ABSTRACT
In this study we describe the epidemiology, clinical signs, and pathology of an outbreak of avian aspergillosis in alternative breeding in the southern region of Rio Grande do Sul, Brazil. Between the fifth and tenth day of life, 360 chicks from a flock of 4000 developed unspecific clinical signs and died. The birds were housed in a reused aviary litter, without previous treatment. In 11 six-day-old female ISA Brown chicks (Gallus gallus domesticus), necropsy revealed firm, yellowish-white, multinodular lesions extending from the pleura to the lung parenchyma. Histologically, a granulomatous, multifocal to coalescent pneumonia was observed. Granulomas were characterized by central necrosis, with heterophil and epithelioid macrophage infiltration and presence of countless Y-shaped intralesional septate hyphae morphologically compatible with Aspergillus spp. The diagnosis through isolation confirmed Aspergillus fumigatus. We highlight the importance of aspergillosis as a primary cause of diseases in the respiratory tract of young birds in alternative breeding. Measures to prevent aspergillosis mainly regarding the reuse of aviary litter are essential in poultry husbandry to prevent economic losses, reduce environmental contamination and mitigate the potential risk to public health.
Keywords:
Aspergillus fumigatus; granulomatous pneumonia; alternative poultry farms; young birds
RESUMO
Descrevem-se os aspectos epidemiológicos e patológicos de um surto de aspergilose aviária em criação alternativa na região sul do Rio Grande do Sul, Brasil. De um lote de 4000 pintainhas, entre o quinto e o 10º dia de vida, 360 aves apresentaram sinais clínicos inespecíficos e morreram. As aves foram alojadas em cama reutilizada do aviário, sem tratamento prévio. Na necropsia de 11 pintainhas (Gallus gallus domesticus), fêmeas, seis dias de idade da linhagem Isa Brown, foram observadas no pulmão lesões multinodulares, branco-amareladas e firmes, que se estendiam da pleura ao parênquima. Histologicamente foi observada pneumonia granulomatosa, multifocal a coalescente. Os granulomas eram caracterizados por necrose central, com infiltrado inflamatório de heterófilos, macrófagos, células epitelioides com presença de inúmeras hifas septadas intralesionais, semelhantes à letra “Y”, morfologicamente compatíveis com Aspergillus spp. O diagnóstico foi confirmado pelo isolamento de Aspergillus fumigatus. Alerta-se para a importância da aspergilose como causa primária de afecções no trato respiratório de aves jovens em criações alternativas. Medidas preventivas relacionadas ao manejo dessas aves são indispensáveis principalmente quanto à reutilização da cama dos aviários, a fim de evitar perdas econômicas, reduzir a contaminação ambiental e o potencial risco à saúde pública.
Palavras-chave:
Aspergillus fumigatus; pneumonia granulomatosa; avicultura alternativa; aves jovens
INTRODUCTION
Aspergillus spp. are saprophytic fungi found in soil, decaying vegetation, seeds, and grains. Some species are opportunistic pathogens of animals and humans, mainly causing secondary respiratory infections, with A. fumigatus being the species most often involved in the pathogenesis of avian aspergillosis (Kunkle, 2003KUNKLE, R.A. Aspergillosis. In: SAIF, Y.M.; BARNES, H.J.; GLISSON, J.R. (Eds.). Diseases of poultry. Iowa: Iowa State University Press, 2003, p.883-895,; Ozhak-Baysan et al., 2010; Arné et al., 2011ARNÉ, P.; THIERRY, S.; WANG, D. et al. Aspergillus fumigatus in poultry. Int. J. Microbiol., v.7, p.1-14, 2011.; Cafarchia et al., 2014CAFARCHIA, C.; CAMARDA, A.; IATTA, R. et al. Environmental contamination by Aspergillus spp. inlaying hen farms and associated health risks for farm workers. J. Med. Microbiol., v.63, p.464-470, 2014.). Aviary litter is rich in organic matter, which activates the proliferation and sporulation of A. fumigatus, producing a high number of conidia, which can remain viable for long periods in this environment and are easily dispersed in the air (Kunkle, 2003; Arné et al., 2011).
