ABSTRACT
Free-range chickens may ingest oocysts of T. gondii present in the environment and consequently harbor virulent strains of this parasite in different tissues, without any clinical signs. Isolation of T. gondii through bioassays on mice and cats from naturally infected chicken tissues has been described in several countries, demonstrating the importance of free-range chickens in the transmission of this parasite. The aim of this study was the genotypic characterization of T. gondii isolates obtained from naturally infected free-range chickens in a rural area of the state of Rio Grande do Sul, Brazil. Brain and heart tissue from 12 chickens seropositive for T. gondii were processed using peptic digestion technique for parasite isolation. From 12 samples subjected to mouse bioassay, nine isolates were obtained. RFLP-PCR genotypic characterization was performed using 11 genetic markers: SAG1, 5'-3'SAG2, alt.SAG2, SAG3, BTUB, GRA6, c22-8, c29-2, L358, PK1 and Apico. Genetic characterization of the isolates revealed the presence of five atypical genotypes according to ToxoDB (# 11, # 55, # 64, # 140 and # 163). Our results showed a wide genetic diversity of T. gondii in free-range chickens in this region.
Keywords:
genotyping; toxoplasmosis; mouse bioassay; PCR-RFLP
RESUMO
Galinhas criadas ao ar livre podem ingerir oocistos de T. gondii presentes no ambiente e, com isso, albergar cepas virulentas desse parasita em diferentes tecidos, sem sinais clínicos. O isolamento de T. gondii por meio de bioensaios em camundongos e gatos, a partir de tecidos de galinhas naturalmente infectadas, tem sido descrito em vários países. Isso demonstra a importância das galinhas caipiras na epidemiologia desse parasita. O objetivo deste trabalho foi caracterizar genotipicamente isolados de T. gondii obtidos de galinhas caipiras naturalmente infectadas em uma área rural do município de Santa Maria, estado do Rio Grande do Sul, Brasil. Fragmentos de cérebro e de coração, de 12 galinhas soropositivas para T. gondii, foram processados pela técnica de digestão péptica para isolamento do parasita. Das 12 amostras submetidas a bioensaio com camundongos, nove isolados foram obtidos. A caracterização genotípica por RFLP-PCR foi realizada utilizando-se 11 marcadores genéticos: SAG1, 5'-3'SAG2, alt.SAG2, SAG3, BTUB, GRA6, c22-8, c29-2, L358, PK1 e Apico e revelou a presença de cinco genótipos atípicos de acordo com o ToxoDB (# 11, # 55, # 64, # 140 e # 163). Os resultados mostraram uma ampla diversidade genética de T. gondii em galinhas caipiras nessa região.
Palavras-chave:
genotipagem; toxoplasmose; bioensaio com camundongos; PCR-RFLP
INTRODUCTION
Toxoplasmosis is one of the most important zoonotic diseases. It is caused by the protozoon Toxoplasma gondii, which has worldwide distribution and can infect many avian and mammal species (Dubey, 2010DUBEY, J.P.; RAJENDRAN, C.; COSTA, D.G.C. et al. New Toxoplasma gondii genotypes isolated from free-range chickens from the Fernando de Noronha, Brazil: unexpected findings. J. Parasitol., v.96, p.709-712, 2010.). Birds presenting T. gondii tissue cysts are a source of infection for cats, which then become the definitive hosts and shed oocysts into the environment (Ruiz and Frenkel, 1980RUIZ, A.; FRENKEL, J.K. Intermediate and transport hosts of Toxoplasma gondii in Costa Rica. Am. J. Trop. Med. Hyg., v.29, p.1161-1166, 1980.). Chickens become infected through ingestion of oocysts and can host virulent strains in different tissues without clinical signs (Dubey, 2002).
