Abstract
Anathallis adenochila is a small epiphytic orchid, endemic to the Atlantic Forest in the southern and southeastern regions of Brazil. Information on the species is scarce and limited to distribution and occurrence data, with no reports about its development. In the present study, plantlets were propagated in vitro, and the influence of different concentrations of macronutrient salts and sucrose on the survival and development of the species was assessed. The analysis were performed on complete Murashige and Skoog (MS) medium and half MS medium (with half-strength macronutrients) containing 10, 30 or 60 g L-1 of sucrose. Plantlet survival, height of the aerial part, and the length of the longest root were significantly greater on half MS medium containing 30 or 60 g L-1 of sucrose. The number of leaves per plantlet was higher in the presence of 60 g L-1 of sucrose, regardless of macronutrient concentration, and the highest number of roots was observed in plantlets cultured on half MS medium with 60 g L-1 of sucrose. This first report of Anathallis adenochila in vitro propagation may contribute to future studies on the physiological and ecological aspects of the life cycle of this species.
conservation; carbon source; micropropagation; orchid; mineral salts
ARTICLES
In vitro propagation of Anathallis adenochila (Loefgr.) F. Barros (Orchidaceae), a species endemic to southern and southeastern Brazil
Delio Endres JúniorI; Márcio Hisayuki SasamoriI; Annette DrosteI, II, * * Corresponding author: annette@feevale.br
IUniversidade Feevale, Laboratório de Biotecnologia Vegetal, Novo Hamburgo, RS, Brazil
IIUniversidade Feevale, Programa de Pós-Graduação em Qualidade Ambiental, Novo Hamburgo, RS, Brazil
ABSTRACT
Anathallis adenochila is a small epiphytic orchid, endemic to the Atlantic Forest in the southern and southeastern regions of Brazil. Information on the species is scarce and limited to distribution and occurrence data, with no reports about its development. In the present study, plantlets were propagated in vitro, and the influence of different concentrations of macronutrient salts and sucrose on the survival and development of the species was assessed. The analysis were performed on complete Murashige and Skoog (MS) medium and half MS medium (with half-strength macronutrients) containing 10, 30 or 60 g L-1 of sucrose. Plantlet survival, height of the aerial part, and the length of the longest root were significantly greater on half MS medium containing 30 or 60 g L-1 of sucrose. The number of leaves per plantlet was higher in the presence of 60 g L-1 of sucrose, regardless of macronutrient concentration, and the highest number of roots was observed in plantlets cultured on half MS medium with 60 g L-1 of sucrose. This first report of Anathallis adenochila in vitro propagation may contribute to future studies on the physiological and ecological aspects of the life cycle of this species.
Key words: conservation, carbon source, micropropagation, orchid, mineral salts
Introduction
The Atlantic Forest is in the third position on the global list of priority areas for the conservation of vascular plants (Myers et al. 2000), epiphytic plants standing out as one of most remarkable characteristic of this biome, with high levels of richness and diversity (Kersten 2010). Considered not only one of the most important global biodiversity hotspots (Myers et al. 2000) but also one of the most endangered biomes in the world, the Atlantic Forest in the state of Rio Grande do Sul, Brazil, has been reduced to 7.48% of its original area of approximately 1,360,000 km2 (Fundação SOS Mata Atlântica and INPE 2011).
Epiphytes are essential for the maintenance of biodiversity and interactive balance of forest communities, positively influencing the ecological processes of ecosystems by providing nutritional resources and specialized microenvironments for the fauna in the canopy and by participating in nutrient cycling mechanisms (Lugo & Scatena 1992; Rocha et al. 2004). In humid tropical and subtropical forest formations, there is great diversity of Orchidaceae species, which account for 70% of all vascular epiphytes in such formations (Moraes et al. 2010). Specifically in the Atlantic Forest, Orchidaceae is represented by 176 genera and 1257 species, of which 719 are endemic to the biome (Barros et al. 2009). Representatives of Orchidaceae are included in the Red Data Book prepared by the International Union for Conservation of Nature and Natural Resources (IUCN 2013) and in Appendix II of the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES 2013).
