ABSTRACT
Glomerular proteinuria is characterized by the loss of high-molecular-weight proteins (HMWPs), while tubulointerstitial proteinuria is characterized by the loss of low-molecular-weight proteins (LMWPs). The objective was to assess the molecular weight of urinary proteins (MWUP) in dogs with naturally acquired CKD and determine the proportion of HMWPs and LMWPs according to CKD stage. Twenty-eight dogs with CKD were recruited and divided into 4 groups based on serum creatinine (Cr) levels (group1: Cr<1,4, n=8; group2: 1,4<Cr<2,0, n=6; group3: 2,1<Cr<5, n=9; group4: Cr>5,0, n=5). The control group consisted of 5 healthy dogs. The MWUP was determined by SDS-PAGE. The urinary protein-to-creatinine ratio (UP/C) was used to quantitatively assess proteinuria. The electrophoresis pattern revealed a proportionally greater loss of HMWPthan of LMWP in all groups with CKD and an increased loss of LMWP in group 4 (P<0.05). These results suggest a predominance of glomerular injuries throughout all stages of CKD in these dogs and an increase in tubulointerstitial injury towards the end-stage of the disease. The results of the present study support the recommendation of SDS-PAGE as an effective technique for the qualitative assessment of proteinuria, as well as a method for assessing the severity and location of renal injury.
Keywords:
electrophoresis; nephrology; proteinuria; renal disease
RESUMO
A proteinúria glomerular é caracterizada pela perda de proteínas de alto peso molecular (PAPM), enquanto a proteinúria tubulointersticial se caracteriza pela perda de proteínas de baixo peso molecular (PBPM). O objetivo do trabalho foi determinar o peso molecular das proteínas urinárias (PMPU) de cães com DRC naturalmente adquirida e a proporção de PAPM e PBPM de acordo com o estágio da DRC. Foram utilizados 28 cães com DRC, divididos em quatro grupos, de acordo com o nível sérico de creatinina (cr) (grupo 1: cr<1,4, n=8; grupo 2: 1,4<cr< 2,0, n=6; grupo 3: 2,1<cr<5, n=9; grupo 4: cr>5,0, n=5). O grupo controle era composto por cinco cães saudáveis. O PMPU foi determinado por SDS-PAGE. A relação proteína:creatinina urinária (RPCU) foi utilizada como um método quantitativo de proteinúria. A eletroforese revelou uma perda proporcionalmente maior de PAPM, quando comparada às PBPM, em todos os grupos de DRC, bem como uma perda crescente de PBPM no grupo 4 (P<0,05). Esses resultados sugerem uma predominância de lesão glomerular em todos os estágios de DRC nesses cães e uma progressão crescente na lesão túbulo-intersticial no estágio terminal da doença. Os resultados deste estudo reafirmam a recomendação de que a eletroforese de proteínas urinárias é uma técnica qualitativa efetiva de avaliação da proteinúria, bem como um método que permite avaliar a extensão e a localização da lesão renal.
Palavras-chave:
eletroforese; nefrologia; proteinúria; doença renal
INTRODUCTION
Chronic kidney disease (CKD) has a high morbidity in elderly canine populations and occurs when there is a structural and/or functional impairment in one or both kidneys that persists for at least three months (Lee, 2004; Roudebush et al., 2009ROUDEBUSH, P.; POLZIN, D.J.; ROSS, S.J. et al. Therapies for feline chronic kidney disease - what is the evidence? J. Feline Med. Surg., v.11, p.195-210, 2009.; Polzin, 2011POLZIN, D.J. Chronic kidney disease in small animals. Vet. Clin. Small Anim. Pract., v.41, p.15-30, 2011.b; Bartges, 2012BARTGES, J.W. Chronic kidney disease in dogs and cats. Vet. Clin. Small Anim., v.42, p.669-692, 2012.). Proteinuria is correlated with the progression of CKD and is defined as the presence of abnormal amounts of protein in the urine and can originate from prerenal, renal or postrenal causes (Lees et al., 2005LEES, G.E.; BROWN, S.A.; ELLIOT, J. et al. Assessment and management of proteinuria in dogs and cats: 2004 ACVIM forum consensus statement (small animal). J. Vet. Intern. Med., v.19, p.377-385, 2005.; Grauer, 2007GRAUER, G.F. Measurement, interpretation, and implications of proteinuria and albuminuria. Vet. Clin. Small Anim. Pract., v.37, p.283-295, 2007.; Polzin, 2007). The origin of pathological renal proteinuria can be glomerular or tubulointerstitial (Lees et al., 2005; Grauer, 2007; Polzin, 2007; Chew et al., 2011CHEW, D.J.; DiBARTOLA, S.P.; SCHENCK, P. Chronic renal failure. In: ______. Canine and feline nephrology and urology, 2.ed Missouri: Elsevier-Saunders; 2011.p. 145-196.; Syme and Elliot, 2011SYME, H.; ELLIOT, J. Proteinuria and microalbuminuria. In: BARTGES, J.; POLZIN, D.J. Nephrology and urology os small animal. West Sussex: Wilet-Blackwell, 2011.p.410-414.).
Proteinuria is of prognostic importance for CKD in dogs and cats (Grauer, 2005aGRAUER, G.F. Canine glomerulonephritis: new thoughts on proteinuria and treatment. J. Small Anim. Pract., v.46, p.469-478, 2005a., 2005b; Jacob et al., 2005JACOB, F.; POLZIN, D.J.; OSBORNE, C.A. et al. Evaluation of the association between initial proteinuria and morbidity rate or death in dogs with naturally occurring chronic renal failure. J. Am. Vet. Med. Assoc., v.226, p.393-400, 2005.; Kriz and Lehir, 2005KRIZ, W.; LEHIR, M. Pathways to nephron loss starting from glomerular diseases-insights from animal models. Kidney Int., v.67, p.404-419, 2005.; Grauer, 2007; Polzin, 2007POLZIN, D.J. 11 guidelines for conservatively treating chronic kidney disease. Vet. Med., v.102, p.788-799, 2007.; Kuwahara et al., 2008KUWAHARA, Y.; NISHII, N.; TAKASU, M. et al. Use of urine albumin/creatinine ratio for estimation of proteinuria in cat and dogs. J. Vet. Med. Sci., v.70, p.865-867, 2008.; Chew et al., 2011CHEW, D.J.; DiBARTOLA, S.P.; SCHENCK, P. Chronic renal failure. In: ______. Canine and feline nephrology and urology, 2.ed Missouri: Elsevier-Saunders; 2011.p. 145-196.; Elliot and Watson, 2010ELLIOT, J.; WATSON, A.D.J. Overview of the IRIS staging system for CKD. London/Sydney, 2010. Available at: <http://.iris-kidney.com>. Accessed 10 May 2011.
http://.iris-kidney.com...
