Abstract
This study presents results of the inventory of algal flora conducted between August 2007 and May 2008 in 18 lakes of the middle Rio Doce lake system, most of which is in the state of Minas Gerais, Brazil. We recorded 481 taxa, increasing the known total phytoplankton diversity of the region (gamma diversity) by 80%. The following classes were represented: Zygnematophyceae (171 taxa), Cyanobacteria (101), Chlorophyceae (71), Bacillariophyceae (42), Euglenophyceae (43), Trebouxiophyceae (24), Dinophyceae (8), Xanthophyceae (8), Chrysophyceae (6), Cryptophyceae (6) and Oedogoniophyceae (1). We identified 221 taxa that were rare (restricted to one or two lakes), and 101 that were considered representative (present in at least nine lakes). Botryococcus braunii, Elakatothrix genevensis, Planktolyngbya limnetica, Peridinium pusillum, Trachelomonas volvocina, Cosmarium contractum, Staurastrum forficulatum, Staurastrum leptocladum, Staurastrum rotula, and Staurodesmus dejectus were present in all lakes. Richness varied from 95 taxa (in Lake Gambazinho) to 168 taxa (in Lake Palmeirinha). Jaccard indices were low, and the highest similarities between lakes were 53% (Ferrugem/Ferruginha), 47% (Central/Almécega) and 46% (Águas Claras/Palmeirinha), demonstrating high environmental and biotic dissimilarities between lakes. Geographic distance was not significantly associated with floristic similarity, suggesting that local factors are more important than are regional ones in shaping the phytoplankton composition of lakes.
algal flora; inventory; tropical lakes
ARTICLES
Phytoplankton diversity in the middle Rio Doce lake system of southeastern Brazil
Cristiane Freitas de Azevedo BarrosI,* * Author for correspondence crisfabarros@gmail.com ; Aline Morena Menezes dos SantosII; Francisco Antônio Rodrigues BarbosaII
IUniversidade do Estado de Minas Gerais, Frutal, MG, Brazil
IIUniversidade Federal de Minas Gerais, Instituto de Ciências Biológicas, Laboratório de Limnologia, Belo Horizonte, MG, Brazil
ABSTRACT
This study presents results of the inventory of algal flora conducted between August 2007 and May 2008 in 18 lakes of the middle Rio Doce lake system, most of which is in the state of Minas Gerais, Brazil. We recorded 481 taxa, increasing the known total phytoplankton diversity of the region (gamma diversity) by 80%. The following classes were represented: Zygnematophyceae (171 taxa), Cyanobacteria (101), Chlorophyceae (71), Bacillariophyceae (42), Euglenophyceae (43), Trebouxiophyceae (24), Dinophyceae (8), Xanthophyceae (8), Chrysophyceae (6), Cryptophyceae (6) and Oedogoniophyceae (1). We identified 221 taxa that were rare (restricted to one or two lakes), and 101 that were considered representative (present in at least nine lakes). Botryococcus braunii, Elakatothrix genevensis, Planktolyngbya limnetica, Peridinium pusillum, Trachelomonas volvocina, Cosmarium contractum, Staurastrum forficulatum, Staurastrum leptocladum, Staurastrum rotula, and Staurodesmus dejectus were present in all lakes. Richness varied from 95 taxa (in Lake Gambazinho) to 168 taxa (in Lake Palmeirinha). Jaccard indices were low, and the highest similarities between lakes were 53% (Ferrugem/Ferruginha), 47% (Central/Almécega) and 46% (Águas Claras/Palmeirinha), demonstrating high environmental and biotic dissimilarities between lakes. Geographic distance was not significantly associated with floristic similarity, suggesting that local factors are more important than are regional ones in shaping the phytoplankton composition of lakes.
Key words: algal flora, inventory, tropical lakes
Introduction
Species richness is considered one of the simplest measures to express and quantify biological complexity in a given region (Nabout et al. 2007). In aquatic environments, species richness is influenced by several factors, including water temperature, mixing patterns of the water column, light, nutrient availability, and herbivory (Reynolds, 1987). Therefore, knowledge of richness patterns is essential to proposals for monitoring strategies and for biodiversity conservation activities (Downing & Leibold 2002; Declerck et al. 2005; Nogueira et al. 2008).
Diversity can be assessed on different scales. Local, or alpha, diversity is given by the total number of species in each habitat. Regional, or gamma, diversity is the total number of species observed in a range of habitats (Magurran 2004). The term beta diversity was introduced by Whittaker in the 1960s. At first the term was used in order to describe changes in species composition along gradients of altitude and humidity through differences in rates of gain and loss of species. However, beta diversity is now defined as the taxonomic difference between samples, whether occurring along an environmental gradient or not (Veech et al. 2002).
The middle Rio Doce lake system is a large freshwater system in southeastern Brazil, formed as a result of a mass of sediments (from the original drainages of Rio Doce and its tributaries) that acted as a natural dam, giving rise to a dense network of lakes (Pflug 1969 cited in de Meis & Tundisi 1997). The biological and ecological importance of the system was recently demonstrated by its recognition as an international Ramsar site, making it the 11th site in Brazil to be added to the Ramsar list of wetlands of international importance (Ramsar 2010).