Anathallis adenochila (Loefgr.) F. Barros is an epiphytic orchid belonging to the subtribe Pleurothallidinae of the subfamily Epidendreae. The species is endemic to the southern and southeastern regions of Brazil, occurring in the Atlantic Forest (Barros et al. 2013). Anathallis adenochila plants are small, measuring only approximately 5 cm in height, and exhibit sympodial growth, as well as having no pseudobulbs. Its flowers are distributed in multi-flowered inflorescences, with sepals joined at the base (Buzzato et al. 2007). Information on the species is scarce and limited to distribution and occurrence data (Buzzato et al. 2007; Brustulin & Schmitt 2008).
Although orchids produce a large amount of seeds, that are fragile and do not have significant endosperm or nutrient material (Mitra 1971). In addition, under natural conditions, only a very limited number of seeds germinate (Stancato & Faria 1996; Pedroso-de-Moraes et al. 2012). This limits the success of the continuity of populations, especially in impacted areas. Associated with this limitation, the loss of habitats due to forest fragmentation makes species vulnerable (Tremblay et al. 2005) and, along with predatory collection, increases the pressure on natural populations.
In vitro tissue culture is an important tool for the genetic conservation of species and for the sustainable use of plants, through propagation, which increases specimen availability (Soares et al. 2009; Unemoto et al. 2007). The asymbiotic germination of orchid seeds under in vitro conditions enables high germination rates (Pedroso-de-Moraes et al. 2009) and the maintenance of the genetic variability of plants (Pinto et al. 2010). Although tissue culture has long been used, Orchidaceae species have different demands and tolerances with regard to abiotic conditions in vitro (Arditti 1967; Stancato & Faria 1996; Faria et al. 2004; Sorace et al. 2008). Most studies aim to improve in vitro culture conditions for species with ornamental and commercial value, which are often subjected to genetic improvement (Faria et al. 2002; Moraes et al. 2002; Faria et al. 2006; Pedroso-de-Moraes et al. 2012), and do not focus on native species that are less significant from an economic point of view (Arditti & Ernest 1992) but important for the conservation of genetic diversity and for the dynamics of the ecosystem.
It is important to understand the physiological demands of the species to be propagated, with the purpose of obtaining better quality plantlets in in vitro cultures. The Murashige and Skoog (MS) medium (Murashige & Skoog 1962) is one of the most widely used culture media for in vitro propagation of several plant species. Some studies have demonstrated that the concentrations of sucrose and macronutrient salts in MS culture medium have different effects on plantlet development and growth, depending on the species and type of explant (Sorace et al. 2008). Such effects are identified based on the evaluation of several parameters, like the development of leaves and roots, as well the biometry of these structures (Araújo et al. 2007; Muller et al. 2007).
The aim of the study was to propagate Anathallis adenochila plants using in vitro culture and evaluate the effect of different concentrations of sucrose and macronutrient salts on plantlet survival and development. To our knowledge, this represents the first attempt at in vitro propagation of the species. Our objective was to contribute to ontogenetic studies and to conservation programs based on the reintroduction of plants into their natural habitat.
Materials and methods
Anathallis adenochila capsules were collected at Henrique Luís Roessler Municipal Park (29°40'54"S; 51°06'56"W; elevation, 16.4 m), a Conservation Unit located in the urban area within the municipality of Novo Hamburgo, in the state of Rio Grande do Sul, Brazil. After being washed in running water with commercial detergent and rinsed in distilled water three times, the capsules were transferred to a laminar flow chamber, where they were washed in 70% ethyl alcohol for 30 s and submerged in 2% sodium hypochlorite for 10 min. The capsules were then washed in autoclaved distilled water four times and opened with a scalpel to remove the seeds. Seeds were placed in 200-ml flasks containing 50 ml of MS culture medium with 50% of the original formulation of macronutrient salts, containing 30 g L-1 of sucrose and 10 g L-1 of activated charcoal, solidified with 6 g L-1 of agar and with the pH adjusted to 5.7 before autoclave sterilization (Unemoto et al. 2007). We then added 1 ml of autoclaved distilled water to each flask (Arditti & Ernest 1992). For 12 months (the time required for obtaining plantlets ≥ 0.5 cm in height), the cultures were kept under controlled conditions: a luminous intensity of 100 µmol m-2/s; a 12/12-h light/dark cycle; and a temperature of 26±1°C.