The main virulence factor related to the pathogenesis of A. fumigatus is the small size of its conidia (2 to 10µm) that allows inhalation and colonization of the lower airways of susceptible hosts (Oca et al., 2017OCA, V.M.; VALDÉS, S.E.; SEGUNDO, C. et al. Aspergillosis, a natural infection in poultry: mycological and molecular characterization and determination of gliotoxin in Aspergillus fumigatus isolates. Avian Dis., v.61, p.77-82, 2017.). High concentrations of conidia in the environment and prolonged host exposure to the fungus, together with anatomical characteristics of the respiratory tract of birds, are also considered important factors in the pathogenesis of aspergillosis (Arné et al., 2011ARNÉ, P.; THIERRY, S.; WANG, D. et al. Aspergillus fumigatus in poultry. Int. J. Microbiol., v.7, p.1-14, 2011.; Chotirmall et al., 2013CHOTIRMALL, S.H.; AL-ALAWI, M.; MIRKOVIC, B. et al. Aspergillus-associated airway disease, inflammation, and the innate immune response. Bio. Med. Res. Int., v.2013, p.1-14, 2013.).
Avian aspergillosis has acute clinical signs in young birds (three days to 20 weeks), with high morbidity and mortality rate. In adult birds, it is chronic leading to decreased productivity and consequently economic losses. Currently, the main form of control of avian aspergillosis in the industrial poultry sector is prevention through the monitoring of hatcheries (Kunkle, 2003KUNKLE, R.A. Aspergillosis. In: SAIF, Y.M.; BARNES, H.J.; GLISSON, J.R. (Eds.). Diseases of poultry. Iowa: Iowa State University Press, 2003, p.883-895,; Arné et al., 2011ARNÉ, P.; THIERRY, S.; WANG, D. et al. Aspergillus fumigatus in poultry. Int. J. Microbiol., v.7, p.1-14, 2011.; Dutta et al., 2017DUTTA, B.; KONCH, P.; GOGOI, S.M. et al. Clinicopathological studies of brooder pneumonia in broiler chicken. Int. J. Chem. Stud., v.5, p.510-512, 2017.), but in the alternative poultry breeding there are few studies about the occurrence of this pathology. The present study aimed to describe an outbreak of aspergillosis in colonial-bred chicks in the southern region of Brazil, highlighting epidemiological and pathological aspects.
CASE REPORT
The outbreak of avian aspergillosis occurred in October 2018 at a breeding located in the Third District of the municipality of Pelotas, Rio Grande do Sul, Brazil. There were approximately 6000 ISA Brown laying hens at the property, separated into two lots, the largest one consisting of 4000 5-day-old animals and the smallest of 2000 hens aged 16 months. The semi-intensive system was used; all animals were housed in a single shed, and the two lots were separated by a metal screen. The new flock of chicks had been housed on reused aviary litter, without prior treatment. In those birds, size differences were observed, and on the fifth day of life some animals had developed lethargy, anorexia, and drooping wings. From the onset of clinical signs to the tenth day of age, 360 birds (9%) died.
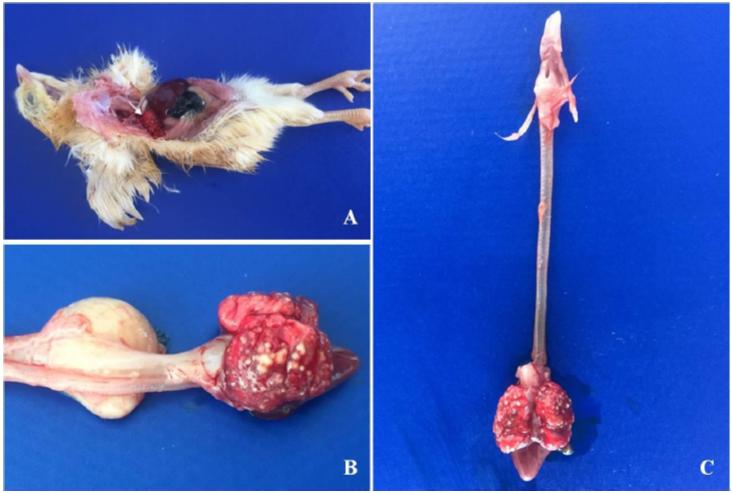
Eleven six-day old female chicks (Gallus gallus domesticus) of the Isa Brown lineage were forwarded to the Laboratório Regional de Diagnóstico (LRD) of Faculdade de Veterinária (FV) at the Universidade Federal de Pelotas (UFPel). In the necropsy, brain and celomatic cavity organ fragments were collected and fixed in 10% buffered formalin. After 48h the organs were embedded in paraffin, cut into sections 3μm thick, and stained with hematoxylin-eosin (HE) and Grocott’s methenamine silver. Lung fragments were sent to the Laboratório de Micologia Veterinária (MicVet), of FV/UFPel for fungal cultures. Briefly, fragments were sown on cycloheximide-free medium (Sabouraud dextrose agar) supplemented with chloramphenicol (0.05 mg/ml) and incubated at 37°C for five days, with daily observation. Macroscopically, exposure of the celomatic cavity revealed firm, multinodular, white-yellowish lesions extending from the pleura to the lung parenchyma (Figure. 1A, 1B, and 1C).