For better understanding of T. gondii epidemiology, parasite isolation through mouse and cat bioassays from naturally infected chickens has been performed (Dubey, 2010DUBEY, J.P.; RAJENDRAN, C.; COSTA, D.G.C. et al. New Toxoplasma gondii genotypes isolated from free-range chickens from the Fernando de Noronha, Brazil: unexpected findings. J. Parasitol., v.96, p.709-712, 2010.). In Brazil, T. gondii isolation has been described in different regions, including the states of São Paulo (Dubey et al., 2002), Rio de Janeiro (Dubey et al., 2003a), Paraná (Dubey et al., 2003b), Amazonas (Dubey et al., 2006), Minas Gerais (Brandão et al., 2006BRANDÃO, G.P.; FERREIRA, A.M.; MELO, M.N. et al. Characterization of Toxoplasma gondii from domestic animals from Minas Gerais, Brazil. Parasite, v.13, p.143-149, 2006.), Pará and Rio Grande do Sul (Dubey et al., 2007), Pernambuco, Rio Grande do Norte, Maranhão, Bahia, Ceará, Sergipe and Alagoas (Oliveira et al., 2009OLIVEIRA, L.N.; COSTA JUNIOR, L.M.; DE MELO, C.F. et al. Toxoplasma gondii isolates from free-range chickens from the Northeast region of Brazil. J. Parasitol., v.95, p.235-237, 2009.), Fernando de Noronha (Dubey et al., 2010), Pantanal (Soares et al., 2011SOARES, R.M.; SILVEIRA, L.H.; SILVA, A.V. et al. Genotyping of Toxoplasma gondii isolates from free range chickens in the Pantanal area of Brazil. Vet. Parasitol., v.178, p.29-34, 2011.), Espírito Santo (Pena et al., 2013PENA, H.F.J.; VITALIANO, S.N.; BELTRAME, M.A.V. et al. PCR-RFLP genotyping of Toxoplasma gondii from chickens from Espírito Santo state, Southeast region, Brazil: New genotypes and a new SAG3 marker allele. Vet. Parasitol., v.192, p.111-117, 2013.), Minas Gerais (Silva et al., 2014SILVA, L.A.; ANDRADE, R.O.; CARNEIRO, A.C.A.V. et al. Overlapping Toxoplasma gondii genotypes circulating in domestic animals and humans in Southeastern Brazil. PLoS ONE, v.9, p.e90237, 2014.), Paraíba (Feitosa et al., 2017) and Santa Catarina (Trevisani et al., 2017TREVISANI, N.; BARROS, L.D.; VIEIRA, N.A. et al. Genotyping of Toxoplasma gondii isolates from naturally infected Gallus domesticus in Santa Catarina state, Brazil. Arq. Bras. Med. Vet. Zootec., v.69, p.139-145, 2017.).
Molecular studies showed that T. gondii had a clonal population structure with three lineages, which were designated types I, II and III, and these lineages were described in both humans and animals (Dardé et al., 1992DARDÉ, M.L.; BOUTEILLE, B.; PESTRE-ALEXANDRE, M. Isoenzyme analysis of 35 Toxoplasma gondii isolates: biological and epidemiological implications. J. Parasitol., v.78, p.786-794, 1992.; Howe and Sibley 1995HOWE, D.K.; SIBLEY, L.D. Toxoplasma gondii comprises three clonal lineages: correlation of parasite genotype with human disease. J. Infect. Dis., v.172, p.1561-1566, 1995.). Polymorphism studies on isolates from animals in Brazil have shown that T. gondii has higher genetic diversity in this country (Ferreira et al., 2001FERREIRA, A.M.; MARTINS, M.S.; VITOR, R.W.A. Virulence for BALB/c mice and antigenic diversity of eight Toxoplasma gondii strains isolated from animals and humans in Brazil. Parasite, v.8, p.99-105, 2001., 2006; Pena et al., 2008PENA, H.F.J; GENNARI, S.M.; DUBEY, J.P. et al. Population structure and mouse virulence of Toxoplasma gondii in Brazil. Int. J. Parasitol., v.38, p.561-569, 2008.; Shwab et al., 2014SHWAB, E.K.; ZHU, X.Q.; MAJUMDAR, D. et al. Geographical patterns of Toxoplasma gondii genetic diversity revealed by multilocus PCR-RFLP genotyping. Parasitology, v.141, p.453-461, 2014.). In Rio Grande do Sul, studies on T. gondii isolates obtained from chicken tissues have shown the existence of seven genotypes, among which five have different combinations of alleles I, II and III (Dubey et al., 2007DUBEY, J.P.; SUNDAR, N.; GENNARI, S.M. et al. Biologic and genetic comparison of Toxoplasma gondii isolates in free-range chickens from the northern Para state and the southern state Rio Grande do Sul, Brazil revealed highly diverse and distinct parasite populations. Vet. Parasitol., v.143, p.182-188, 2007.). Therefore, the aim of the present study was to genetically characterize T. gondii isolates from naturally infected free-range chickens raised in a rural area of Santa Maria county, of the state of Rio Grande do Sul, Brazil.