Plantlets measuring 0.5-0.7 cm in height were selected, 270 of which were cultured in 200-ml flasks containing 50 ml of MS medium, with the same concentrations of activated charcoal and agar and the same pH used in the initial culture stage. We analyzed combinations of two concentrations of the original formula of macronutrient salts in the MS medium (50% and 100%) and three concentrations of sucrose (10, 30 and 60 g L-1). For each combination of salt and sucrose concentration, we cultured five plantlets per flask, totaling 11 flasks, which were kept for 240 days under the same conditions of luminosity and temperature used in the initial culture stage, and subculture was performed at 120 days.
After 240 days, plantlets were removed from the flasks and washed in running water. The following parameters were evaluated: survival; height of the aerial part; number of leaves; number of roots; longest root length; and fresh mass. Measurements were taken using a caliper. The survival data obtained were transformed into percentages. The survival data obtained for sucrose concentrations in each salt concentration were compared by the Student-Newman-Keuls test, with a probability level of 5%. The survival data obtained for salt concentrations in each sucrose concentration were compared by the Mann-Whitney test, with a probability level of 5%. The values for height of the aerial part, number of leaves, number of roots, longest root length and fresh mass were log (x+1) transformed (for fresh mass) or log (√(x+1)) transformed (for the remaining parameters). Means obtained for sucrose concentrations in each salt concentration were submitted to ANOVA followed by Tukey's test, with a probability level of 5%. Means of these parameters obtained for salt concentrations in each sucrose concentration were compared by Student's t-test, with a probability level of 5%.
Results and discussion
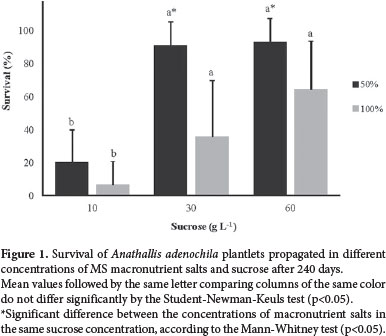
The higher percentages of survival were observed in plantlets treated with 30 or 60 g L-1 of sucrose associated with 50% of the original concentration of macronutrient salts (Fig. 1). In the presence of 10 g L-1 of sucrose combined with 50% or 100% of macronutrients, only 20% and 6% of the plantlets survived, respectively. On media with lower percentages of sucrose, plantlet necrosis was preceded by chlorosis, which can occur in tissues cultured in vitro in the presence of sucrose concentrations lower than 20 g L-1 (Grattapaglia & Machado 1998). Cells do not find normal luminosity conditions and carbon dioxide concentration adequate for photosynthesis when in culture (Skrebsky et al. 2004), requiring a supply of carbohydrates as an energy source, although they can exhibit some degree of autotrophic growth (Pinto et al. 2010). The fact that the higher survival rates were observed for treatments in which macronutrients were at half of their original concentration may be related to nitrogen concentration in the MS medium and to the metabolic function of this element, because high concentrations of ammonium and nitrate may inhibit plantlet germination and growth, as observed for the orchid Vanilla planifolia Andrews (Pedroso-de-Moraes et al. 2012).
Morphological parameters showed the influence of macronutrient and sucrose concentrations on the growth and development of Anathallis adenochila plantlets. Height of the aerial part and longest root length were highest for the treatments with 50% of the original macronutrient concentration and 30 or 60 g L-1 of sucrose. The highest number of leaves per plantlet was obtained with the addition of 60 g L-1 of sucrose to the MS medium, at 100% and 50% of macronutrient concentration. However, the number of roots per plantlet was highest for the treatment with 60 g L-1 of sucrose and 50% of macronutrient salts (Tab. 1 and Fig. 2).