Limnological research in the middle Rio Doce lake system was initiated in the 1970s (Tundisi & Saijo 1997). Since then, various aspects have been investigated (Barbosa & Tundisi 1980; Henry & Barbosa 1989; Rocha et al. 1989; Tundisi & Saijo 1997), increasing knowledge of the geological, morphological, physical, chemical, and biological characteristics of the lakes. However, specific studies on phytoplankton were mainly focused on Lake Dom Helvécio and Lake Carioca (Hino et al. 1986; Taniguchi et al. 2003; Barros et al. 2006; Souza et al. 2008). However, a recent survey evaluated plankton diversity in a larger number of lakes (Maia-Barbosa et al. 2006). Therefore, there is still little information on phytoplankton diversity in this lake system, as shown by Barbosa et al. (1994). The present study aims to contribute to the knowledge of phytoplankton diversity of this lake complex by presenting the results of an inventory of the algal flora in 18 lakes.
Material and methods
Study area
The Rio Doce basin is located at in southeastern Brazil, between the state of Minas Gerais (86% of the total area) and the state of Espírito Santo (14% of the total area), encompassing 83,400 km2 (Marques & Barbosa, 2002). Two lake systems compose this basin: one, at the middle course, comprising ca. 250 lakes, distinct in their trophic status (Maillard et al. 2011), and another, at the lower course, comprising ca. 70 lakes (Cavati & Fernandes 2008). Approximately 50 lakes in the middle course are protected within Rio Doce State Park, a conservation unit created in 1939 and representing the largest contiguous remnant of the Atlantic Forest in Minas Gerais (359.76 km2). Lakes located in the surrounding area are affected mainly by hardwood (Eucalyptus spp.) plantations and pastureland, among several municipalities.
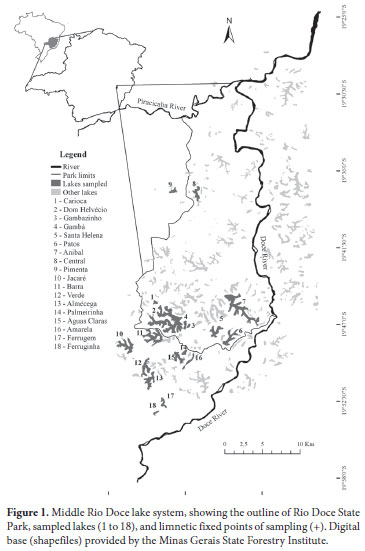
For the purposes of this study, 18 lakes were selected: eight located inside the Rio Doce State Park limits and ten in the surrounding areas (Fig. 1; Tab. 1). In selecting the lakes, we took into account their greatest physiographic differences and their accessibility, mainly during the rainy (summer) season. The climate of the region is classified as tropical semi-humid with 4-5 months of dry weather, exhibiting mesothermal characteristics (Nimer, 1989) with temperatures of approximately 25ºC. According to Tundisi (1997), the monthly precipitation is highest in December (350 mm) and lowest in July and August (10 mm).
Samplings
Field work was conducted quarterly, in August 2007, November 2007, February 2008, and May 2008. Samples were collected from a fixed point in the limnetic region of each lake. Samplings were authorized by the Minas Gerais State Forestry Institute (permit no. 005/07). Water transparency was estimated in situ by Secchi disk measurements (Cole 1983). Samples were collected for total phosphorus quantification (Mackereth et al. 1978).
Samples for qualitative analysis of phytoplankton were collected by successive vertical and horizontal throws with a 20-µm mesh plankton net, then fixed with 4% formaldehyde solution. For each qualitative sample (four samples/lake), eight slides were analyzed, for a total of 32 slides per lake. Organisms were identified under light microscopy down to the lowest possible taxonomic level using a specific bibliography: Föster (1969; 1974), Prescott et al. (1975; 1977; 1981; 1982), Komárek & Fott (1983), Sant'Anna (1984), Komárek & Anagnostidis (1989; 1999), Menezes et al. (1995), and Bicudo & Menezes (2006). Samples for quantitative analyses were collected with van Dorn bottles at three depths (100%, 10%, and 1% of incident light, as defined with Secchi disk measurements) and fixed with Lugol's solution. Quantitative analysis followed the method described by Utermöhl (1958).
Data analysis
For each lake, species richness was assessed on the basis of the number of taxa identified, considering the qualitative and quantitative data. Species richness for the sampled region (gamma diversity) was estimated with the first-order jackknife estimator (Nabout et al. 2007), using Stimate S software (Colwell 2006). Beta diversity was estimated by the difference between gamma diversity (total species recorded for the set of lakes) and average alpha diversity (mean species richness per lake), as suggested by Crist et al. (2003):
β = γ - mean α
where β is beta diversity, γ is gamma diversity, and α is alpha diversity.