; Syme and Elliot, 2011SYME, H.; ELLIOT, J. Proteinuria and microalbuminuria. In: BARTGES, J.; POLZIN, D.J. Nephrology and urology os small animal. West Sussex: Wilet-Blackwell, 2011.p.410-414.). There is increasing evidence that proteinuria causes tubulointerstitial and perhaps glomerular injuries, resulting in a progressive loss of nephrons (Burton and Harris, 1996BURTON, C.; HARRIS, K.P.G. The role of proteinuria in the progression of chronic renal failure. Am. J. Kidney Dis., v.27, p.765-775, 1996.; Finco et al., 1999FINCO, D.R.; BROWN, S.A.; BROWN, C.A. et al. Progression of chronic renal disease in the dog. J. Vet. Int. Med., v.13, p.516-528, 1999.; Grauer, 2005a, 2005b, 2007; Jepson et al., 2009JEPSON, R.E.; BRODBELT, D.; VALLANCE, C. et al. Evaluation of predictors of the development of azotemia in cats. J. Vet. Int. Med., v.23, p.806-813, 2009.; Littman, 2011LITTMAN, M.P. Protein-losing nephropathy in small animals. Vet. Clin. Small Anim. Pract., v.41, p.31-62, 2011.). Studies have shown that dogs with CKD and persistent proteinuria are at greater risk of experiencing a fatal uremic crisis than nonproteinuric patients (Jacob et al., 2005).
Under physiological conditions, insignificant amounts of proteins with molecular weight greater than or equal to 70kDa pass through the glomerular barrier (Lulich and Osborne, 1990LULICH, J.P.; OSBORNE, C.A. Interpretation of urine protein-creatinine ratio in dogs with glomerular and nonglomerular disorders. Comp. Cont. Educ. Pract. Vet., v.12, p.59-72, 1990.; Russo et al., 2002RUSSO, L.M.; BARKRIS, G.L.; COMPER, W.D. Renal handling of albumin: a critical review of basic concepts and perspective. Am. J. Kidney Dis., v.39, p.899-919, 2002.; D´Amico and Bazzi, 2003; Syme and Elliot, 2011SYME, H.; ELLIOT, J. Proteinuria and microalbuminuria. In: BARTGES, J.; POLZIN, D.J. Nephrology and urology os small animal. West Sussex: Wilet-Blackwell, 2011.p.410-414.). Only a small portion of intermediate-molecular-weight proteins (especially albumin) and almost no high-molecular-weight proteins (HMWPs) reach the tubule (Russo et al., 2002; D´Amico and Bazzi, 2003). Conversely, the majority of low molecular-weight proteins (LMWPs) reach the proximal tubule. All proteins that reach the tubule are eliminated in negligible amounts in the urine due to tubular reabsorption (D´Amico and Bazzi, 2003; Lees et al., 2005LEES, G.E.; BROWN, S.A.; ELLIOT, J. et al. Assessment and management of proteinuria in dogs and cats: 2004 ACVIM forum consensus statement (small animal). J. Vet. Intern. Med., v.19, p.377-385, 2005.). Therefore, the presence of HMWP and/or excess albumin characterizes glomerular proteinuria, whereas tubular proteinuria is defined by the presence of LMWP (D´Amico and Bazzi, 2003; Zaragoza et al., 2003ZARAGOZA, C.; BARRERA, R.; CENTENO, F. et al. Characterization of renal damage in canine leptospirosis by sodium dodecyl suphate-polyacrylamide gel electrophoresis (SDS-PAGE) and western blotting of the urinary proteins. J. Comp. Pathol., v.129, p.169-178, 2003.; Hart, 2005HART, S.G.E. Assessment of renal injury in vivo. J. Pharma. Toxic. Methods, v.52, p.30-45, 2005.). Mild increases in albumin can result from either increased glomerular filtration or reduced tubular reabsorption (Hart, 2005).
Loss of glomerular permselectivity is associated with a proportionally greater increase in the passage of albumin and HMWP than that of LMWP (D´Amico and Bazzi, 2003). A moderate increase in glomerular permeability occurs in the initial stages of some glomerular diseases (minimal-change nephropathy, primary focal segmental glomerulosclerosis, diabetic nephropathy, membranous nephropathy). In these cases, there is a greater increase in transglomerular passage of intermediate-molecular-weight proteins, including albumin, than that of HMWP (D´Amico and Bazzi, 2003; Hart, 2005HART, S.G.E. Assessment of renal injury in vivo. J. Pharma. Toxic. Methods, v.52, p.30-45, 2005.), and since they are only partially reabsorbed by cells of the proximal tubule, they are eventually eliminated in the urine (Rego et al., 2001REGO, A.B.; KOGIKA, M.M.; SANTORO, M.L. et al. Eletroforese das proteínas urinárias de cães normais e de cães com doença renal em gel de sódio-dodecil-sulfato de poliacrilamida (SDS-PAGE). Vet. Notícias, v.7, p.65-72, 2001. Smets et al., 2010SMETS, P.M.Y.; MEYER, E.; MADDENS, B.E.J. et al. Urinary markers in healthy young and ages dogs and dogs with chronic kidney disease. J. Vet. Int. Med., v.24, p.65-72, 2010.).