When using the original macronutrient formulation, plantlet fresh mass was significantly higher in 60 g L-1 of sucrose than in the lower concentrations tested. In the treatments with 50% of the original macronutrient concentration, higher fresh masses were observed in the presence of 30 and 60 g L-1 of sucrose (Tab. 1). It should be noted that, in the media with 60 g L-1 of sucrose, plantlet growth was visually remarkable (Fig. 2), and that the increase in the number of leaves and roots was reflected in the increase in fresh mass, which, in the media with 50% of the original macronutrient concentration, was three and ten times as high as fresh mass in the media with 30 and 10 g L-1 of sucrose, respectively (Tab. 1).
In general, the exogenous carbohydrate supply is essential for in vitro plant growth and development, because it provides carbons that will be used in respiration and that are precursors for the synthesis of structural and functional compounds (Caldas et al. 1998; Thorpe et al. 2008). Sucrose concentrations of 20-30 g L-1 are commonly used in in vitro culture studies of Orchidaceae (Arditti 1974). Although sucrose concentrations > 50 g L-1 could be considered harmful to the development of plantlets of some species, due to the excessive osmotic potential of the medium (Arditti & Ernst 1992; Paiva-Neto & Otoni 2003) and to the inhibition of the photosynthetic process (Yamada & Sato 1978; Capellades et al. 1991; Hdider & Desjardins 1994), there have been reports that the addition of 60 g L-1 of sucrose to the medium positively influences the development of epiphytic orchids. That has been observed in Oncidium varicosum Lindl., which showed significantly higher values for growth of the aerial part, number of roots, longest root length, and fresh mass in MS medium containing 50% of the original macronutrient concentration and 60 g L-1 of sucrose than in the same medium with sucrose concentrations of 0, 10, 20, 30 or 90 g L-1 (Rego-Oliveira et al. 2003). Faria et al. (2004) reported that Dendrobium nobile Lindl. plantlets developed in an MS medium with 50% of the original macronutrient formulation and 60 g L-1 of sucrose exhibit greater vertical growth, as well as high multiplication rate and fresh mass accumulation, when compared with plantlets treated with lower sucrose concentrations (0-30 g L-1). The authors found that the different sucrose concentrations did not influence the formation of plantlet roots, except when there was no sucrose in the medium, a situation in which there was no root growth.
The increase in plantlet growth and development in the presence of high sucrose concentrations does not represent a standard behavior for Orchidaceae. Positive effects of relatively low concentrations of sucrose have also been reported for epiphytic orchids such as Caularthron bicornutum Raf., which showed a higher number of roots and greater height of the aerial part in MS media containing 10-30 g L-1 of sucrose (Pivetta et al. 2010). In Oncidium baueri Lindl., treatment at 50% of the macronutrient concentration with 40 g L-1 of sucrose has been shown to result in higher values for height of the aerial part, fresh mass and root length, in addition to benefiting root formation, when compared with treatments with 30 or 60 g L-1 of sucrose (Sorace et al. 2008). Cattleya loddigesii Lindl. plantlets showed greater height of the aerial part and fresh mass in 16-30 g L-1 of sucrose when associated with the growth regulator gibberellic acid. However, for the formation of roots, 60 g L-1 of sucrose has been shown to be the most beneficial concentration (Rezende et al. 2009). Similar concentrations of sucrose (20-30 g L-1) have been found to increase growth in Cattleya violacea (Kunth) Rolfe (Galdiano Júnior et al. 2013). Hybrid plantlets of Cattleya labiata Lindl. x Laelia itambana Pabst and Cattleya warneri T. Moore x Laelia purpurata Lindl. & Paxton have been found to develop better in 20 g L-1 of sucrose than in other concentrations (Fráguas et al. 2003; Moreira et al. 2007).
