moments (Figure 3). Pregnancy and parturition in dairy cows have a huge influence in the lipid metabolism and liver function, which increase the vulnerability to oxidative stress. Thereby, alterations in PON1 serum activity (involved in oxidative protection) could be used as a marker to detect diseases in cows during peripartum (Kulka et al., 2014KULKA, M., BEŁTOWSKI, J., KLUCIŃSKI, W. et al. Serum paraoxonase-1 activity of dairy Holstein-Fresian cows in different lactation stages-preliminary study. Pol. J. Vet. Sci., v.17, p.143-147, 2014.). Higher PON1 levels in induced heifers, at pre-lactation period, indicated that the protocol did not induce an inflammatory condition in the animals. Low levels of PON1 activity for calving heifers during the prepartum period indicate that the gestation is more challenging for animals regarding the inflammatory status, than the lactation induction, and may be an indicator that pregnant animals are more likely to be affected by diseases than induced.
Concentrations of paraoxonase-1 (PON1) activity for control and induced heifers during pre-lactation (weeks -3, -2 and -1) and post-lactation (weeks 1, 2 and 3).
Differently from the observed for PON-1, albumin levels were higher (P< 0.05) for the control group than for induced, at both moments (Figure 4). Albumin corresponds to approximately 50 to 65% of the total plasma proteins, and these concentrations can be affected by nutrition, hepatic function, amino acids bioavailability, dehydration and weight loss (Contreras and Sordillo, 2011CONTRERAS, G.A.; SORDILLO, L.M. Lipid mobilization and inflammatory responses during the transition period of dairy cows. Comp. Immunol. Microbiol. Infect. Dis., v.34, p.281-289, 2011.).
Concentrations of albumin for induced (
 ) heifers during pre-lactation (weeks -3, -2 and -1) and post-lactation (weeks 1, 2 and 3).
) heifers during pre-lactation (weeks -3, -2 and -1) and post-lactation (weeks 1, 2 and 3). ) and control (
) and control (
It is important to emphasize that the observed values were within physiological concentrations for the species, i.e. 2.7 to 3.8g/dL (Cozzi et al., 2011). Although, the lower albumin concentrations in induced heifers during pre-lactation can be a consequence of their metabolic status. The fact that induced heifers also had higher NEFA concentrations indicates lipomobilization, which can be explained by the daily routine and preparation for the beginning of lactation.
It is reasonable to believe that the AIL protocols would result in an intense hepatic metabolism, as a consequence of the daily hormone administration, although this hypothesis has not been investigated in dairy cows. Radavelli et al. (2016RADAVELLI, W.M.; CAMPIGOTTO, G.; MACHADO, G. et al. Effect of lactation induction on milk production and composition, oxidative and antioxidant status, and biochemical variables. Comp. Clin. Pathol., v.25, p.639-648, 2016.), have previously reported elevated ALT and GGT levels in ewes submitted to AIL protocol. However, in the present study the enzymes were differentialy regulated. While increased levels of ALT were observed in Control heifers in both periods (P< 0.05) (Figure 5), GGT levels were significantly higher in induced heifers and oscilated during the evaluated period (P< 0.05) (Figure 6). Although differentially modulated, the levels of both enzymes were within the physiological values for cattle, ALT 0-38U/L and GGT 0-39 U/L (Stojević et al., 2005STOJEVIĆ, Z.; PIRŠLJIN, J.; MILINKOVIĆ-TUR, S. et al. Activities of AST, ALT and GGT in clinically healthy dairy cows during lactation and in the dry period. Vet. Arch., v.75, p.67-73, 2005.).
Concentrations of ALT for control and induced heifers during pre-lactation (weeks -3, -2 and -1) and post-lactation (weeks 1, 2 and 3).
Concentrations of GGT for control (
 ) heifers during pre-lactation (weeks -3, -2 and -1) and post-lactation (weeks 1, 2 and 3).
) heifers during pre-lactation (weeks -3, -2 and -1) and post-lactation (weeks 1, 2 and 3). ) and induced (
) and induced (
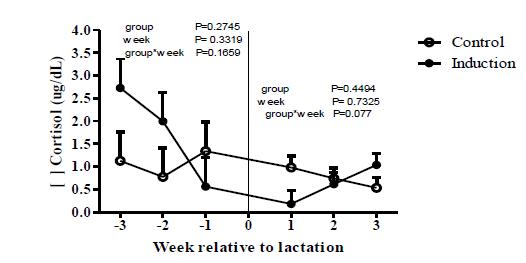
Cortisol release in dairy cows can be one of the primary adaptive mechanisms to adverse conditions, inducing short-term mobilization of body reserves and regulating inflammatory responses (Gross et al., 2015GROSS, J.J.; WELLNITZ, O. BRUCKMAIER, R.M. Cortisol secretion in response to metabolic and inflammatory challenges in dairy cows 1. J. Anim. Sci., v.93, p.3395-3401, 2015.). In the present study, no significant effects of group, week and their interaction (P> 0.05) were seen in both the pre- and post-lactation period (Figure 7).
Concentrations of cortisol for control and induced heifers during pre-lactation (weeks -3, -2 and -1) and post-lactation (weeks 1, 2 and 3).
Initially, it was expected that cortisol concentrations would be higher in induced heifers, since the AIL protocol involves intensive daily management, and daily corticoid injections on the last three days. Therefore, considering cortisol levels, our results suggest that the handling of the animals during the AIL protocol is not more stressful than the peripartum period.