A qualitative analysis of urinary proteins, based on molecular weight, can be used to determine the origin of proteinuria and the location of renal injuries (Gorg et al., 1985GORG, A.; POSTEL, W.; WESER, J. et al. Horizontal SDS eletrophoresis in ultrathin pore-gradient gels for the analysis of urinary proteins. Sci. Tools, v.32, p.5-9, 1985.; Weber, 1988WEBER, M.H. Urinary protein analysis. J. Cromathogr., v.429, p.315-344, 1988.; Lapin et al., 1989LAPIN, A.; GABL, F.; KOPSA, H. Diagnostic use of an analysis of urinary proteins by practicable sodium dodecyl sulfate eletrophoresis method and rapid two-dimensional electrophoresis. Electrophoresis, v.10, p.589-595, 1989.; Schultze and Jensen, 1989SCHULTZE, A.E.; JENSEN, R.K. Sodium dodecyl sulfate polyacrilamide gel eletrophoresis of canine urinary proteins for analysis and differentiation of tubular and glomerular diseases. Vet. Clin. Pathol., v.18, p.93-97, 1989.). Several studies have demonstrated the effectiveness of sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) for the qualitative analysis of proteins (Weber, 1988; Schultze and Jensen, 1989; Waller et al., 1989WALLER, K.V.; WARD, K.M.; MAHAN, J.D.; WISMATT, D.K. Current concepts in proteinuria. Clin. Chem., v.35, p.755-765, 1989.; Kawakami et al., 1990KAWAKAMI, H.; MURAKAMI, T.; KAJIL, T. Normal values for 24-h urinary protein excretion: total and low molecular weigth proteins with a sex-related difference. Clin. Nephrol., v.33, p.232-236, 1990.). SDS-PAGE separates proteins based on their molecular weight (Waller et al., 1989), and its results correlate well with histologic renal lesions, demonstrating the potential value of this technique for detecting and locating such lesions (Schultze and Jensen, 1989).
The use of SDS-PAGE can reduce the need for kidney biopsy in the diagnosis of kidney disease in humans (Lau and Woo, 2002LAU, Y.K.; WOO, K.T. SDS-PAGE is underutilized as a tool for investigating renal patients. Nephron, v.90, p.227-229, 2002.). Another study showed that tubular proteinuria is an indicator of the severity of tubulointerstitial injury and that it is associated with the intensity of azotemia in dogs with progressive glomerular disease (Lazaretti et al., 2006LAZARETTI, P.; BAHR, A.; STEINER, J.M. et al. Qualitative changes in proteinuria correlate with changes in kidney structure and functional as X-linked hereditary nephropathy (XLHN) progresses in affected male dogs. J. Vet. Int. Med., v.20, p.737-738, 2006.). The aim of this study was to assess the molecular weight of urinary proteins in dogs with naturally acquired CKD using SDS-PAGE, determine the proportion of proteins with high (> 60kDa) and low (< 60kDa) molecular weights at different stages of kidney disease and characterize proteinuria according to the stage of CKD.
MATERIALS AND METHODS
This prospective observational study was conducted in accordance with all guidelines of Universidade Federal Fluminense and with approval from the institutional animal care committee under the protocol with registered permit number 274. All clients were informed about the study and gave their consent. Patient study groups included healthy dogs and dogs with naturally acquired CKD. The control group consisted of five clinically healthy dogs (4 males and 1 female) over seven years of age, with no known preexisting disease, and whose blood tests (CBC, BUN, creatinine, alanine aminotransferase, alkaline phosphatase, cholesterol, triglycerides, proteins and fractions, glucose, phosphorous, calcium, sodium, potassium), urinalyses, blood pressure and abdominal ultrasound were all normal.
The inclusion criteria for the CKD groups were based on serum creatinine in accordance with the previous guidelines developed by the International Renal Interest Society (IRIS) (Elliot and Watson, 2010ELLIOT, J.; WATSON, A.D.J. Overview of the IRIS staging system for CKD. London/Sydney, 2010. Available at: <http://.iris-kidney.com>. Accessed 10 May 2011.
http://.iris-kidney.com...
): CKD stage 1, nonazotemic dogs, but with other renal abnormalities; CKD stage 2, serum creatinine 1.4 - 2.0mg/dL; CKD stage 3, serum creatinine levels between 2.1 and 5,0mg/dL; and CKD stage 4, serum creatinine level greater than 5.0mg/dL. All animals had CKD that was diagnosed at least 3 months previously. Animals were divided into patient groups based on serum creatinine levels.
Eight non azotemic (creatinine < 1.4mg/dL) patients (7 males and 1 female) were included in group 1, all of which showed at least one permanent change to kidney structure upon ultrasound examination (calcifications, nephrolithiasis, diminished corticomedullary differentiation, reduced size or irregular shape). Six dogs (3 males and 3 females) with serum creatinine between 1.4 and 2.0mg/dL were included in group 2, whereas group 3 contained nine dogs (6 males and 3 females) with serum creatinine levels between 2.1 and 5.0mg/dL; group 4 consisted of five dogs (3 males and 2 females) whose serum creatinine levels were greater than 5.0mg/dL. These twenty-eight dogs included in the CKD groups had stable renal function and had at least two serum creatinine tests performed one to two weeks apart when they were well-hydrated, and they were selected regardless of breed, sex and age.
The exclusion criteria used were patients with suspected acute kidney injury, obstructive diseases of the lower and upper urinary tracts, obesity, diagnoses of any endocrine disorder, and/or use of chronic medication for other diseases (e.g., epilepsy, neoplasia, chronic inflammatory diseases, hemoparasites). Blood and urine samples were obtained from all animals. Abdominal ultrasound was also performed, and systolic blood pressure was measured by the Doppler method in all dogs. An aliquot of 15(L of concentrated urine was diluted for SDS-PAGE. Urine samples were obtained by cystocentesis and subjected to complete urinalysis, determination of urine protein:creatinine ratio and centrifugation (2000 rpm/ 10 minutes). Samples were frozen and stored at -20oC until gel electrophoresis was performed.