The reduction in the concentration of macronutrient salts of the original formulation of the MS medium has a beneficial effect on several parameters of orchid development, as observed for Cattleya cinnabarina (Bateman ex Lindl.) Van der Berg (Stancato & Faria 1996), Phalaenopsis Blume (Griesbach 2002), Catasetum fimbriatum (E. Morren) Lindl. & Paxton (Rego-Oliveira & Faria 2005) and Oncidium baueri (Sorace et al. 2008). Pedroso-de-Moraes et al. (2012) demonstrated that the development of Vanilla planifolia is influenced by the availability of nitrogen salts. The authors reported higher germination percentages and velocity, as well as greater plantlet growth, at 25% of the original concentrations of ammonium nitrate and potassium nitrate in MS medium. Nitrogen is a constituent of molecules such as amino acids, enzymes and proteins, its bioavailability and absorption by tissues therefore being related to plant metabolism. High salt concentrations tend to inhibit rooting phases, especially the stage of root growth. Therefore, dilutions of macronutrient formulations have enabled more efficient root formation (Grattapaglia & Machado 1998), as found for the orchids Anathallis adenochila (in the present study), Cattleya nobilior Rchb.f. (Araújo et al. 2005), and Vanilla planifolia (Pedroso-de-Moraes et al. 2012), as well as for the bromeliads Pitcairnia flammea Lindl. and Vriesea philippocoburgii Wawra (Mercier & Kerbauy 1991).
Ex vitro plantlet acclimatization is an important stage, and its success depends on, among other factors, the physiological conditions of the plants. Because in vitro carbohydrate availability is a chemical condition very different from that found during acclimatization (Faria et al. 2004), plantlets that receive great amounts of sucrose as a source of energy for their metabolic activities may show reduced photosynthesis during acclimatization (Rolland et al. 2002) and often undergo necrosis. In the present study, plantlets were acclimatized and, at this writing, had been developing, exhibiting normal phenotypes, for more than 12 months. Plantlets grown on MS media with 50% of the macronutrient salts and containing 30 or 60 g L-1 of sucrose flourished even before acclimatization, in the in vitro culture phase. This first report of in vitro propagation of Anathallis adenochila may contribute to future studies on physiological and ecological aspects of the life cycle of this epiphytic species that is endemic to Brazil.
Acknowledgments
This study received financial support from Feevale University, which also provided the necessary infrastructure, the Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS, Foundation for the Support of Research in the State of Rio Grande do Sul; PROBIC, Institutional Scientific Initiation Grant Program research grant to DEJ) and the Brazilian Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, National Council for Scientific and Technological Development; PIBIC, Institutional Scientific Initiation Grant Program; research grant to MHS).
Received: May 2, 2013
Accepted: November 26, 2013
- Araújo, A.G.; Pasqual, M.; Rodrigues, V.A.; Silva, A.B. & Soares, G.A. 2005. Concentração de KNO3 e NH4NO3 no crescimento in vitro de plântulas de orquídea. Plant Cell Culture & Micropropagation 1 (1): 31-36.
- Araújo, A.G.; Pasqual, M.; Castro, E.M.; Rodrigues, F.A.; Santos, D.N. & Dutra, L. F. 2007. Efeito da concentração de sacarose e qualidade de luz na propagação in vitro de plântulas de orquídea. Plant Cell Culture & Micropropagation 3 (2): 96-102.
- Arditti, J. 1967. Factors affecting the germination of orchid seeds. Botanical Review 33: 1-97.
- Arditti, J. 1974. Orchid Biology. Reviews and perspectives. Ithaca, Cornell University Press.
- Arditti, J. & Ernest, R. 1992. Micropropagation of Orchids 1 ed. Irvine, John Wiley & Sons.
- Barros, F.; Rodrigues, V.T. & Batista, J.A.N. 2009. Orchidaceae. In: Stehmann, J.R.; Forzza, R.C.; Salino, A.; Sobral, M.; Costa, D.P. & Kamino, L.H.Y. (eds.). Plantas da Floresta Atlântica Rio de Janeiro, Instituto de Pesquisa Jardim Botânico do Rio de Janeiro.