MATERIAL AND METHODS
Between March 2013 and February 2014, blood samples were collected from 597 chickens in 74 farms in nine different locations in a rural area of the municipality of Santa Maria county, which is located in the central area of the state of Rio Grande do Sul, Brazil, situated at coordinates 29° 41′ 2″ S and 53° 48′ 25″ W.
After serological analyses, 12 positive chickens were selected randomly to attempt to isolate the parasite. All of them were euthanized in accordance with the guidelines established through the International Guiding Principles for Biomedical Research Involving Animals. Brain and heart tissues from each bird were collected aseptically and subjected to mouse bioassay. All the experimental practices involving animals were approved by the Ethics Committee for Animal Experimentation at the Federal University of Santa Maria (UFSM), under protocol number 049/2012.
For serological examination of chickens and mice from bioassays, immunofluorescence antibody test (IFAT) was performed as previously described (Camargo, 1974CAMARGO, M.E. Introdução as técnicas de imunofluorescência. Rev. Bras. Patol. Clín., v.10, p.87-107, 1974.). Titers of IgG ≥ 16 and ≥ 64 were considered to be positive for T. gondii in mice and chickens, respectively.
A pool of brain and heart tissues were subjected to digestion in accordance with a protocol previously described (Dubey, 1998DUBEY, J.P. Refinement of pepsin digestion method for isolation of Toxoplasma gondii from infected tissues. Vet. Parasitol., v.74, p.75-77, 1998.). The final solution was mixed with 1,000U of penicillin and 100µl of streptomycin/ml and was inoculated intraperitoneally into four mice. The mice were observed daily and those that showed clinical signals (lacrimation, weight loss, diarrhea or abdominal distention) were euthanized. Peritoneal lavage was performed to verify the presence of tachyzoites. Mice that did not develop clinical signals or died more than 60 days after inoculation were euthanized for blood and brain sample collection. Brain tissue was squashed between a coverslip and a glass slide for tissue cyst detection. Serum samples were used for IgG anti-T. gondii detection by means of IFAT. Brain and peritoneal fluid from mice, in which it was possible to detect tissue cysts and tachyzoites respectively, were used for DNA extraction with a commercial kit (Wizard® Genomic DNA purification kit, Promega, USA) in accordance with the manufacturer’s instructions. Toxoplasma gondii genotyping was performed by multilocus PCR-RFLP in accordance with the protocol described by Su et al. (2006SU, C.; ZHANG, X.; DUBEY, J.P. Genotyping of Toxoplasma gondii by multilocus PCR-RFLP markers: a high resolution and simple method for identification of parasites. Int. J. Parasitol., v.36, p.841-848, 2006.). Reference strains (GT1, PTG, CTG, TgCgCa1, MAS, TgCatBr5, TgCatBr64 and TgTsCr1) were used as positive controls and ultrapure water as a negative control. All the products of enzymatic digestion were subjected to electrophoresis on 2.5% agarose gel and were viewed under UV light and photo documented. The results obtained were compared, identified and classified based on the genotypes present in ToxoDB at http://toxodb.org/toxo/. The phylogenetic tree was constructed using the SplitsTree 4.0 software, with the neighbor-joining method (Huson and Bryant 2006HUSON, D.H.; BRYANT, D. Application of phylogenetic networks in evolutionary studies. Mol. Biol. Evol., v.23, p.254-267, 2006.).