Measurements of total protein and creatinine were obtained from the supernatant of centrifuged urine samples with inactive sediment (without red blood cells, white blood cells, epithelial cells, bacteria, urine crystals, amorphous debris), and UPC was determined by the simple division of values obtained, as described by White et al. (1984WHITE, J.V.; OLIVIER, N.B.; REIMANN, K.; JOHNSON, C. Use of protein-creatinine ratio in a single specimen for quantitative estimation of canine proteinuria. J. Am. Vet. Med. Assoc., v.185, p.882-885, 1984.). Urinary creatinine was determined using the Creatinine K test kit (Creatinine K test kit, Ref.96 - Labtest® Diagnóstica SA, Brazil). Urinary proteins were assessed using a pyrogallol red protein dye-binding assay (Sensiprotkit, Ref. 36-200 - Labtest® Diagnóstica SA, Brazil) as described by Watanabe et al. (1986WATANABE, N.; KAMEL, S.; OHKUBO, A. et al. Urinary protein as measured with a pyrogalol red-molybdate complex, manually and in a Hitachi 726 automated analyser. Clin. Chem., v.32, p.1551-1554, 1986.). Each animal’s UPC was determined at least twice with a minimum 2-week interval to establish persistent proteinuria (Polzin, 2011POLZIN, D.J. Chronic kidney disease in small animals. Vet. Clin. Small Anim. Pract., v.41, p.15-30, 2011.). The proteinuria classification was based on substaging guidelines developed by IRIS (Lees et al., 2005LEES, G.E.; BROWN, S.A.; ELLIOT, J. et al. Assessment and management of proteinuria in dogs and cats: 2004 ACVIM forum consensus statement (small animal). J. Vet. Intern. Med., v.19, p.377-385, 2005.; Elliot and Watson, 2010ELLIOT, J.; WATSON, A.D.J. Overview of the IRIS staging system for CKD. London/Sydney, 2010. Available at: <http://.iris-kidney.com>. Accessed 10 May 2011.
http://.iris-kidney.com...
).
Electrophoretic assays were performed using homogeneous SDS-PAGE in a mini-vertical electrophoresis system (Model no. LCV-10X10NC, LoccusBiotecnologia®, São Paulo, Brazil) containing a set of vertical plates (stacking and separating gels), as described by Laemmli (1970LAEMMLI, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, v.227, p.680-685, 1970.), following concentration of urinary proteins with a Minicon® B15 Static Concentrator (Minicon® B15 Static Concentrator, Ref. 9031 - Merck Millipore, Billerica, MA). After urine concentration a correction factor was calculated for each sample according to the difference between the initial and final volume (urine concentration factor). Pierce™ Blue Prestained Protein Molecular Weight Marker Mix (No. 26681 - Thermo Scientific®, Asheville, USA) ranging from 18.3 to 215kDa, was used as a standard.
The stacking gel consisted of 4% polyacrylamide (99.32% acrylamide and 0.68% N,N'-Methylenebisacrylamide) in 0.25M Tris-HCl buffer - pH 6.8, containing 0.1% SDS, 0.1% APS (ammonium persulfate solution) and 0.02% TEMED® (N,N,N',N'-Tetramethylethylenediamine - Sigma-Aldrich), whereas the separating gel was composed of 15% polyacrylamide in 3 M Tris-HCl buffer - pH 8.8, containing 0.033% SDS, 0.084% APS and 0.0084% TEMED®. Gels were 0.75mm thick, 11cm wide and approximately 10cm high. Each gel contained 12 wells.
In preparation for electrophoresis, 15(L of concentrated urine samples were subjected to a 3:4 dilution in sample buffer (0.25M Tris-HCl - pH 6.8; 23% glycerol; 2.7% SDS; 6.7% β-mercaptoethanol; and 6.7% Bromophenol Blue). The mixture was boiled for 5 minutes before being loaded onto the gel. Each well received the total volume of sample solution (20(L) obtained, for a total of 10 urine samples per gel, leaving one well for the molecular weight standard and one for gel differentiation.
Electrophoretic runs were performed using the preset voltages of 140 and 190 volts for the stacking and separating gels, respectively, with a total running time of approximately 3 hours. The gels were fixed in a 40% methanol and 3% glycerol solution and were subsequently stained using 0.125% Coomassie Brilliant Blue R-250 (in 50% methanol and 10% acetic acid) for 30 minutes. Excess dye was removed by soaking the gels in a destaining solution (25% methanol and 10% acetic acid) until the background of the gel was completely destained.
Gels were scanned, and a digital image was obtained using a printer/scanner (HP Laser Jet M1120 MFP® - Hewlett-Packard Company, Houston, USA). Visual assessment and comparison to molecular weight standards and migration distance on the gel were used to estimate the molecular weight of each protein band. Each image was also subjected to densitometric analysis using Scion Image Software (Release Beta 3b®, Scion Corporation, USA). Densitometric analysis was used to determine the proportions of HMWPs and LMWPs, as described by Osborne et al. (1995OSBORNE, C.A.; STEVENS, J.B.; LULICH, J.P. et al. Clinician´s analysis of urinalysis. In: OSBORNE, C.A.; FINCO, D.R. (Eds.). Canine and feline nephrology and urology. Baltimore: Lea and Febiger; 1995. p.136-205.) and Zaragoza et al. (2003ZARAGOZA, C.; BARRERA, R.; CENTENO, F. et al. Characterization of renal damage in canine leptospirosis by sodium dodecyl suphate-polyacrylamide gel electrophoresis (SDS-PAGE) and western blotting of the urinary proteins. J. Comp. Pathol., v.129, p.169-178, 2003.). The amount of protein was comparatively estimated for each electrophoretic run using the densitometric analysis provided by the software. To determine the amount of protein in a comparative unit (CU) based on molecular weight, the percentage of each protein band was multiplied by the amount of proteins estimated by the software, and the result was subsequently divided by the urine’s concentration factor and volume of concentrated urine used for the run, thereby resulting in a value with a comparative unit of protein per μL of urine (CU/μL). The amount of protein in CU/μL was calculated for total proteins, HMWPs and LMWPs.
SAS System® software was used to perform statistical analyses. The effect of the CKD group was assessed through analysis of variance (ANOVA), and Duncan's test was used for the comparison of group means at a 5% significance level, after having been previously tested.
RESULTS
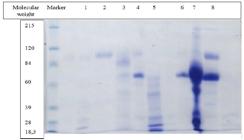
The digital image obtained of one gel is shown in Figure 1 as an illustration of the electrophoretic product. Tables 1, 2 and 3 show the mean, standard deviation, minimum and maximum values for the proportion of HMWPs and LMWPs, amount of proteins, HMWPs and LMWPs in CU/μL, urine specific gravity (USG) and UPC.