- Barros, F.; Vinhos, F.; Rodrigues, V.T.; Barbarena, F.F.V.A.; Fraga, C.N.; Pessoa, E.M. 2013. Orchidaceae in Lista de Espécies da Flora do Brasil Jardim Botânico do Rio de Janeiro. Disponível em: http://floradobrasil.jbrj.gov.br/2012/FB011075 (Accessed in 21/03/2013).
- Brustulin, J. & Schmitt, J.L. 2008. Composição florística, distribuição vertical e floração de orquídeas epifíticas em três parques municipais do estado do Rio Grande do Sul, Brasil. Pesquisas, Botânica 59: 143-158.
- Buzzato, C.R.; Freitas, E.M.; Silva, A.P.M. & Lima, L.F.P. 2007. Levantamento florístico das Orchidaceae ocorrentes na Fazenda São Maximiliano, Município de Guaíba, Rio Grande do Sul. Revista Brasileira de Biociências 5 (2-3): 19-25.
- Caldas, L.S.; Haridasan, P.; Ferreira, M.E. 1998. Meios nutritivos, Pp. 87-132. In: Torres, A.C.; Caldas, L.S. & Buso, J.A. (eds.) 1998. Cultura de tecidos e transformação genética de plantas 2 ed. Brasília, Embrapa.
- Capellades, M.; Lemeur, R. & Debergh, P. 1991. Effects of sucrose on starch accumulation and rate of photosynthesis in Rosa cultured in vitro Plant Cell, Tissue and Organ Culture 25 (1): 21-26.
- CITES - Convention on International Trade in Endangered Species of Wild Fauna and Flora. 2013. Convention on International Trade in Endangered Species of Wild Fauna and Flora - Appendix II Disponível em: http://www.cites.org./eng/disc/E-Text.pdf (Accessed in 08/04/2013).
- Faria, R.T.; Santiago, D.C.; Saridakis, D.P.; Albino, U.B. & Araujo, R. 2002. Preservation of the Brazilian orchid Cattleya walkeriana Gardner using in vitro propagation. Crop Breeding and Applied Biotechnology 2 (3): 489-492.
- Faria, R.T.; Rodrigues, F.N.; Oliveira, L.V.R. & Muller, C. 2004. In vitro Dendrobium nobile growth and rooting in different sucrose concentrations. Horticultura Brasileira 22 (4): 780-783.
- Faria, R.T.; Dalio, R.J.D.; Unemoto, L.K. & Silva, G.L. 2006. Propagação in vitro de Oncidium baueri Lindl. (Orchidaceae) sem uso de ágar. Acta Scientiarum Agronomy 28 (1): 71-74.
- Fráguas C.B.; Villa, F.; Souza, A.V.; Pasqual, M. & Dutra, L.F. 2003. In vitro growth of orchid seedlings obtained from hybridization between Cattleya labiata and Laelia itambana Revista Ceres 50: 719-726.
- Fundação SOS Mata Atlântica & INPE - Instituto Nacional de Pesquisas Espaciais. 2011. Atlas dos remanescentes florestais da Mata Atlântica, Período 2008-2010 São Paulo, Fundação SOS Mata Atlântica & São José dos Campos, INPE.
- Galdiano Júnior, R.F.; Mantovani, C.; Cassano, A.O. & Lemos, E.G.M. 2013. Desenvolvimento inicial e crescimento in vitro de Cattleya violacea (Kunth) Rolfe em diferentes concentrações de sacarose. Acta Amazonica 43(2): 127-134.
- Grattapaglia, D. & Machado, M.A. 1998. Micropropagação. Pp. 183-260. In: Torres, A.C.; Caldas, L.S. & Buso, J.A. (eds.) 1998. Cultura de tecidos e transformação genética de plantas 2 ed. Brasília, Embrapa.
- Griesbach, R.J. 2002. Development of Phalaenopsis orchids for the mass market. In: Jainick, J. & Whipkey, A. (eds.) 2002. Trends in New Crops and New Uses Alexandria, ASHS Press.