RESULTS
Out of 597 chicken serum samples, 294 (49.2%) were positive for T. gondii, with titers ranging from 64 to 4096 (Camillo et al., 2018CAMILLO, G.; MACHADO, M.E.A.; WEBER, A. et al. Prevalência de anticorpos e fatores de risco associados à infecção por Toxoplasma gondii em galinhas domésticas da zona rural de Santa Maria, Rio Grande do Sul. Pesqui. Vet. Bras., v.38, p.1351-1357, 2018.). In mouse bioassays, nine isolates were obtained (Table 1). Four isolates (TgCkBRSM01, TgCkBRSM02, TgCkBRSM03 and TgCkBRSM04) caused acute infection in mice, which manifested clinical signs between 10 and 15 days post-inoculation, with tachyzoites observed in the peritoneal lavage. Cysts in brain tissue were observed in mice that were inoculated with the other five isolates (TgCkBRSM05, TgCkBRSM06, TgCkBRSM07, TgCkBRSM08 and TgCkBRSM09), but without clinical signs.
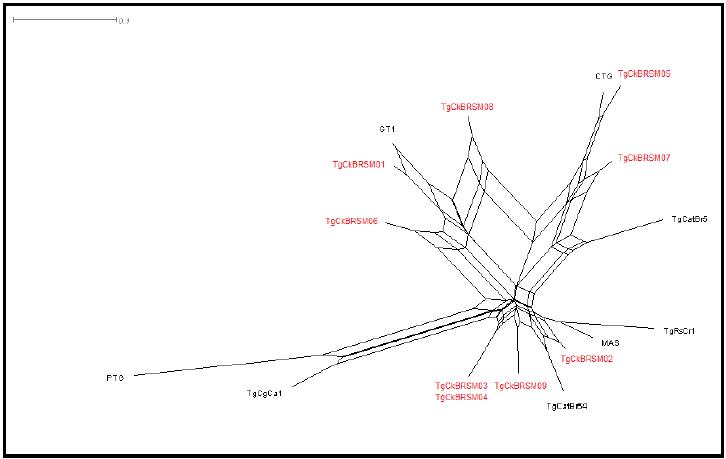
Genotyping results showed the presence of five genotypes (ToxoDB #11, #55, #64, #140 and 163), with no clonal types (Table 2). In three isolates it was not possible to determine the genotype, since DNA amplification did not occur in all markers (Table 2). Genotype #11 was observed in chickens from farms at different locations, while genotypes #55, #64 and #140 were observed from farms at the same location (Table 1 and 2). According to the phylogenetic tree (Figure 1), the isolates observed in this study were genetically closer to the clonal types I and III than to type II.
DISCUSSION
According to the results, the presence of the parasite was spread across the municipality of Santa Maria, since the same T. gondii genotype could be found infecting birds from different locations. Moreover, the parasite could be isolated from asymptomatic chickens and showed different levels of virulence in mice, which is in accordance with a previous study (Dubey et al., 2002DUBEY, J.P.; Graham, D.H.; BLACKSTON, C.R. et al. Biological and genetic characterisation of Toxoplasma gondii isolates from chickens (Gallus domesticus) from São Paulo, Brazil: unexpected findings. Int. J. Parasitol., v.32, p.99-105, 2002.). Four isolates (TgCkBRSM01, TgCkBRSM02, TgCkBRSM03 and TgCkBRSM04) exhibited high virulence in mice, causing death between 10 and 12 days post-inoculation. T. gondii virulence may show different levels depending on the strain, parasite stage and severity of infection (Dubey et al., 2004). Many studies have demonstrated that T. gondii isolates from asymptomatic chickens in Brazil are more pathogenic for mice than are isolates from Europe and North America, irrespective of the genotype (Dubey et al., 2006).