Differences in relation to water transparency and total phosphorus levels were determined using ANOVA. Spearman's correlation coefficient was used in order to test for relationships between the morphometric features (area, depth, and margin development index) and physico-chemical variables. Hierarchical cluster analysis using Jaccard distance and Ward's method (Ward 1963) were performed in order to assess similarity between lakes in terms of the phytoplankton species composition. These statistical analyses were conducted using Past 1.90 software (Hammer et al. 2001).
Results and discussion
Morphometric features, such as depth, margin development index, and area, varied among lakes (Table 1). Secchi depth and total phosphorus concentrations also differed among lakes (F=996.888; p=0.000 and F=730.533; p=0.000, respectively) and correlated with depth (p<0.05; r=0.630 for Secchi disk measurements and r=0.596 for total phosphorus concentrations). Lakes that were shallower (<5 m: Pimenta, Central, Amarela, Ferrugem, and Ferruginha) showed lower Secchi disappearance depths (>1.2 m) and higher levels of total phosphorus (>30 µg/L; especially Lakes Amarela and Ferrugem, in which total phosphorus was >50 µg/L), than did the lakes that were deeper (>7 m: Dom Helvécio, Águas Claras, Almécega, Gambá, and Verde), which showed greater water transparency (down to 2.2 m) and lower values of the trophy indicator (below 21 µg/L of phosphorus).
Richness extrapolation indices, such as the jackknife, although not usually used for phytoplankton (Nabout et al. 2007; Nogueira et al. 2008), can be important tools to assess the representativeness of the sampling effort. In the present study, a total of 481 taxa were recorded (Fig. 2), corresponding to 77% of the expected richness, estimated using first-order jackknife (jackknife 1 = 624). The relationship between the observed and estimated values for richness indicated that our methods were appropriate for a diversity survey. In addition, the known phytoplankton diversity in the middle Rio Doce lake system was high in comparison with the 267 species reported for seven lakes in the system by Maia-Barbosa et al. (2006). Gamma diversity increased by 80% with the expansion of the number of studied environments, reinforcing the observed environmental and biotic heterogeneity.
Eleven classes were identified: Zygnematophyceae (171 taxa), Cyanobacteria (101), Chlorophyceae (71), Bacillariophyceae (42), Euglenophyceae (43), Trebouxiophyceae (24), Dinophyceae (8), Xanthophyceae (8), Chrysophyceae (6), Cryptophyceae (6) and Oedogoniophyceae (1); Appendix 1Appendix 1. The predominance of desmids, especially those of the genera Cosmarium,Staurastrum and Staurodesmus, in the middle Rio Doce lakes was previously reported by Reynolds (1997), who attributed the dominance of this group to oligotrophic conditions and good preservation of the lakes. It has also been suggested that the thermal stratification pattern known as atelomixis (characterized by unusual, irregular circulation periods of short duration) is a key factor for desmid prevalence (Barbosa & Padisák 2002; Souza et al. 2008), because it allows these species, which have relatively high specific density (Padisák et al. 2003), to remain within the upper layers of the water column.
Of the 481 taxa identified, 221 were rare, occurring exclusively in one or two lakes, and 101 exhibited high frequency, occurring in at least nine lakes (Tab. 2). In addition, 10 species were common to all lakes: Botryococcus braunii Kützing, Elakatothrix genevensis (Reverdin) Hindák, Planktolyngbya limnetica (Lemmerman) Komárkova-Legnerová and Cronberg, Peridinium pusillum (Pénard) Lemmerman, Trachelomonas volvocina Ehrenberg, Cosmarium contractum Kirchner, Staurastrum forficulatum Lundell, Staurastrum leptocladum Nordstedt, Staurastrum rotula Nordstedt, and Staurodesmus dejectus (Brébisson) Teiling.
Phytoplankton richness ranged from 95 taxa (Lake Gambazinho) to 168 taxa (Lake Palmeirinha). In general, the most representative groups for each lake were the same observed for the data set: Zygnematophyceae > Chlorophyceae > Cyanobacteria; exceptions occurred for Lake Amarela, where the number of Euglenophyceae species (25) exceeded that of Cyanobacteria species (15), and for Lake Gambazinho, which had more Bacillariophyceae species (22) than Cyanobacteria species (12) (Fig. 3). The predominance of Euglenophyceae in Lake Amarela, previously reported by Reynolds (1997), is associated with its late successional stage, shallowness, and broad macrophyte coverage of the surface area, mainly with Nymphaea sp., Utricularia sp., and Eleocharis sp.
Lake Amarela showed the highest number of exclusive taxa (26), followed by Lakes Gambazinho (15), Jacaré (13), and Palmeirinha (13). The lowest numbers of exclusive species were observed in Lakes Ferrugem (1), Carioca (2), and Ferruginha (2).