- Hdider, C. & Desjardins, Y. 1994. Effects of sucrose on photosynthesis and phosphoenolpyruvate carboxylase activity of in vitro cultured strawberry plantlets. Plant Cell, Tissue and Organ Culture 36 (1): 27-33.
- IUCN - International Union for Conservation of Nature. 2013. IUCN Red List of Threatened species Disponível em: http://www.redlist.org/ (Accessed in 08/04/2013).
- Kersten, R.A. 2010. Epífitas vasculares - Histórico, participação taxonômica e aspectos relevantes, com ênfase na Mata Atlântica. Hoehnea 37 (1): 9-38.
- Lugo, A.E. & Scatena, F.N. 1992. Epiphytes and climate change research in the Caribbean: a proposal. Selbyana13: 123-130.
- Mercier, H. & Kerbauy, G.B. 1991. Effects of nitrogen source on growth rates and levels of endogenous cytokinins and chlorophyll in protocorms of Epidendrum fulgens Journal of Plant Physiology 138: 195-199.
- Mitra, G.C. 1971. Studies on seeds, shoot tips and stem disc of an orchid grown in aseptic culture. Indian Journal of Experimental Biology 9: 79-85.
- Moreira, B.M.T; Tomba, E.C. & Zonetti P.C. 2007. Crescimento in vitro de plântulas de orquídea (Laelia purpurata Lindl var venosa x Cattleya warneri T. Moore alba) sob diferentes concentrações de sacarose e frutose. SaBios - Revista de Saúde e Biologia 2: 16-21.
- Moraes, L.M.; Cavalcanti, L.C.D. & Faria, R.T. 2002. Substratos para aclimatação de plântulas de Dendrobium nobile Lindl. (Orchidaceae) propagadas in vitro Acta Scientiarum Agronomy 25 (5): 1397-1400.
- Moraes, C.P.; Domingues, E.; Prezzi, L.E.; Leal, T.S.; Zambon, R.I.; Brescansin, R.L.; Ramos, P.A.B. 2010. Florística e fitossociologia da família Orchidaceae no Centro de Educação Ambiental "Francisco Mendes", município de Mogi Guaçu, SP, Brasil. Scientia Plena 6 (1): 1-5
- Muller, T. S.; Dewes, D.; Karsten, J.; Schuelter, A. R. & Stefanello, S. 2007. Crescimento in vitro e aclimatação de plântulas de Miltonia flavescens Ciências Agrárias 29 (4): 775-782.
- Murashige, T. & Skoog, F. 1962. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiologia Plantarum 15: 473-497.
- Myers, N., Mittermeier, R.A., Mittermeier, C.G., Fonseca, G.A.B. & Kent, J. 2000. Biodiversity Hotspots for conservation priorities. Nature 403:853-858.
- Paiva-Neto, V.B. & Otoni, W.C. 2003. Carbon sources and their osmotic potential in plant tissue culture: does it matter? Scientia Horticulturae 97: 193-202
- Pedroso-de-Moraes, C.; Diogo, J.A.; Pedro, N.P.; Canabrava, R.I.; Martini, G.A. & Marteline, M.A. 2009. Desenvolvimento in vitro de Cattleya loddigesii Lindl. (Orchidaceae) utilizando os fertilizantes comerciais. Revista Brasileira de Biociências 7: 67-69.
- Pedroso-de-Moraes, C.; Souza-Leal, T.; Panosso, A. R. & Souza, M. C. 2012. Efeitos da escarificação química e da concentração de nitrogênio sobre a germinação e o desenvolvimento in vitro de Vanilla planifolia Jack ex Andr. (Orchidaceae: Vanilloideae). Acta Botanica Brasilica 26 (3): 714-719.
- Pinto, J.R.S.; Freitas, R.M.O. & Praxedes, S.C. 2010. Stimulation of in vitro development of Cattleya granulosa by sucrose. General and Applied Plant Physiology 36 (3-4): 183-188.
- Pivetta, K.F.L.; Martins, T.A.; Galdiano Júnior, R.F.; Gimenes, R.; Faria, R.T. & Takane, R.J. 2010. Crescimento in vitro de plântulas de Caularthron bicornutum em diferentes concentrações de sacarose. Ciência Rural 40 (9): 1897-1902.