Chicks. Isa Brown, 6-day-old. 1A. Celomatic cavity: In situ observation of celomatic cavity’s organs from 11 necropsied birds. In the pulmonary pleura there were numerous nodules, multifocal to coalescent, yellowish-white (arrow). No involvement of air sacs or injuries to other organs was observed. 1B and 1C. Lungs: Multifocal nodular to coalescent lesions, yellowish-white, firm to the cut, compromising pleura and pulmonary parenchyma.
Histologically, there was a multifocal-to-coalescent granulomatous pneumonia (Figure 2A). The granulomas were characterized by central necrosis (Figure 2B), with infiltration of heterophiles, macrophages, and epithelioid cells; numerous Y-shaped intralesional septate hyphae were observed, morphologically consistent with Aspergillus spp. (Figure 2C). Grocott’s methenamine-silver nitrate stain clearly showed hyphae of regular diameter, with septations into dichotomous branches, often at acute angles (Figure 2D).
In the mycological culture, characteristic colonies of Aspergillus fumigatus were observed (Figure 3A). Microscopy revealed fungal hyphae bifurcated at an acute angle of 45°, conidiophores with a swollen apex (vesicle), conidia, metulae, and phialides (Figure 3B). Direct examination of the colonies was performed by placing a filament of fungal culture between the slide and coverslip with a sterile loop and staining with lactophenol cotton blue. Slides were then observed at a magnification of 100 to 400x.
Chicks. Isa Brown, 6-day-old. 2A. Lung: Numerous granulomas with multifocal to coalescent distribution throughout the lung parenchyma. HE, obj. 4X. 2B. Lung: Granuloma characteristic of aspergillosis, exhibiting central necrosis. HE, obj. 10X. 2C. Pulmonary granuloma: Intralesional septate hyphae, similar to the letter "Y" (arrows), morphologically compatible with Aspergillus spp., located in the necrosis areas in the central region of granulomatous lesions. HE, obj. 40X. 2D. Pulmonary granuloma: Evidence of septate fungal hyphae through silver impregnation of the external wall of the fungus. Grocott, obj. 40X.
Mycological culture of lung samples from 11 chicks, Isa Brown, six days old. Figure 3A. Dark gray, filamentous fungal colonies characteristics of Aspergillus fumigatus. Sabouraud dextrose agar. 3B. Bifurcated fungal mycelia at an acute angle of 45°, conidiophores with a swollen apex, conidia, metulae, and phialides. Lactophenol cotton blue, obj 400X.
DISCUSSION
The diagnosis of avian aspergillosis was based on epidemiological data, characteristic macroscopic and histological lesions with the presence of intralesional fungal hyphae, together with the isolation of A. fumigatus. Avian aspergillosis has a greater economic impact on the industrial poultry sector (mortality and economic losses) and causes mortality in wild birds (Arné et al., 2011ARNÉ, P.; THIERRY, S.; WANG, D. et al. Aspergillus fumigatus in poultry. Int. J. Microbiol., v.7, p.1-14, 2011.; Oca et al. 2017OCA, V.M.; VALDÉS, S.E.; SEGUNDO, C. et al. Aspergillosis, a natural infection in poultry: mycological and molecular characterization and determination of gliotoxin in Aspergillus fumigatus isolates. Avian Dis., v.61, p.77-82, 2017.). However, the impact of this pathogenic agent on alternative poultry breeding is unknown.