The fact that the incidence of exclusive species was highest in Lakes Amarela and Gambazinho indicates the great importance of the shoreline regions, given that, in the case of Lake Gambazinho, the main representative species were diatoms of the order Pennales (6 taxa) and large desmids (5 taxa), the latter also prevailing in Lake Amarela (12 taxa), together with Euglenophyceae (7 taxa). Those two lakes are quite distinct from the other lakes of the region and from those presented here. In Lake Amarela, high amounts of organic matter reflect its advanced state of eutrophication, as evidenced by the total phosphorous and transparency values. Lake Gambazinho has a polymictic pattern of thermal stratification, in contrast to the more common warm-monomictic pattern of thermal stratification observed in the majority of the middle Rio Doce lakes. Such characteristics seem to have been responsible for the higher numbers of typically periphytic species in the samples collected from these lakes.
We obtained low Jaccard indices. The highest similarities were 53% for Lake Ferrugem versus Lake Ferruginha, followed by 47% for Lake Central versus Lake Almécega, and 46% for Lake Águas Claras versus Lake Palmeirinha. Lake Amarela showed the lowest similarity with the other lakes, ranging from 14% to 25%.
On the basis of phytoplankton species composition, we identified five clusters of lakes (Fig. 4): cluster 1-Lakes Aníbal, Carioca, Pimenta, Dom Helvécio, and Santa Helena; cluster 2-Lakes Central, Almécega, Ferrugem, and Ferruginha; cluster 3-Lakes Águas Claras, Palmeirinha, Patos, Barra, Jacaré, and Verde; cluster 4-Lakes Gambá and Gambazinho, and cluster 5-Lake Amarela. Geographic distance between lakes did not correlate significantly with interlake similarity in phytoplankton composition (r = 0.03; p > 0.05).
With the exception of a few pairs of lakes that are geographically proximal and were all included in the same cluster-Gambá and Gambazinho (500 m apart); Ferrugem and Ferruginha (1200 m apart); and Águas Claras and Palmeirinha (1500 m apart)-it should be noted that the clusters were composed of lakes that were distant from one another, some located within the Rio Doce State Park and others located in the surrounding areas. In addition, the difference between the gamma diversity and the mean alpha diversity, considered here as an estimate of beta diversity, was high: 347 species. This suggests that the phytoplankton species composition of such lakes is more dependent on local factors than on regional factors.
Considering that diversity and rarity are important criteria for assessing the conservation value of a given region (Coesel 2001), the possibility of protecting the middle Rio Doce lake system as a whole should be considered. That will require specific strategies for the environments surrounding the state park, with the primary objective of maintaining phytoplankton diversity at the regional level.
Acknowledgments
This study received financial support from the Conselho Nacional de Desenvolvimento Científico e Tecnológico/Ministério da Ciência e Tecnologia (MCT/CNPq, Ministry of Science and Technology/National Council for Scientific and Technological Development), as part of the funding of the Brazilian Long-Term Ecological Research (LTER) program, Site 4 (Grant no. 520031/98-9), and from the Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG, Foundation for the Support of Research in the state of Minas Gerais; doctoral scholarship to C.F.A.B.) We are grateful to Diego G. F. Pujoni and Maria Betânia G. Souza for their valuable suggestions, as well as to the staff of the Limnology Laboratory of the Instituto de Ciências Biológicas/Universidade Federal de Minas Gerais (ICB/UFMG, Life Sciences Institute/Federal University of Minas Gerais) for their contributions to the sampling and analysis.
References
Barbosa, F.A.R. & Tundisi, J.G. 1980. Primary production of phytoplankton and environmental characteristics of a shallow quaternary lake at Eastern Brazil. Archiv fur Hydrobiologie 90(2):139-161.
Barbosa, F.A.R.; Godinho, A.L.; Gentil, J.G. & Pinto-Coelho, R.M. 1994. Limnologia e ictiologia. Pp. 17-23. In: Instituto Estadual de Florestas/MG (Ed.). Anais do Workshop sobre pesquisas prioritárias para o Parque Estadual do Rio Doce. Instituto Estadual de Florestas IEF/ENGEVIX, Belo Horizonte.
Barbosa, F.A.R. & Padisák, J. 2002. The forgotten lake stratification pattern: atelomixis, and its ecological importance. Verhandlungen des Internationalen Verein Limnologie 28:1385-1395.
Barros, C.F.A.; Souza, M.B.G. & Barbosa, F.A.R. 2006. Seasonal forces driving phytoplankton size structure in a tropical deep lake (Dom Helvécio Lake, South-East Brasil). Acta Limnologica Brasiliensia 18(1):55-66.
Bicudo, C.E.M. & Menezes, M. 2006. Gêneros de algas de águas continentais do Brasil: Chave para Identificação e Descrições. São Carlos, Editora Rima.
Cavati, B. & Fernandes, V.O. 2008. Algas perifíticas em dois ambientes do baixo rio Doce (lagoa Juparanã e rio Pequeno - Linhares, Estado do Espírito Santo, Brasil): variação espacial e temporal. Acta Scientiarium 30(4):439-448.
Coesel, P.F.M. 2001. A method for quantifying conservation value in lentic freshwater habitats using desmids as indicator organisms. Biodiversity and Conservation 10(2):177-187.
Cole, G.A. 1983. Textbook of limnology. St. Louis: The C.V. Mosby Company.