Digital image of gel 4 with SDS-PAGE of urinary proteins of 8 dogs with CKD and the molecular weight marker.
Six dogs in group 1 had USG below 1.025. Three animals had borderline proteinuria (UPC between 0.2 and 0.5), and two were proteinuric (UPC > 0.5). Patients with CKD showed greater urinary protein loss than patients in the control group. Values for protein, HMWP and LMWP in CU/μL and UPC were higher than those observed in the control group, despite the lower USG. Loss of HMWP (> 60kDa %) was higher than that observed in the control group.
Urine specific gravity was below 1.025 in all animals in group 2, and one patient (16.67%) was within the isosthenuric range. One dog had borderline proteinuria and four were proteinuric. Higher values of protein, HMWP and LMWP in CU/μL, as well as UPC, revealed increased protein loss in group 2 when compared to that of group 1 and the control group. A predominant loss of HMWP was also noted.All dogs in group 3 had low USG, and five (55.56%) of them were within the isosthenuric range. All animals were proteinuric. Loss of urinary protein was greatest in group 3 when compared to that in all the other groups in terms of protein, HMWP in CU/μL and UPC. HMWPs were predominantly lost.
All animals in group 4 had a low USG (below 1.020), and three (60.0%) of them were within the isosthenuric range. Based on UPC, all animals had proteinuria. The higher values of protein, HMWP and LMWP in CU/μL observed in this group show that protein loss was greater than that in groups 1 and 2 and the control group. Total protein loss (protein, HMW in CU/μL and UPC) was lower than that in group 3; however, the loss of LMWP was greater. A predominance of HMWP was noted. Based on analysis of variance, a significant difference was noted between the CKD groups regarding UPC and the amount of LMWP in CU/μL (p<0.05), groups 3 and 4 had higher values for these parameters. No significant differences were noted among the CKD groups with respect to USG; however, a significant difference was observed when comparing all CKD groups to the controls. Low USG was found in six animals (75%) in group 1 and all animals in groups 2, 3 and 4.
When assessing proteinuria through UPC, a significant difference was noted between groups 2 and 3, with more severe proteinuria found in the latter. Equivalent proteinuria was observed in groups 1 and 2 and the control group, and in groups 3 and 4. Proteinuria was more severe in groups with advanced CKD and occurred in all animals in groups 3 and 4. Moreover, the proportion of animals with true proteinuria in each group also increased up to groups 3 and 4 (1 = 25%; 2 = 66.67%; 3 = 100%; 4 = 100%). Considering all animals with CKD, 70.4% showed true proteinuria (UPC > 0.5), 14.8% had UPC values between 0.2 and 0.5 (borderline proteinuria), and only 14.8% had no proteinuria.
The loss of LMWP in CU/(L was significantly higher in group 4 than in groups 1 and 2 and the control group. Group 3 was statistically similar to all groups. Although no significant differences were found for the remaining variables, certain trends could be noted (Figure 2). When considering the proportions of proteins, group 3 showed the greatest trend toward losing HMWP. The loss of HMWP was predominant in all groups (Figure 3).
Urinary protein loss expressed in comparative unit (CU/µL) in dogs in the control group and groups1, 2, 3 and 4 of CKD regarding the amount of proteins in CU/μL, it was noted that total protein and HMWP had similar trends, with greater losses observed in group 3, followed by groups 4, 2, 1 and the control group, in descending order.HMWP - high-molecular-weight protein, LMWP - low-molecular-weight protein.
Urinary protein loss expressed as average percentage in dogs in the control group and groups 1, 2, 3 and 4 of CKD. HMWP - high-molecular-weight protein, LMWP - low-molecular-weight protein.
DISCUSSION
The identification of the origin of urinary proteins can be useful in investigating the cause of CKD in early stages and factors involved in the progression of the disease, while also reflecting the location and severity of the injury (Hart, 2005HART, S.G.E. Assessment of renal injury in vivo. J. Pharma. Toxic. Methods, v.52, p.30-45, 2005.; Raila, et al., 2007RAILA, J.; AUPPERLE, H.; RAILA, G. et al. Renal pathology and urinary protein excretion in a 14-month-old bernese mountain dog with chronic renal failure. J. Vet. Med., v.54, p.131-135, 2007.; Smets et al., 2010SMETS, P.M.Y.; MEYER, E.; MADDENS, B.E.J. et al. Urinary markers in healthy young and ages dogs and dogs with chronic kidney disease. J. Vet. Int. Med., v.24, p.65-72, 2010.). This study demonstrates that proteinuria is a consistent finding in dogs with CKD and suggests that glomerular injury is predominant in all stages of CKD and that tubular injuries occur thereafter, becoming more intense in later stages of the disease (Gorg et al., 1985GORG, A.; POSTEL, W.; WESER, J. et al. Horizontal SDS eletrophoresis in ultrathin pore-gradient gels for the analysis of urinary proteins. Sci. Tools, v.32, p.5-9, 1985.; Weber, 1988WEBER, M.H. Urinary protein analysis. J. Cromathogr., v.429, p.315-344, 1988.; Lapin et al., 1989LAPIN, A.; GABL, F.; KOPSA, H. Diagnostic use of an analysis of urinary proteins by practicable sodium dodecyl sulfate eletrophoresis method and rapid two-dimensional electrophoresis. Electrophoresis, v.10, p.589-595, 1989.; Schultze and Jensen, 1989SCHULTZE, A.E.; JENSEN, R.K. Sodium dodecyl sulfate polyacrilamide gel eletrophoresis of canine urinary proteins for analysis and differentiation of tubular and glomerular diseases. Vet. Clin. Pathol., v.18, p.93-97, 1989.).