Although A. fumigatus is considered an opportunistic fungus associated with other pathogens, it has been described as a primary cause of granulomatous pneumonia and airsacculitis in birds, affecting even immunocompetent individuals (Kunkle, 2003KUNKLE, R.A. Aspergillosis. In: SAIF, Y.M.; BARNES, H.J.; GLISSON, J.R. (Eds.). Diseases of poultry. Iowa: Iowa State University Press, 2003, p.883-895,; McClenny, 2005; Arné et al., 2011ARNÉ, P.; THIERRY, S.; WANG, D. et al. Aspergillus fumigatus in poultry. Int. J. Microbiol., v.7, p.1-14, 2011.; Dutta et al., 2017DUTTA, B.; KONCH, P.; GOGOI, S.M. et al. Clinicopathological studies of brooder pneumonia in broiler chicken. Int. J. Chem. Stud., v.5, p.510-512, 2017.; Oca et al. 2017OCA, V.M.; VALDÉS, S.E.; SEGUNDO, C. et al. Aspergillosis, a natural infection in poultry: mycological and molecular characterization and determination of gliotoxin in Aspergillus fumigatus isolates. Avian Dis., v.61, p.77-82, 2017.). Unlike mammals that develop pulmonary aspergillosis when in immunosuppressive conditions, birds are more susceptible to infections by fungi of the Aspergillus genus, mainly due to anatomical characteristics of the respiratory tract observed in this species (Kunkle, 2003; Tell, 2005TELL, L.A. Aspergillosis in mammals and birds: impact on veterinary medicine. Med. Mycol., v.43, p.71-73, 2005.; Arné et al., 2011). In the present report, in the analyzes carried out, there was no growth of other fungal and/or bacterial agents, reinforcing that A. fumigatus was the primary cause of chick mortality.
Younger birds are more susceptible to infections, especially in the first days of life and when exposed to high concentrations of Aspergillus spp. conidia. Infections tend to have high morbidity and mortality (Kunkle, 2003KUNKLE, R.A. Aspergillosis. In: SAIF, Y.M.; BARNES, H.J.; GLISSON, J.R. (Eds.). Diseases of poultry. Iowa: Iowa State University Press, 2003, p.883-895,; Arné et al., 2011ARNÉ, P.; THIERRY, S.; WANG, D. et al. Aspergillus fumigatus in poultry. Int. J. Microbiol., v.7, p.1-14, 2011.; Dutta et al., 2017DUTTA, B.; KONCH, P.; GOGOI, S.M. et al. Clinicopathological studies of brooder pneumonia in broiler chicken. Int. J. Chem. Stud., v.5, p.510-512, 2017.). In the present report, the disease occurred acutely and severely, with 9% mortality. This mortality rate and the clinicopathological presentation of this outbreak are probably attributable to the form of infection (inhaled), as well as to other factors that influenced the natural immune resistance of the affected chicks, such as age, transport stress and environmental contamination. Similar data have been described in alternative poultry breeding, with different morbidity and mortality rates varying according to the conditions of each breeding analyzed (Eassa et al., 2017EASSA, S.H.; MOHAMMED, M.H.; OMER, A.M. Case Report: Prevalence and significance of aspergillosis in commercial broiler chicken: Pathological study. Iraqi J. Vet. Sci., v.31, p.113-116, 2017.).
The reuse of aviary litter is standard practice in industrial aviculture, but treatment prior to reuse for new lots, and for chicks it is recommended to exchange (Beernaert et al., 2010BEERNAERT, L.A.; PASMANS, F.; VAN WAEYENBERGHE, L. et al. Aspergillus infections in birds: a review. Avian Pathol., v.39, p.325-331, 2010.; Chotirmall et al., 2013CHOTIRMALL, S.H.; AL-ALAWI, M.; MIRKOVIC, B. et al. Aspergillus-associated airway disease, inflammation, and the innate immune response. Bio. Med. Res. Int., v.2013, p.1-14, 2013.). When reused inappropriately, the litter becomes an important source of infection for birds, since it presents conditions of humidity, temperature and substrate are favorable to the development and permanence of Aspergillus spp. in the aviary for long periods (Kunkle, 2003KUNKLE, R.A. Aspergillosis. In: SAIF, Y.M.; BARNES, H.J.; GLISSON, J.R. (Eds.). Diseases of poultry. Iowa: Iowa State University Press, 2003, p.883-895,; Throne Steinlage et al., 2003; McClenny, 2005; Arné et al., 2011ARNÉ, P.; THIERRY, S.; WANG, D. et al. Aspergillus fumigatus in poultry. Int. J. Microbiol., v.7, p.1-14, 2011.).
In this case, the probable source of infection was the reuse of aviary litter handled incorrectly, being reused for chicks and without previous treatment. As observed in the present report, in many alternative creations, countless practices adopted in the birds and aviaries management are not carried out according to what is recommended. Considering this epidemiological finding, colonization of the respiratory tract of the affected chicks was caused by a high concentration of A. fumigatus conidia in environment, as well as additional factors that increased the susceptibility of this flock. In addition to the microclimate of the aviary, the climate of the municipality of Pelotas, with high relative humidity rates (80%) and average temperatures (24°C) in October 2018 (INMET, 2018) favored the development of fungal agents in the environment.