Colwell, R.K. 2006. Estimate S - Statistical estimation of species richness and shared species from samples. Version 8. University of Connecticut. Available in: http://purl.oclc.org/estimates. (Accessed 19/March/2010).
Crist, T.O.; Veech, J.A.; Gering, J.C. & Summerville, K.S. 2003. Partitioning species diversity across landscapes and regions: a hierarchical analysis of α, β, and γ diversity. The American Naturalist 162(6):734-743.
Declerck, S.; Vandekerkhove, J.; Johansson, L.; Muylaert, K.; Conde-Porcuna, J.M.; Van Der Gucht, K.; Pérez-Martinéz, C.; Lauridsen, T.; Schwenk, K.; Zwart, G.; Rommens, W.; López-Ramos, J.; Jeppesen, E.; Vyverman, W.; Brendonck, L. & De Meester, L. 2005. Multi-group biodiversity in shallow lakes along gradients of phosphorus and water plant cover. Ecology 86(7):1905-1915.
De Meis, M.R.M. & Tundisi, J.G. 1997. Geomorphological and limnological process as a basis for lake typology. The middle Rio Doce lake system. Pp. 25-50. In: Tundisi, J.G. & Saijo, Y. (Ed.). Limnological Studies in the Rio Doce Valley Lakes. São Carlos, Brazilian Academy of Sciences.
Downing, A.L. & Leibold, M.A. 2002. Ecosystem consequences of species richness and composition in pond food webs. Nature 416:837-841.
Förster, K. 1969. Amazonische Desmidieen. 1. Areal Santarém. Amazoniana 2(1-2):5-232.
Förster, K. 1974. Amazonische Desmidieen. 2. Areal Maués-n Abacaxis. Amazoniana 5(2):135-242.
Hammer, O.; Harper, D.A.T. & Ryan, P.D. 2001. Paleontological statistics software package for education and data analysis. Paleontologia electronic 4:9.
Henry, R. & Barbosa, F.A.R. 1989. Thermal structure, heat content and stability of two lakes in The National Park of Rio Doce Valley (Minas Gerais, Brazil). Hydrobiologia 171(3):189-199.
Hino, K.; J.G. Tundisi & Reynolds, C.S. 1986. Vertical distribution of phytoplankton in a stratified lake (Lago Dom Helvécio, Southeastern Brazil), with special reference to the metalimnion. Japanese Journal of Limnology 47(3):239-246.
Komárek, J. & K. Anagnostidis, 1989. Modern approach to the classification system of cyanophytes, 4: Nostocales. Algological Studies 82(3):247-345.
Komárek, J. & Anagnostidis, K. 1999. Cyanoprokaryota I. Teil Chroococcales. Pp. 1-548. In: Ettl, H.; Gärtner, G.; Heynig H. & Mollenhauer, D. (Ed.). Süsswasserflora von Mitteleuropa 19. Verlag: G. Fischer.
Komárek, J. & Fott, B. 1983. Chlorophyceae (Grünalgen), Ordiniung: Chlorococcales. Pp. 1-1.044. In: Huber-Pestalozzi, G.; Heynig H. & Mollenhauer, D. (Eds.). Das phytoplankton des Sübwassers: Systematik und Biologie. Stuttgart: E. Schwiezerbat'sche Verlagsbuchlandlung.
Mackereth, F.J.H.; Heron, J. & Talling, J.F. 1978. Water analysis. Ambleside: Scientific Publication Nº 36, Freshwater Biological Association.
Magurran, A.F. 2004. Measuring Biological Diversity. Oxford, Blackwell.
Maia-Barbosa, P.M.; Barros, C.F.A.; Souza, M.B.G.; Faria, V.R.; Barbosa, L.G.; Brito, S.L.; Souza, R.A.; Rietzler, A.; Eskinazi-Sant'anna, E. & Barbosa, F.A.R. 2006. The middle Rio Doce lakes, southeast Brazil: biodiversity and its controlling forces at local and regional scales. Verhandlungen des Internationalen Verein Limnologie 29:1-5.
Maillard, P.; Pivari, M.O. & Luis, C.H.P. 2011. Remote Sensing for Mapping and Monitoring Wetlands and Small Lakes in Southeast Brazil. In: Chemin, Y. (Ed.). Remote Sensing of Planet Earth. Croatia, Intechweb.org.
Marques, M.M. & Barbosa, F.A.R. 2002. Áreas prioritárias para a conservação da diversidade aquática no trecho médio da bacia do Rio Doce, MG. Naturalia 27:211-229.
Menezes, M.; Nascimento, E.P. & Fonseca, C.G. 1995. Flora dos Estados de Goiás e Tocantins - Criptógamos v. 4: Euglenophyceae. Goiânia, Editora UFG.
Nabout, J.C.; Nogueira, I.S.; Oliveira, L.G. & Morais, R.R. 2007. Phytoplankton diversity (alpha, beta, and gamma) from the Araguaia River tropical floodplain lakes (central Brazil). Hydrobiologia 557:455-461.