The severity of CKD in dogs appears to be mediated by both glomerular and tubulointerstitial injuries (Raila, et al., 2007RAILA, J.; AUPPERLE, H.; RAILA, G. et al. Renal pathology and urinary protein excretion in a 14-month-old bernese mountain dog with chronic renal failure. J. Vet. Med., v.54, p.131-135, 2007.; Yabuki et al., 2010YABUKI, A.; MITANI, S.; FUJIKI, M. et al. Comparative study of chronic kidney disease in dogs and cats: induction of myofibroblasts. Res. Vet. Sci., v.88, p.294-299, 2010.). Both types of injuries are important for the pathophysiology and progression of kidney disease in humans, and increasing evidence points to glomerular proteinuria causing tubular injury (Satirapoj et al., 2012SATIRAPOJ, B.; NAST, C.C.; ADLER, S. Novel insights into relationship between glomerular pathology and progressive kidney disease. Adv. Chronic Kidney Dis., v.19, p.93-100, 2012.), although the opposite has also been shown to occur (Eardley and Cockwell, 2005EARDLEY, K.S.; COCKWELL, P. Macrophages and progressive tubulointerstitial disease. Kidney Int., v.68, p.437-455, 2005.).
The results obtained from the control group confirm that, under normal conditions, only a small amount of protein is eliminated in the urine. No significant difference or changes above the normal range were observed regarding UPC. Analysis of proteinuria by electrophoresis in the control group and group 1 showed a greater amount of HMWP and LMWP in the urine of animals included in the latter group.
The increase in HMWP was proportionally greater than that observed for LMWP. These results suggest that the identification of the type of proteinuria plays an important role in the diagnosis of early CKD, when USG and UPC may still be within normal ranges (Uechi et al., 1994UECHI, M.; NOGAMI, Y.; TERUI, H. et al. Evaluation of urinary enzymes in dogs with early renal disorder. J. Vet. Med. Sci., v.56, p.555-556, 1994.). An established normal range for electrophorectic variables, however, is still lacking. The presence of HMWP and LMWP in the urine of healthy control dogs may be explained by physiological protein constituents in the urine (Weber, 1988WEBER, M.H. Urinary protein analysis. J. Cromathogr., v.429, p.315-344, 1988.) but these normal ranges have yet to be determined. When analyzing the mean UPC, it was possible to note a trend toward increased severity of proteinuria up to group 3 (Figure 4). The trend observed for UPC was statistically confirmed in groups 2 and 3.
Urinary protein loss expressed in UPC (urine protein:creatinine ratio) in dogs of the control group and groups 1, 2, 3 and 4 of CKD.
The major limitation of this study was the small population size which may allow some data obtained to not be representative of the general population. Another limitation was the lack of histopathological investigations, which could confirm our conclusions. When analyzing the concentrations of total proteins and HMWPs in CU/μL, it was possible to note a trend toward greater protein loss up to group 3, followed by a slight reduction in loss in group 4.
These data suggest an increase in proteinuria up to group 3 and a reduction in group 4 (Figure 5). The increasing trend can be justified by the fact that proteinuria is probably one of the perpetuating factors of CKD (Grauer, 2005aGRAUER, G.F. Canine glomerulonephritis: new thoughts on proteinuria and treatment. J. Small Anim. Pract., v.46, p.469-478, 2005a., 2007; Littman, 2011LITTMAN, M.P. Protein-losing nephropathy in small animals. Vet. Clin. Small Anim. Pract., v.41, p.31-62, 2011.). The slight reduction in group 4 may be explained by the reduced renal mass that decreases total proteinuria, although proteinuria per nephron remains high (Lees et al., 2005LEES, G.E.; BROWN, S.A.; ELLIOT, J. et al. Assessment and management of proteinuria in dogs and cats: 2004 ACVIM forum consensus statement (small animal). J. Vet. Intern. Med., v.19, p.377-385, 2005.; Polzin, 2007POLZIN, D.J. 11 guidelines for conservatively treating chronic kidney disease. Vet. Med., v.102, p.788-799, 2007.; Elliot and Watson, 2010ELLIOT, J.; WATSON, A.D.J. Overview of the IRIS staging system for CKD. London/Sydney, 2010. Available at: <http://.iris-kidney.com>. Accessed 10 May 2011.
http://.iris-kidney.com...
). The glomerular or tubular origin of injury may be suggested by determining the proportions of high- and low-molecular-weight proteins (Gorg et al., 1985GORG, A.; POSTEL, W.; WESER, J. et al. Horizontal SDS eletrophoresis in ultrathin pore-gradient gels for the analysis of urinary proteins. Sci. Tools, v.32, p.5-9, 1985.; Weber, 1988WEBER, M.H. Urinary protein analysis. J. Cromathogr., v.429, p.315-344, 1988.; Lapin et al., 1989LAPIN, A.; GABL, F.; KOPSA, H. Diagnostic use of an analysis of urinary proteins by practicable sodium dodecyl sulfate eletrophoresis method and rapid two-dimensional electrophoresis. Electrophoresis, v.10, p.589-595, 1989.; Schultze and Jensen, 1989SCHULTZE, A.E.; JENSEN, R.K. Sodium dodecyl sulfate polyacrilamide gel eletrophoresis of canine urinary proteins for analysis and differentiation of tubular and glomerular diseases. Vet. Clin. Pathol., v.18, p.93-97, 1989.).
A greater amount of LMWP loss occurred in stage 4 CKD, suggesting a worse degree of tubular injury. The present study showed an increasing proportion of HMWPs, with maximum values observed in group 3 (65.74%), whereas the maximum concentration of LMWPs was found in group 4 (3.44 UC/μL). These data support the concept presented in other studies that have suggested that glomerular injury is predominant in all stages of CKD with tubular injuries occurring as a consequence of that injury. The literature supports this observation by stating that glomerular damage initially results in large amounts of albumin in the urine and, as it progresses, there is an increase in the amount of HMWP, whereas tubular injury is characterized by increased amounts of LMWP (Schultze and Jensen, 1989SCHULTZE, A.E.; JENSEN, R.K. Sodium dodecyl sulfate polyacrilamide gel eletrophoresis of canine urinary proteins for analysis and differentiation of tubular and glomerular diseases. Vet. Clin. Pathol., v.18, p.93-97, 1989.; Lulich and Osborne, 1990LULICH, J.P.; OSBORNE, C.A. Interpretation of urine protein-creatinine ratio in dogs with glomerular and nonglomerular disorders. Comp. Cont. Educ. Pract. Vet., v.12, p.59-72, 1990.; Rego et al., 2001REGO, A.B.; KOGIKA, M.M.; SANTORO, M.L. et al. Eletroforese das proteínas urinárias de cães normais e de cães com doença renal em gel de sódio-dodecil-sulfato de poliacrilamida (SDS-PAGE). Vet. Notícias, v.7, p.65-72, 2001.; D´Amico and Bazzi, 2003; Zaragoza et al., 2003ZARAGOZA, C.; BARRERA, R.; CENTENO, F. et al. Characterization of renal damage in canine leptospirosis by sodium dodecyl suphate-polyacrylamide gel electrophoresis (SDS-PAGE) and western blotting of the urinary proteins. J. Comp. Pathol., v.129, p.169-178, 2003.; Hart, 2005HART, S.G.E. Assessment of renal injury in vivo. J. Pharma. Toxic. Methods, v.52, p.30-45, 2005.).