T. gondii serological results of free-range chickens (Gallus gallus domesticus) from rural area of Santa Maria, Rio Grande do Sul, Brazil and mouse bioassays results from serological positive chickens
Phylogenetic tree from T. gondii strains isolated from chickens (Gallus gallus domesticus) naturally infected from Santa Maria, state of Rio Grande do Sul, Brazil. Reference genotypes are marked in black and in red the genotypes achieved in the present study.
Genotyping results showed the presence of five genotypes, according to ToxoDB (#11, #55, #64, #140 and #163) and all of them were classified as atypical strains. Like in other previous studies conducted in Brazil, T. gondii populational structure was found to be highly diverse, compared with those seen in North America and Europe, where clonal strains (types I, II and III) are commonly found (Dubey et al., 2008DUBEY, J.P.; VELMURUGAN, G.V.; CHOCKALINGAM, A. et al. Genetic diversity of Toxoplasma gondii isolates from chickens from Brazil. Vet. Parasitol., v.157, p.299-305, 2008.).
Many studies have shown that the phylogenetic populations are highly differentiated, which suggests that recombination is the most important factor in the diversity of strains in South America (Pena et al., 2008PENA, H.F.J; GENNARI, S.M.; DUBEY, J.P. et al. Population structure and mouse virulence of Toxoplasma gondii in Brazil. Int. J. Parasitol., v.38, p.561-569, 2008.). In Brazil, genotype #11, which was observed in two isolates in the present study, had already been described in cats in the states of Paraná and São Paulo (Dubey et al., 2004DUBEY, J.P.; NAVARRO, I.T.; SREEKUMAR, C. et al. Toxoplasma gondii infections in cats from Paraná, Brazil: seroprevalence, tissue distribution, and biologic and genetic characterization of isolates. J. Parasitol., v.90, p.721-726, 2004.; Pena et al., 2006; Dubey et al., 2008) and in chickens in Rio de Janeiro and Paraná (Dubey et al., 2003a, 2003b). This genotype has also been observed in chickens in Argentina (Rajendran et al., 2012RAJENDRAN, C.; SU, C.; DUBEY, J.P. Molecular genotyping of Toxoplasma gondii from Central and South America revealed high diversity within and between populations. Infect. Genet. Evol., v.12, p.359-368, 2012.). In the present study, genotype #11 was found on two different farms, but they were at the same location (around 20km apart) and showed similar degrees of virulence in mice.
Genotype #55, which was observed in one isolate in the present study, has already been described in cats in São Paulo (Pena et al., 2008PENA, H.F.J; GENNARI, S.M.; DUBEY, J.P. et al. Population structure and mouse virulence of Toxoplasma gondii in Brazil. Int. J. Parasitol., v.38, p.561-569, 2008.), and in this previous study genotype #55 caused death in mice between 14 and 27 days post-inoculation. However, in our study, mice inoculated with this genotype started to show clinical signs from the 7th day post-inoculation onwards, which might suggest that this strain is more pathogenic.
Genotypes #55, #64 and #140 were isolated from chickens in different farms, within the same locality, which suggests that populational diversity was present in nearby locations (10km apart). Phenotypic differences were also observed between these isolates, among which only genotype #140 caused chronic infection in mice, with tissue cysts in the brain and Genotype #140 has already been described in chickens in Nicaragua (Rajendran et al., 2012RAJENDRAN, C.; SU, C.; DUBEY, J.P. Molecular genotyping of Toxoplasma gondii from Central and South America revealed high diversity within and between populations. Infect. Genet. Evol., v.12, p.359-368, 2012.) Genotype #163 was observed in one isolate and caused neurological signs in mice (walking in circles and motor incoordination), 40 days after inoculation. This same genotype has been described in chickens in the archipelago of Fernando de Noronha, and the authors of the study observed that not all the isolates were pathogenic to mice (Dubey et al., 2010DUBEY, J.P.; RAJENDRAN, C.; COSTA, D.G.C. et al. New Toxoplasma gondii genotypes isolated from free-range chickens from the Fernando de Noronha, Brazil: unexpected findings. J. Parasitol., v.96, p.709-712, 2010.).