Nimer, E. 1989. Climatologia do Brasil. IBGE.
Nogueira, I.S., Nabout, J.C.; Oliveira, J.E. & Silva, K.D. 2008. Diversidade (alfa, beta e gama) da comunidade fitoplanctônica de quatro lagos artificiais urbanos do município de Goiânia, GO. Hoehnea 35(2):219-233.
Padisák, J.; Soróczki-Pintér, E. & Reznersinking, Z. 2003. Properties of some phytoplankton shapes and the relation of form resistance to morphological diversity of plankton - an experimental study. Hydrobiologia 500:243-257.
Prescott, G.W.; Croasdale, H.T. & Vinard, W.C. 1975. A synopsis of North American desmids Part II. Desmidiaceae: Placodermae Section 1. USA, University of Nebraska Press.
Prescott, G.W.; Croasdale, H.T. & Vinard, W.C. 1977. A synopsis of North American desmids Part II. Desmidiaceae: Placodermae Section 2. USA, University of Nebraska Press.
Prescott, G.W.; Croasdale, H.T.; Vinard, W.C. & Bicudo, C.E.M. 1981. A synopsis of North American desmids Part II. Desmidiaceae: Placodermae Section 3. USA, University of Nebraska Press.
Prescott, G.W., Croasdale, H.T. & Vinard, W.C. 1982. A synopsis of North American desmids Part II. Desmidiaceae: Placodermae Section 4. USA, University of Nebraska Press.
Ramsar. 2010. The Ramsar List of wetlands of international importance. Available in http://www.ramsar.org/pdf/sitelist_order.pdf. (Accessed 16/May/2011).
Reynolds, C.S. 1987. The response of phytoplankton communities to change lake environments. Schweizerische Zeitschrift fur Hydrologie 49(2):220-236.
Reynolds, C.S. 1997. On the vertical distribution of phytoplankton in the middle Rio Doce valley lakes. Pp. 227-241. In: Tundisi, J.G. & Saijo, Y. (Ed.). Limnological studies on the Rio Doce valley lakes, Brazil. São Carlos, Brazilian Academy of Sciences.
Rocha O.; Tundisi, T.M. & Tundisi, J.G. 1989. The metalimnetic layer of Lake D. Helvécio: Plankton Distribution. Japanese Journal of Limnology 50(2):168-169.
Sant'anna, C.L. 1984. Bibliotheca Phycologica. Chlorococcales (Chlorophyceae) do Estado de São Paulo, Brasil. Germany, J.Cramer.
Souza, M.B.G.; Barros, C.F.A.; Barbosa, F.; Hajinal, E. & Padisák, J. 2008. Role of atelomixis in phytoplankton assemblages' replacement in Dom Helvécio Lake, South-East Brazil. Hydrobiologia 607:211-224.
Taniguchi, G.M.; Rocha, O. & Senna, P.A.C. 2003. A comunidade fitoplanctônica de um lago tropical no sudeste do Brasil (lago Dom Helvécio, estado de Minas Gerais). Caderno de Pesquisa Série Biologia, Santa Cruz do Sul 15(1):29-55.
Tundisi, J.G. 1997. Climate; Pp. 7-11. In: Tundisi, J.G. & Saijo, Y. (Ed.). Limnological Studies on the Rio Doce Valley Lakes, Brazil. São Paulo: Brazilian Academy of sciences, University of São Paulo, School Engineering at S. Carlos and Center for Water Resources and Applied Ecology.
Tundisi, J.G. & Saijo, Y. 1997. Limnological studies in the Rio Doce valley lakes, Brazil. São Paulo, Brazilian Academy of sciences, University of São Paulo, School Engineering at S. Carlos and Center for Water Resources and Applied Ecology.
Utermöhl, H., 1958. Zur vervollkomnung der quantitativen phytoplankton-methodik. Verhandlungen Internationale Vereinigung für Theoretische und Angewandte Limnologie 9:1-38.
Veech, J.A.; Summerville, K.S., Crist, T.O. & Gering, J.C. 2002. The additive partitioning of species diversity: recent revival of an old idea. Oikos 99:3-9.
Ward, J.H. 1963. Hierarchical grouping to optimize an objective function. Journal of the American Statistical Association 58(301):236-244.
Wetzel, R.G. 2001. Limnology: Lake and River ecosystems. 3 ed. USA, Academic Press.
Submitted: 22 June, 2011
Accepted: 15 January, 2013
Appendix 1
Appendix 1 - Click to enlargeAppendix 1
- Barbosa, F.A.R. & Tundisi, J.G. 1980. Primary production of phytoplankton and environmental characteristics of a shallow quaternary lake at Eastern Brazil. Archiv fur Hydrobiologie 90(2):139-161.
- Barbosa, F.A.R. & Padisák, J. 2002. The forgotten lake stratification pattern: atelomixis, and its ecological importance. Verhandlungen des Internationalen Verein Limnologie 28:1385-1395.