Tubular injury can occur as a consequence of glomerular injury because the increased amount of proteins in the tubules overwhelms the tubular reabsorptive capacity and can also be accompanied by lysosomal rupture and apoptosis. Moreover, the mere presence of proteins triggers the release of inflammatory factors that further aggravate tubular damage (Russo et al., 2002RUSSO, L.M.; BARKRIS, G.L.; COMPER, W.D. Renal handling of albumin: a critical review of basic concepts and perspective. Am. J. Kidney Dis., v.39, p.899-919, 2002.; Grauer, 2005aGRAUER, G.F. Canine glomerulonephritis: new thoughts on proteinuria and treatment. J. Small Anim. Pract., v.46, p.469-478, 2005a.; Smets et al., 2010SMETS, P.M.Y.; MEYER, E.; MADDENS, B.E.J. et al. Urinary markers in healthy young and ages dogs and dogs with chronic kidney disease. J. Vet. Int. Med., v.24, p.65-72, 2010.; Satirapoj et al., 2012SATIRAPOJ, B.; NAST, C.C.; ADLER, S. Novel insights into relationship between glomerular pathology and progressive kidney disease. Adv. Chronic Kidney Dis., v.19, p.93-100, 2012.).
Total urinary protein loss expressed in comparative units (CU/µL) in dogs of the control group and groups 1, 2, 3 and 4 of CKD.
CONCLUSIONS
The present study allowed us to conclude that proteinuria based on the UPC ratio is more intense up to stage 3 of CKD, when it reaches a maximum. HMWP elimination increases until stage 3 of CKD, and maximum loss of LMWP occurs in the most advanced stage of CKD. The loss of HMWP is predominant throughout all stages of CKD in dogs, and the loss of LMWP is more severe at the most advanced stage of the disease, suggesting predominant glomerular injury in all stages of CKD. In addition to the determination of proteinuria through UPC, the qualitative assessment of urinary proteins through electrophoresis can aid in the diagnosis of kidney disease, since it enables an initial assessment of the severity and probable location of renal injury.
ACKNOWLEDGEMENTS
We thank Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPQ) who funded the study and all the dogs and their owners who allowed our study to be performed.
et al.,
REFERENCES
- BARTGES, J.W. Chronic kidney disease in dogs and cats. Vet. Clin. Small Anim., v.42, p.669-692, 2012.
- BURTON, C.; HARRIS, K.P.G. The role of proteinuria in the progression of chronic renal failure. Am. J. Kidney Dis., v.27, p.765-775, 1996.
- CHEW, D.J.; DiBARTOLA, S.P.; SCHENCK, P. Chronic renal failure. In: ______. Canine and feline nephrology and urology, 2.ed Missouri: Elsevier-Saunders; 2011.p. 145-196.
- D´AMICO, G.; BAZZI, C. Pathophysiology of proteinuria. Kidney Int., v.63, p.809-825, 2003.
- EARDLEY, K.S.; COCKWELL, P. Macrophages and progressive tubulointerstitial disease. Kidney Int., v.68, p.437-455, 2005.
- ELLIOT, J.; WATSON, A.D.J. Overview of the IRIS staging system for CKD. London/Sydney, 2010. Available at: <http://.iris-kidney.com>. Accessed 10 May 2011.
» http://.iris-kidney.com - FINCO, D.R.; BROWN, S.A.; BROWN, C.A. et al. Progression of chronic renal disease in the dog. J. Vet. Int. Med., v.13, p.516-528, 1999.
- GORG, A.; POSTEL, W.; WESER, J. et al. Horizontal SDS eletrophoresis in ultrathin pore-gradient gels for the analysis of urinary proteins. Sci. Tools, v.32, p.5-9, 1985.
- GRAUER, G.F. Canine glomerulonephritis: new thoughts on proteinuria and treatment. J. Small Anim. Pract., v.46, p.469-478, 2005a.
- GRAUER, G.F. Early detection of renal damage and disease in dogs and cats. Vet. Clin. Small Anim. Pract., v.35, p.581-596, 2005b.
- GRAUER, G.F. Measurement, interpretation, and implications of proteinuria and albuminuria. Vet. Clin. Small Anim. Pract., v.37, p.283-295, 2007.
- HART, S.G.E. Assessment of renal injury in vivo. J. Pharma. Toxic. Methods, v.52, p.30-45, 2005.
- International Renal Interest Society - IRIS. IRIS Staging of CKD [Online]. 2010. Available from: http://www.iris-kidney.com Accessed 10 May 2013.
» http://www.iris - JACOB, F.; POLZIN, D.J.; OSBORNE, C.A. et al. Evaluation of the association between initial proteinuria and morbidity rate or death in dogs with naturally occurring chronic renal failure. J. Am. Vet. Med. Assoc., v.226, p.393-400, 2005.
- JEPSON, R.E.; BRODBELT, D.; VALLANCE, C. et al. Evaluation of predictors of the development of azotemia in cats. J. Vet. Int. Med., v.23, p.806-813, 2009.
- KAWAKAMI, H.; MURAKAMI, T.; KAJIL, T. Normal values for 24-h urinary protein excretion: total and low molecular weigth proteins with a sex-related difference. Clin. Nephrol., v.33, p.232-236, 1990.
- KRIZ, W.; LEHIR, M. Pathways to nephron loss starting from glomerular diseases-insights from animal models. Kidney Int., v.67, p.404-419, 2005.