CONCLUSIONS
Based upon the parameters investigated, lactation induction protocol is efficient to initiate milk production in dry dairy heifers with no considerable changes in energetic, and hepatic metabolism, as well as inflammatory status when compared to primiparous in physiological lactation. Also, lactation induction is a viable alternative procedure to have dairy cows producing milk with no parturition requirement, since their 25% lower milk production is taken into consideration.
ACKNOWLEDGMENTS
The authors acknowledge Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), the Núcleo de Pesquisa Ensino e Extensão em Pecuária (NUPEEC) for financial support, Granjas 4 Irmãos for assistance with the project.
REFERENCES
- ABOIN, A.C.; COOKE, R.F.; VIEIRA, F.V.R. et al. Effects of bovine somatotropin injection on serum concentrations of progesterone in non-lactating dairy cows. Livest. Sci., v.154, p.240-245, 2013.
- BAUMAN, D.E.; VERNON, R.G. Effects of exogenous bovine somatotropin on lactatation. Annu. Rev. Nutr., v.13, p.437-461, 1993.
- BELL, A.W.; BAUMAN, D.E. Adaptations of glucose metabolism during pregnancy and lactation. J. Mamm. Gland Biol Neoplasia, v.2, p.265-278, 1997.
- BROWNE, R.W.; KOURY, S.T.; MARION, S. et al. Accuracy and biological variation of human serum paraoxonase 1 activity and polymorphism (Q192R) by kinetic enzyme assay. Clin. Chemist., v.53, p.310-317, 2007.
- CONTRERAS, G.A.; SORDILLO, L.M. Lipid mobilization and inflammatory responses during the transition period of dairy cows. Comp. Immunol. Microbiol. Infect. Dis., v.34, p.281-289, 2011.
- COZZI, G.; RAVAROTTO, L.; GOTTARDO, F. et al. Reference values for blood parameters in Holstein dairy cows: effects of parity, stage of lactation, and season of production. J. Dairy Sci., v.94, p.3895-3901, 2001.
- FREITAS, P.R.C.; COELHO, S.G.; RABELO, E. et al. Indução artificial de lactação em bovinos. Rev. Bras. Zootec., v.39, p.2268-2272, 2010.
- GROSS, J.J.; WELLNITZ, O. BRUCKMAIER, R.M. Cortisol secretion in response to metabolic and inflammatory challenges in dairy cows 1. J. Anim. Sci., v.93, p.3395-3401, 2015.
- GRUMMER, R.R. Impact of changes in organic nutrient metabolism on feeding the transition dairy cow impact of changes in organic nutrient metabolism feeding the transition dairy cowl. J. Anim. Sci., v.73, p.2820-2833, 1995.
- HART, I.C.; CHADWICK, P.M.; BOONE, T.C. et al. A comparison of the growth-promoting, lipolytic, diabetogenic and immunological properties of pituitary and recombinant-DNA-derived bovine growth hormone (somatotropin). Biochem. J., v.224, p.93-100, 1984.
- HUZZEY, J.M.; NYDAM, D.V.; GRANT, R.J. et al. Associations of prepartum plasma cortisol, haptoglobin, fecal cortisol metabolites, and nonesterified fatty acids with postpartum health status in Holstein dairy cows. J. Dairy Sci., v.94, p.5878-5889, 2011.
- KULKA, M., BEŁTOWSKI, J., KLUCIŃSKI, W. et al. Serum paraoxonase-1 activity of dairy Holstein-Fresian cows in different lactation stages-preliminary study. Pol. J. Vet. Sci., v.17, p.143-147, 2014.
- LANNA, D.P.; HOUSEKNECHT, K.L.; HARRIS, D.M. et al. Effect of somatotropin treatment on lipogenesis, lipolysis, and related cellular mechanisms in adipose tissue of lactating cows. J. Dairy Sci., v.78, p.1703-1712, 1995.
- LEBLANC, S.J. Interactions of metabolism, inflammation, and reproductive tract health in the postpartum period in dairy cattle. Reprod. Domest. Anim., v.47, p.18-30, 2012.
- MELLADO, M.; NAZARRE, E.; OLIVARES, L. et al. Milk production and reproductive performance of cows induced into lactation and treated with bovine somatotropin. Anim. Sci., v.82, p.555-559, 2006.
- PRITCHARD, T.; COFFEY, M.; MRODE, R. et al. Genetic parameters for production, health, fertility and longevity traits in dairy cows. Animal, v.7, p.34-46, 2013.
- RADAVELLI, W.M.; CAMPIGOTTO, G.; MACHADO, G. et al. Effect of lactation induction on milk production and composition, oxidative and antioxidant status, and biochemical variables. Comp. Clin. Pathol., v.25, p.639-648, 2016.
- RENNÓ, F.P.; BARLETTA, R.V.; JUNIOR, J.E.F. et al. Escore de condição corporal e sua relação com a produtividade, saúde e bem estar de vacas em lactação. In: SIMPÓSIO INTERNACIONAL DE BOVINOCULTURA LEITEIRA, 3., 2011, Viçosa. Anais... Viçosa: [s.n.], 2011. p.335-370. (Resumo).
- STOJEVIĆ, Z.; PIRŠLJIN, J.; MILINKOVIĆ-TUR, S. et al. Activities of AST, ALT and GGT in clinically healthy dairy cows during lactation and in the dry period. Vet. Arch., v.75, p.67-73, 2005.
Publication Dates
-
Publication in this collection
08 May 2020 -
Date of issue
Mar-Apr 2020
History
-
Received
31 Jan 2019 -
Accepted
11 July 2019