- Barros, C.F.A.; Souza, M.B.G. & Barbosa, F.A.R. 2006. Seasonal forces driving phytoplankton size structure in a tropical deep lake (Dom Helvécio Lake, South-East Brasil). Acta Limnologica Brasiliensia 18(1):55-66.
- Bicudo, C.E.M. & Menezes, M. 2006. Gêneros de algas de águas continentais do Brasil: Chave para Identificação e Descrições São Carlos, Editora Rima.
- Cavati, B. & Fernandes, V.O. 2008. Algas perifíticas em dois ambientes do baixo rio Doce (lagoa Juparanã e rio Pequeno - Linhares, Estado do Espírito Santo, Brasil): variação espacial e temporal. Acta Scientiarium 30(4):439-448.
- Coesel, P.F.M. 2001. A method for quantifying conservation value in lentic freshwater habitats using desmids as indicator organisms. Biodiversity and Conservation 10(2):177-187.
- Cole, G.A. 1983. Textbook of limnology St. Louis: The C.V. Mosby Company.
- Colwell, R.K. 2006. Estimate S - Statistical estimation of species richness and shared species from samples. Version 8. University of Connecticut. Available in: http://purl.oclc.org/estimates (Accessed 19/March/2010).
- Crist, T.O.; Veech, J.A.; Gering, J.C. & Summerville, K.S. 2003. Partitioning species diversity across landscapes and regions: a hierarchical analysis of α, β, and γ diversity. The American Naturalist 162(6):734-743.
- Declerck, S.; Vandekerkhove, J.; Johansson, L.; Muylaert, K.; Conde-Porcuna, J.M.; Van Der Gucht, K.; Pérez-Martinéz, C.; Lauridsen, T.; Schwenk, K.; Zwart, G.; Rommens, W.; López-Ramos, J.; Jeppesen, E.; Vyverman, W.; Brendonck, L. & De Meester, L. 2005. Multi-group biodiversity in shallow lakes along gradients of phosphorus and water plant cover. Ecology 86(7):1905-1915.
- De Meis, M.R.M. & Tundisi, J.G. 1997. Geomorphological and limnological process as a basis for lake typology. The middle Rio Doce lake system. Pp. 25-50. In: Tundisi, J.G. & Saijo, Y. (Ed.). Limnological Studies in the Rio Doce Valley Lakes São Carlos, Brazilian Academy of Sciences.
- Downing, A.L. & Leibold, M.A. 2002. Ecosystem consequences of species richness and composition in pond food webs. Nature 416:837-841.
- Förster, K. 1969. Amazonische Desmidieen. 1. Areal Santarém. Amazoniana 2(1-2):5-232.
- Förster, K. 1974. Amazonische Desmidieen. 2. Areal Maués-n Abacaxis. Amazoniana 5(2):135-242.
- Hammer, O.; Harper, D.A.T. & Ryan, P.D. 2001. Paleontological statistics software package for education and data analysis. Paleontologia electronic 4:9.
- Henry, R. & Barbosa, F.A.R. 1989. Thermal structure, heat content and stability of two lakes in The National Park of Rio Doce Valley (Minas Gerais, Brazil). Hydrobiologia 171(3):189-199.
- Hino, K.; J.G. Tundisi & Reynolds, C.S. 1986. Vertical distribution of phytoplankton in a stratified lake (Lago Dom Helvécio, Southeastern Brazil), with special reference to the metalimnion. Japanese Journal of Limnology 47(3):239-246.
- Komárek, J. & K. Anagnostidis, 1989. Modern approach to the classification system of cyanophytes, 4: Nostocales. Algological Studies 82(3):247-345.
- Komárek, J. & Anagnostidis, K. 1999. Cyanoprokaryota I. Teil Chroococcales. Pp. 1-548. In: Ettl, H.; Gärtner, G.; Heynig H. & Mollenhauer, D. (Ed.). Süsswasserflora von Mitteleuropa 19. Verlag: G. Fischer.
- Komárek, J. & Fott, B. 1983. Chlorophyceae (Grünalgen), Ordiniung: Chlorococcales Pp. 1-1.044. In: Huber-Pestalozzi, G.; Heynig H. & Mollenhauer, D. (Eds.). Das phytoplankton des Sübwassers: Systematik und Biologie. Stuttgart: E. Schwiezerbat'sche Verlagsbuchlandlung.
- Mackereth, F.J.H.; Heron, J. & Talling, J.F. 1978. Water analysis Ambleside: Scientific Publication Nş 36, Freshwater Biological Association.
- Magurran, A.F. 2004. Measuring Biological Diversity Oxford, Blackwell.
- Maia-Barbosa, P.M.; Barros, C.F.A.; Souza, M.B.G.; Faria, V.R.; Barbosa, L.G.; Brito, S.L.; Souza, R.A.; Rietzler, A.; Eskinazi-Sant'anna, E. & Barbosa, F.A.R. 2006. The middle Rio Doce lakes, southeast Brazil: biodiversity and its controlling forces at local and regional scales. Verhandlungen des Internationalen Verein Limnologie 29:1-5.