In a previous study on naturally infected chickens in the state of Rio Grande do Sul, Brazil, Dubey et al. (2007DUBEY, J.P.; SUNDAR, N.; GENNARI, S.M. et al. Biologic and genetic comparison of Toxoplasma gondii isolates in free-range chickens from the northern Para state and the southern state Rio Grande do Sul, Brazil revealed highly diverse and distinct parasite populations. Vet. Parasitol., v.143, p.182-188, 2007.) found 19 isolates and suggested that clonal lineages I and III, or lineages close to them, were circulating across the state. It was observed that only one isolate had allele type I for all markers, while three isolates had allele type III. All the other isolates had combinations of different alleles, and comparison among them showed that diversity within close geographical areas was present. This diversity can be explained in terms of the presence of mixed infection in intermediate hosts, such as chickens, which can contribute towards genetic cross-breeding between different lineages of the parasite in definitive hosts (Dubey et al., 2006). The success of clonal lineages may have resulted from simultaneous infection via the oral route. Sexual recombination promotes transmission through successive hosts, thus leading to clonal expansion (Su et al., 2003).
Genotyping studies on T. gondii using PCR-RFLP have been conducted around the world and have contributed towards expanding the epidemiological information available regarding the diversity of this parasite. Based on these studies, it has been suggested that comparisons between genotypes and clinical manifestations of toxoplasmosis in humans should be investigated (Saeij et al., 2005SAEIJ, J.P.J.; BOYLE, J.P.; BOOTHROYD, J.C. Differences among the three major strains of Toxoplasma gondii and their specific interactions with the infected host. Trends Parasitol., v.21, p.476-481, 2005.; Pena et al., 2008PENA, H.F.J; GENNARI, S.M.; DUBEY, J.P. et al. Population structure and mouse virulence of Toxoplasma gondii in Brazil. Int. J. Parasitol., v.38, p.561-569, 2008.). According to Pena et al. (2008), epidemiological studies are able to reveal the populational diversity that is possibly related to higher virulence. Therefore, further studies should be conducted to evaluate the relationship between the genotypes observed in the present study and the epidemiological characteristics of the disease in animal and human populations.
CONCLUSION
Our results showed that there was high genotypic diversity among the T. gondii isolates obtained from naturally infected free-range chickens raised in a rural area, corroborating with the genetic diversity that has been observed in the state of Rio Grande do Sul and in Brazil. The genotypes characterized in this study are atypical strains, with different combinations between alleles. No clonal lineages were observed in any of the isolates.
ACKNOWLEDGEMENTS
Funding: This work was supported by CAPES (Coordination Office for Improvement of Higher-Education Personnel)
REFERENCES
- BRANDÃO, G.P.; FERREIRA, A.M.; MELO, M.N. et al. Characterization of Toxoplasma gondii from domestic animals from Minas Gerais, Brazil. Parasite, v.13, p.143-149, 2006.
- CAMARGO, M.E. Introdução as técnicas de imunofluorescência. Rev. Bras. Patol. Clín., v.10, p.87-107, 1974.
- CAMILLO, G.; MACHADO, M.E.A.; WEBER, A. et al. Prevalência de anticorpos e fatores de risco associados à infecção por Toxoplasma gondii em galinhas domésticas da zona rural de Santa Maria, Rio Grande do Sul. Pesqui. Vet. Bras., v.38, p.1351-1357, 2018.