Correct management, control of the aviary microclimate (temperature and humidity), and appropriate cleaning procedures (removal of feces, use of disinfectants) are essential to controlling environmental contamination by Aspergillus spp. (Ozhak-Baysan et al., 2010; Arné et al., 2011ARNÉ, P.; THIERRY, S.; WANG, D. et al. Aspergillus fumigatus in poultry. Int. J. Microbiol., v.7, p.1-14, 2011.; Cafarchia et al., 2014CAFARCHIA, C.; CAMARDA, A.; IATTA, R. et al. Environmental contamination by Aspergillus spp. inlaying hen farms and associated health risks for farm workers. J. Med. Microbiol., v.63, p.464-470, 2014.). In the case reported herein, after the diagnosis of aspergillosis deaths ceased once the aviary litter was changed, a fact that corroborates the hypothesis that the source of infection in this outbreak was the practice of reusing litter without prior sanitization. Due to the high cost of specific antifungals, only management changes were used to control mortality in the affected flock. Although there are numerous antifungal protocols for the treatment of aspergillosis, they are financially unfeasible for commercial poultry breeding (Beernaert et al., 2010BEERNAERT, L.A.; PASMANS, F.; VAN WAEYENBERGHE, L. et al. Aspergillus infections in birds: a review. Avian Pathol., v.39, p.325-331, 2010.).
Avian aspergillosis is important not only as an opportunistic pathogen, but also as a primary agent of respiratory tract disorders in young poultry. Preventive measures should be taken, especially regarding the reuse of aviary litter (a key predisposing factor for the contamination of susceptible hosts), to reduce economic losses and mitigate risks to public health.
REFERÊNCIAS
- ARNÉ, P.; THIERRY, S.; WANG, D. et al. Aspergillus fumigatus in poultry. Int. J. Microbiol., v.7, p.1-14, 2011.
- BEERNAERT, L.A.; PASMANS, F.; VAN WAEYENBERGHE, L. et al. Aspergillus infections in birds: a review. Avian Pathol., v.39, p.325-331, 2010.
- CAFARCHIA, C.; CAMARDA, A.; IATTA, R. et al. Environmental contamination by Aspergillus spp. inlaying hen farms and associated health risks for farm workers. J. Med. Microbiol., v.63, p.464-470, 2014.
- CHOTIRMALL, S.H.; AL-ALAWI, M.; MIRKOVIC, B. et al. Aspergillus-associated airway disease, inflammation, and the innate immune response. Bio. Med. Res. Int., v.2013, p.1-14, 2013.
- DUTTA, B.; KONCH, P.; GOGOI, S.M. et al. Clinicopathological studies of brooder pneumonia in broiler chicken. Int. J. Chem. Stud., v.5, p.510-512, 2017.
- EASSA, S.H.; MOHAMMED, M.H.; OMER, A.M. Case Report: Prevalence and significance of aspergillosis in commercial broiler chicken: Pathological study. Iraqi J. Vet. Sci., v.31, p.113-116, 2017.
- INMET - Instituto Nacional de Meteorologia. Boletim agroclimatológico, mensal de outubro - 2018. Bol. Agroclimatol., v.53, p.1-41, 2018.
- KUNKLE, R.A. Aspergillosis. In: SAIF, Y.M.; BARNES, H.J.; GLISSON, J.R. (Eds.). Diseases of poultry. Iowa: Iowa State University Press, 2003, p.883-895,
- MCCLENNY, N. Laboratory detection and identification of Aspergillus species by microscopic observation and culture: the traditional approach. Med. Mycol., v.43, p.125-128, 2005.
- OCA, V.M.; VALDÉS, S.E.; SEGUNDO, C. et al. Aspergillosis, a natural infection in poultry: mycological and molecular characterization and determination of gliotoxin in Aspergillus fumigatus isolates. Avian Dis., v.61, p.77-82, 2017.
- OZHAK-BAYSAN, B.; ALASTRUEY-IZQUIERDO, A.; SABA, R.; et al. Aspergillus alliaceus and Aspergillus flavus co-infection in an acute myeloid leukemia patient. Med. Mycol., v.48, p.995-999, 2010.
- TELL, L.A. Aspergillosis in mammals and birds: impact on veterinary medicine. Med. Mycol., v.43, p.71-73, 2005.
- THRONE STEINLAGE, S.J.; SANDER, J.E.; BROWN, T.P.; et al. Disse minated mycosis in layer cockerels and pullets. Avian Dis., v.47, p.229-233, 2003.
Publication Dates
-
Publication in this collection
14 Aug 2020 -
Date of issue
Jul-Aug 2020
History
-
Received
02 Mar 2020 -
Accepted
04 May 2020