- KUWAHARA, Y.; NISHII, N.; TAKASU, M. et al. Use of urine albumin/creatinine ratio for estimation of proteinuria in cat and dogs. J. Vet. Med. Sci., v.70, p.865-867, 2008.
- LAEMMLI, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, v.227, p.680-685, 1970.
- LAPIN, A.; GABL, F.; KOPSA, H. Diagnostic use of an analysis of urinary proteins by practicable sodium dodecyl sulfate eletrophoresis method and rapid two-dimensional electrophoresis. Electrophoresis, v.10, p.589-595, 1989.
- LAU, Y.K.; WOO, K.T. SDS-PAGE is underutilized as a tool for investigating renal patients. Nephron, v.90, p.227-229, 2002.
- LAZARETTI, P.; BAHR, A.; STEINER, J.M. et al. Qualitative changes in proteinuria correlate with changes in kidney structure and functional as X-linked hereditary nephropathy (XLHN) progresses in affected male dogs. J. Vet. Int. Med., v.20, p.737-738, 2006.
- LEES, G.E. Early diagnosis of renal disease and renal failure. Vet. Clin. Small Anim. Pract., v.34, p.867-885, 2004.
- LEES, G.E.; BROWN, S.A.; ELLIOT, J. et al. Assessment and management of proteinuria in dogs and cats: 2004 ACVIM forum consensus statement (small animal). J. Vet. Intern. Med., v.19, p.377-385, 2005.
- LITTMAN, M.P. Protein-losing nephropathy in small animals. Vet. Clin. Small Anim. Pract., v.41, p.31-62, 2011.
- LULICH, J.P.; OSBORNE, C.A. Interpretation of urine protein-creatinine ratio in dogs with glomerular and nonglomerular disorders. Comp. Cont. Educ. Pract. Vet., v.12, p.59-72, 1990.
- OSBORNE, C.A.; STEVENS, J.B.; LULICH, J.P. et al. Clinician´s analysis of urinalysis. In: OSBORNE, C.A.; FINCO, D.R. (Eds.). Canine and feline nephrology and urology. Baltimore: Lea and Febiger; 1995. p.136-205.
- POLZIN, D.J. 11 guidelines for conservatively treating chronic kidney disease. Vet. Med., v.102, p.788-799, 2007.
- POLZIN, D.J. Chronic kidney disease in small animals. Vet. Clin. Small Anim. Pract., v.41, p.15-30, 2011.
- RAILA, J.; AUPPERLE, H.; RAILA, G. et al. Renal pathology and urinary protein excretion in a 14-month-old bernese mountain dog with chronic renal failure. J. Vet. Med., v.54, p.131-135, 2007.
- REGO, A.B.; KOGIKA, M.M.; SANTORO, M.L. et al. Eletroforese das proteínas urinárias de cães normais e de cães com doença renal em gel de sódio-dodecil-sulfato de poliacrilamida (SDS-PAGE). Vet. Notícias, v.7, p.65-72, 2001.
- ROUDEBUSH, P.; POLZIN, D.J.; ROSS, S.J. et al. Therapies for feline chronic kidney disease - what is the evidence? J. Feline Med. Surg., v.11, p.195-210, 2009.
- RUSSO, L.M.; BARKRIS, G.L.; COMPER, W.D. Renal handling of albumin: a critical review of basic concepts and perspective. Am. J. Kidney Dis., v.39, p.899-919, 2002.
- SATIRAPOJ, B.; NAST, C.C.; ADLER, S. Novel insights into relationship between glomerular pathology and progressive kidney disease. Adv. Chronic Kidney Dis., v.19, p.93-100, 2012.
- SCHULTZE, A.E.; JENSEN, R.K. Sodium dodecyl sulfate polyacrilamide gel eletrophoresis of canine urinary proteins for analysis and differentiation of tubular and glomerular diseases. Vet. Clin. Pathol., v.18, p.93-97, 1989.
- SMETS, P.M.Y.; MEYER, E.; MADDENS, B.E.J. et al. Urinary markers in healthy young and ages dogs and dogs with chronic kidney disease. J. Vet. Int. Med., v.24, p.65-72, 2010.
- SYME, H.; ELLIOT, J. Proteinuria and microalbuminuria. In: BARTGES, J.; POLZIN, D.J. Nephrology and urology os small animal. West Sussex: Wilet-Blackwell, 2011.p.410-414.
- UECHI, M.; NOGAMI, Y.; TERUI, H. et al. Evaluation of urinary enzymes in dogs with early renal disorder. J. Vet. Med. Sci., v.56, p.555-556, 1994.
- WALLER, K.V.; WARD, K.M.; MAHAN, J.D.; WISMATT, D.K. Current concepts in proteinuria. Clin. Chem., v.35, p.755-765, 1989.
- WATANABE, N.; KAMEL, S.; OHKUBO, A. et al. Urinary protein as measured with a pyrogalol red-molybdate complex, manually and in a Hitachi 726 automated analyser. Clin. Chem., v.32, p.1551-1554, 1986.
- WEBER, M.H. Urinary protein analysis. J. Cromathogr., v.429, p.315-344, 1988.
- WHITE, J.V.; OLIVIER, N.B.; REIMANN, K.; JOHNSON, C. Use of protein-creatinine ratio in a single specimen for quantitative estimation of canine proteinuria. J. Am. Vet. Med. Assoc., v.185, p.882-885, 1984.
- YABUKI, A.; MITANI, S.; FUJIKI, M. et al. Comparative study of chronic kidney disease in dogs and cats: induction of myofibroblasts. Res. Vet. Sci., v.88, p.294-299, 2010.
- ZARAGOZA, C.; BARRERA, R.; CENTENO, F. et al. Characterization of renal damage in canine leptospirosis by sodium dodecyl suphate-polyacrylamide gel electrophoresis (SDS-PAGE) and western blotting of the urinary proteins. J. Comp. Pathol., v.129, p.169-178, 2003.
Publication Dates
-
Publication in this collection
14 Aug 2020 -
Date of issue
Jul-Aug 2020
History
-
Received
31 Jan 2019 -
Accepted
01 Apr 2020