- DARDÉ, M.L.; BOUTEILLE, B.; PESTRE-ALEXANDRE, M. Isoenzyme analysis of 35 Toxoplasma gondii isolates: biological and epidemiological implications. J. Parasitol., v.78, p.786-794, 1992.
- DUBEY, J.P. A review of toxoplasmosis in wild birds. Vet. Parasitol., v.106, p.121-153, 2002.
- DUBEY, J.P. Refinement of pepsin digestion method for isolation of Toxoplasma gondii from infected tissues. Vet. Parasitol., v.74, p.75-77, 1998.
- DUBEY, J.P. Toxoplasma gondii infections in chickens (Gallus domesticus): prevalence, clinical disease, diagnosis and public health significance. Zoonoses Public Health, v.57, p.60-73, 2010.
- DUBEY, J.P.; GENNARI, S.M.; LABRUNA, M.B. et al. Characterization of Toxoplasma gondii isolates in free-range chickens from Amazon, Brazil. J. Parasitol., v.92, p.36-40, 2006.
- DUBEY, J.P.; Graham, D.H.; BLACKSTON, C.R. et al. Biological and genetic characterisation of Toxoplasma gondii isolates from chickens (Gallus domesticus) from São Paulo, Brazil: unexpected findings. Int. J. Parasitol., v.32, p.99-105, 2002.
- DUBEY, J.P.; GRAHAM, D.H.; SILVA, D.S. et al. Toxoplasma gondii isolates of free-ranging chickens from Rio de Janeiro, Brazil: mouse mortality, genotype, and oocyst shedding by cats. J. Parasitol., v.89, p.851-853, 2003a.
- DUBEY, J.P.; NAVARRO, I.T.; GRAHAM, D.H. et al. Characterization of Toxoplasma gondii isolates from free range chickens from Parana, Brazil. Vet. Parasitol., v.117, p.229-234, 2003b.
- DUBEY, J.P.; NAVARRO, I.T.; SREEKUMAR, C. et al. Toxoplasma gondii infections in cats from Paraná, Brazil: seroprevalence, tissue distribution, and biologic and genetic characterization of isolates. J. Parasitol., v.90, p.721-726, 2004.
- DUBEY, J.P.; RAJENDRAN, C.; COSTA, D.G.C. et al. New Toxoplasma gondii genotypes isolated from free-range chickens from the Fernando de Noronha, Brazil: unexpected findings. J. Parasitol., v.96, p.709-712, 2010.
- DUBEY, J.P.; SUNDAR, N.; GENNARI, S.M. et al. Biologic and genetic comparison of Toxoplasma gondii isolates in free-range chickens from the northern Para state and the southern state Rio Grande do Sul, Brazil revealed highly diverse and distinct parasite populations. Vet. Parasitol., v.143, p.182-188, 2007.
- DUBEY, J.P.; VELMURUGAN, G.V.; CHOCKALINGAM, A. et al. Genetic diversity of Toxoplasma gondii isolates from chickens from Brazil. Vet. Parasitol., v.157, p.299-305, 2008.
- FEITOSA, T.F.; VILELA, V.L.; ALMEIDA-NET, J.L. et al. First report of typical Brazilian Toxoplasma gondii genotypes from isolates of free-range chickens (Gallus gallus domesticus) circulating in the state of Paraíba, Northeast Brazil. Parasitol. Res., v.116, p.2265-2270, 2017.
- FERREIRA, A.M.; MARTINS, M.S.; VITOR, R.W.A. Virulence for BALB/c mice and antigenic diversity of eight Toxoplasma gondii strains isolated from animals and humans in Brazil. Parasite, v.8, p.99-105, 2001.
- FERREIRA, A.M.; VITOR, R.W.A.; GAZZINELLI, R.T. et al. Genetic analysis of natural recombinant Brazilian Toxoplasma gondii strains by multilocus PCR-RFLP. Infect. Genet. Evol., v.6, p.22-31, 2006.