- Rego-Oliveira, L.V.; Faria, R.T.; Fonseca, I.C.B. & Saconato, C. 2003. Influência da fonte e concentração de carboidrato no crescimento vegetativo e enraizamento in vitro de Oncidium varicosum Lindl. (Orchidaceae). Ciências Agrárias 24 (2): 265-272.
- Rego-Oliveira, L.V. & Faria, R.T. 2005. In vitro propagation of Brazilian orchids using traditional culture media and commercial fertilizers formulations. Acta Scientiarum Agronomy 27: 1-5.
- Rezende, J.C.; Ferreira, E.A.; Pasqual, M.; Villa, F. & Santos, F.C. 2009. Desenvolvimento in vitro de Cattleya loddigesii sp: adição de reguladores de crescimento e sacarose. Agrarian 2 (3): 99-114.
- Rocha, C.F.D., Cogliatti-Carvalho, L., Nunes-Freitas, A.F., Rocha-Pessoa, T.C., Dias, A.S., Ariani, C.V., Morgado, L.N. 2004. Conservando uma larga proporção da diversidade biológica através da conservação de Bromeliaceae. Vidalia 2: 52-68.
- Rolland, F.; Moore, B. & Sheen, J. 2002. Sugar sensing and signaling in plants. Plant Cell 14: 185-205.
- Skrebsky, E.C.; Nicoloso, F.T. & Ferrão, G. da E. 2004. Sacarose e período de cultivo in vitro na aclimatização ex vitro de ginseng brasileiro (Pfaffia glomerata Spreng. Pedersen). Ciência Rural 34 (5): 1471-1477.
- Soares, J.D.R.; Araújo, A.G.; Pasqual, M.; Rodrigues, F.A. & Assis, F.A.. 2009. Concentrações de sais do meio Knudson C e de ácido giberélico no crescimento in vitro de plântulas de orquídea. Ciência Rural 39 (3): 772-777.
- Sorace, M.; Faria, R.T.; Damasceno Júnior, C.V.; Gomes, G.P.; Barbosa, C.M.; Vieira, F.G.N.; Silva, G.L.; Takahashi, L.S.A. & Schnitzer, J.A. 2008. Crescimento in vitro de Oncidium baueri (Orchidaceae) em diferentes concentrações de macronutrientes e sacarose. Ciências Agrárias 29 (4): 775-782.
- Stancato, G.C. & Faria, R.T. 1996. In vitro growth and mineral nutrition of the lithophytic orchid Laelia cinnabarina Batem (Orchidaceae): effects of macro and microelements. Lindleyana 11 (1): 41-43.
- Thorpe, T.; Stasolla C.; Yeung, E.C.; Klerk, G.J.; Roberts, A. & George, E.F. 2008. The components of Plant Tissue Culture Media II: Organic Additions, Osmotic and pH Effects, and Support Systems. In: George, E.F.; Hall, M.A. & Klerk, G.J. Plant Propagation by Tissue Culture 3 ed. Dordrecht, Springer.
- Tremblay, R.L.; Ackerman, J.D.; Zimmerman, J.K. & Calvo, R.N. 2005. Variation in sexual reproduction in orchids and its evolutionary consequences: a spasmodic journey to diversification. Biological Journal of the Linnean Society 84: 1-54.
- Unemoto, L.K.; Faria, R.T.; Vieira, A.O.S. & Dalio, R.J.D. 2007. Propagação in vitro de orquídeas brasileiras em meio de cultura simplificado. Revista Brasileira de Agrociência 13 (2): 267-269.
- Yamada, Y. & Sato, F. 1978. The photoautotrophic culture of chlorophyllous cell. Plant and Cell Physiology 19 (4): 691-699.
Publication Dates
-
Publication in this collection
08 Jan 2015 -
Date of issue
Dec 2014
History
-
Received
02 May 2013 -
Accepted
26 Nov 2013