- Maillard, P.; Pivari, M.O. & Luis, C.H.P. 2011. Remote Sensing for Mapping and Monitoring Wetlands and Small Lakes in Southeast Brazil. In: Chemin, Y. (Ed.). Remote Sensing of Planet Earth Croatia, Intechweb.org.
- Marques, M.M. & Barbosa, F.A.R. 2002. Áreas prioritárias para a conservação da diversidade aquática no trecho médio da bacia do Rio Doce, MG. Naturalia 27:211-229.
- Menezes, M.; Nascimento, E.P. & Fonseca, C.G. 1995. Flora dos Estados de Goiás e Tocantins - Criptógamos v. 4: Euglenophyceae. Goiânia, Editora UFG.
- Nabout, J.C.; Nogueira, I.S.; Oliveira, L.G. & Morais, R.R. 2007. Phytoplankton diversity (alpha, beta, and gamma) from the Araguaia River tropical floodplain lakes (central Brazil). Hydrobiologia 557:455-461.
- Nimer, E. 1989. Climatologia do Brasil IBGE.
- Nogueira, I.S., Nabout, J.C.; Oliveira, J.E. & Silva, K.D. 2008. Diversidade (alfa, beta e gama) da comunidade fitoplanctônica de quatro lagos artificiais urbanos do município de Goiânia, GO. Hoehnea 35(2):219-233.
- Padisák, J.; Soróczki-Pintér, E. & Reznersinking, Z. 2003. Properties of some phytoplankton shapes and the relation of form resistance to morphological diversity of plankton - an experimental study. Hydrobiologia 500:243-257.
- Prescott, G.W.; Croasdale, H.T. & Vinard, W.C. 1975. A synopsis of North American desmids Part II. Desmidiaceae: Placodermae Section 1 USA, University of Nebraska Press.
- Prescott, G.W.; Croasdale, H.T. & Vinard, W.C. 1977. A synopsis of North American desmids Part II. Desmidiaceae: Placodermae Section 2 USA, University of Nebraska Press.
- Prescott, G.W.; Croasdale, H.T.; Vinard, W.C. & Bicudo, C.E.M. 1981. A synopsis of North American desmids Part II. Desmidiaceae: Placodermae Section 3 USA, University of Nebraska Press.
- Prescott, G.W., Croasdale, H.T. & Vinard, W.C. 1982. A synopsis of North American desmids Part II. Desmidiaceae: Placodermae Section 4 USA, University of Nebraska Press.
- Reynolds, C.S. 1987. The response of phytoplankton communities to change lake environments. Schweizerische Zeitschrift fur Hydrologie 49(2):220-236.
- Rocha O.; Tundisi, T.M. & Tundisi, J.G. 1989. The metalimnetic layer of Lake D. Helvécio: Plankton Distribution. Japanese Journal of Limnology 50(2):168-169.
- Sant'anna, C.L. 1984. Bibliotheca Phycologica. Chlorococcales (Chlorophyceae) do Estado de São Paulo, Brasil Germany, J.Cramer.
- Souza, M.B.G.; Barros, C.F.A.; Barbosa, F.; Hajinal, E. & Padisák, J. 2008. Role of atelomixis in phytoplankton assemblages' replacement in Dom Helvécio Lake, South-East Brazil. Hydrobiologia 607:211-224.
- Taniguchi, G.M.; Rocha, O. & Senna, P.A.C. 2003. A comunidade fitoplanctônica de um lago tropical no sudeste do Brasil (lago Dom Helvécio, estado de Minas Gerais). Caderno de Pesquisa Série Biologia, Santa Cruz do Sul 15(1):29-55.
- Tundisi, J.G. 1997. Climate; Pp. 7-11. In: Tundisi, J.G. & Saijo, Y. (Ed.). Limnological Studies on the Rio Doce Valley Lakes, Brazil São Paulo: Brazilian Academy of sciences, University of São Paulo, School Engineering at S. Carlos and Center for Water Resources and Applied Ecology.
- Tundisi, J.G. & Saijo, Y. 1997. Limnological studies in the Rio Doce valley lakes, Brazil São Paulo, Brazilian Academy of sciences, University of São Paulo, School Engineering at S. Carlos and Center for Water Resources and Applied Ecology.
- Utermöhl, H., 1958. Zur vervollkomnung der quantitativen phytoplankton-methodik. Verhandlungen Internationale Vereinigung für Theoretische und Angewandte Limnologie 9:1-38.
- Veech, J.A.; Summerville, K.S., Crist, T.O. & Gering, J.C. 2002. The additive partitioning of species diversity: recent revival of an old idea. Oikos 99:3-9.
- Ward, J.H. 1963. Hierarchical grouping to optimize an objective function. Journal of the American Statistical Association 58(301):236-244.
- Wetzel, R.G. 2001. Limnology: Lake and River ecosystems 3 ed. USA, Academic Press.
Appendix 1


Publication Dates
-
Publication in this collection
22 July 2013 -
Date of issue
June 2013
History
-
Received
22 June 2011 -
Accepted
15 Jan 2013