- HOWE, D.K.; SIBLEY, L.D. Toxoplasma gondii comprises three clonal lineages: correlation of parasite genotype with human disease. J. Infect. Dis., v.172, p.1561-1566, 1995.
- HUSON, D.H.; BRYANT, D. Application of phylogenetic networks in evolutionary studies. Mol. Biol. Evol., v.23, p.254-267, 2006.
- OLIVEIRA, L.N.; COSTA JUNIOR, L.M.; DE MELO, C.F. et al. Toxoplasma gondii isolates from free-range chickens from the Northeast region of Brazil. J. Parasitol., v.95, p.235-237, 2009.
- PENA, H.F.J.; SOARES, R.M.; AMAKU, M. et al. Toxoplasma gondii infection in cats from São Paulo state, Brazil: seroprevalence, oocyst shedding, isolation in mice, and biologic and molecular characterization. Res. Vet. Sci., v.81, p.58-67, 2006.
- PENA, H.F.J.; VITALIANO, S.N.; BELTRAME, M.A.V. et al. PCR-RFLP genotyping of Toxoplasma gondii from chickens from Espírito Santo state, Southeast region, Brazil: New genotypes and a new SAG3 marker allele. Vet. Parasitol., v.192, p.111-117, 2013.
- PENA, H.F.J; GENNARI, S.M.; DUBEY, J.P. et al. Population structure and mouse virulence of Toxoplasma gondii in Brazil. Int. J. Parasitol., v.38, p.561-569, 2008.
- RAJENDRAN, C.; SU, C.; DUBEY, J.P. Molecular genotyping of Toxoplasma gondii from Central and South America revealed high diversity within and between populations. Infect. Genet. Evol., v.12, p.359-368, 2012.
- RUIZ, A.; FRENKEL, J.K. Intermediate and transport hosts of Toxoplasma gondii in Costa Rica. Am. J. Trop. Med. Hyg., v.29, p.1161-1166, 1980.
- SAEIJ, J.P.J.; BOYLE, J.P.; BOOTHROYD, J.C. Differences among the three major strains of Toxoplasma gondii and their specific interactions with the infected host. Trends Parasitol., v.21, p.476-481, 2005.
- SHWAB, E.K.; ZHU, X.Q.; MAJUMDAR, D. et al. Geographical patterns of Toxoplasma gondii genetic diversity revealed by multilocus PCR-RFLP genotyping. Parasitology, v.141, p.453-461, 2014.
- SILVA, L.A.; ANDRADE, R.O.; CARNEIRO, A.C.A.V. et al. Overlapping Toxoplasma gondii genotypes circulating in domestic animals and humans in Southeastern Brazil. PLoS ONE, v.9, p.e90237, 2014.
- SOARES, R.M.; SILVEIRA, L.H.; SILVA, A.V. et al. Genotyping of Toxoplasma gondii isolates from free range chickens in the Pantanal area of Brazil. Vet. Parasitol., v.178, p.29-34, 2011.
- SU, C.; EVANS, D.; COLE, R.H. et al. Recent expansion of Toxoplasma through enhanced oral transmission. Science, v.299, p.414-416, 2003.
- SU, C.; ZHANG, X.; DUBEY, J.P. Genotyping of Toxoplasma gondii by multilocus PCR-RFLP markers: a high resolution and simple method for identification of parasites. Int. J. Parasitol., v.36, p.841-848, 2006.
- TREVISANI, N.; BARROS, L.D.; VIEIRA, N.A. et al. Genotyping of Toxoplasma gondii isolates from naturally infected Gallus domesticus in Santa Catarina state, Brazil. Arq. Bras. Med. Vet. Zootec., v.69, p.139-145, 2017.
Publication Dates
-
Publication in this collection
14 Aug 2020 -
Date of issue
Jul-Aug 2020
History
-
Received
03 Oct 2019 -
Accepted
11 Feb 2020