ABSTRACT
Collema leptosporum was originally included in Collemataceae as part of the Collema fasciculare group, an informal group that also included C. fasciculare, C. papuanorum, and C. uviforme. However, molecular data from C. fasciculare showed that this species belongs to Arctomiaceae, and all species in this informal group were relocated to Arctomia, although no molecular data were generated and analyzed for C. leptosporum, C. papuanorum and C. uviforme. To investigate the phylogenetic relationships of Collema leptosporum, currently Arctomia leptospora, we analyzed three DNA loci and examined morphological and anatomical features of specimens collected near the type locality. Genetic data suggest that this species is not included in Arctomiaceae and should be treated as a new genus in Collemataceae. Hondaria gen. nov. is characterized by having the longest transversely-septate ascospores in the family ((100-)120-175(-200) × 2-4(-5) µm). This study also suggests that the structures characterizing the C. fasciculare group are a result of convergent evolution, since this group includes species from different distantly related species.
Keywords:
Arctomia leptospora; Collema leptosporum; C. fasciculare group; jelly lichens; South America biodiversity
Introduction
The landscape of the west-central region of Brazil, especially near the border with Bolivia and Paraguay, is composed of a mosaic of vegetation formations that include the Pantanal wetlands, the Brazilian savanna (Cerrado) and the Chaco (Pott & Pott 1994Pott A, Pott VJ. 1994. Plantas do Pantanal. Corumbá, EMBRAPA/CPAP.). The first paper on the diversity of lichenized fungi describe the species from this region based on an analysis of material obtained during the First Regnellian Expedition 1892-1894 (Malme 1897Malme GOA. 1897. Die Flechten der ersten Regnellschen Expedition. I. Einleitung. Die Gattung Pyxine (Fr.) Nyl. Bihang till Kongl. Svenska Vetenskaps-Akademiens Handlingar 23 III: 1-52.). More than 20 new species were described by the Swedish botanist Gustaf Malme (Spielmann & Canêz 2012Spielmann AA, Cânez LS. 2012. Breve histórico sobre a taxonomia de liquens no Estado de Mato Grosso do Sul, Brasil. Glalia 4: 53-60.), including the jelly lichen Collema leptosporum (Collemataceae, Peltigerales; Malme 1924). This species was originally reported from Corumbá then subsequently from the Campo Grande municipality in Brazil (Oliva et al. 1992Oliva MLV, Mendes CR, Bueno NR, Honda NK, Sampaio MU, Sampaio CAM. 1992. Cysteine proteinase inhibitors in lichen (Collema leptosporum Malme). Brazilian Journal of Medical and Biological Research 25: 999-1002.; Prado et al. 1999Prado SRT, Gorin PAJ, Stuelp PM, Honda NK, Iacomini M. 1999. An unusual juxtaposition of polysaccharide components of Collema leptosporum. Carbohydrate Polymers 40: 271-276. ), and from the Chaco region in Paraguay (Degelius 1974Degelius G. 1974. The lichen genus Collema with special reference to the extra-European species. Symbolae Botanicae Upsaliensis 20: 1-215.).
The ascospores of Collema leptosporum are acicular and transversely septate, like C. fasciculare, C. papuanorum, and C. uviforme. So, these species were included in an informal subgeneric group, the C. fasciculare group, due to the combination of a “crustose” thallus with corticicolous habit and the very long ascospores (Degelius 1974Degelius G. 1974. The lichen genus Collema with special reference to the extra-European species. Symbolae Botanicae Upsaliensis 20: 1-215.). Later, all four species were excluded from the Collemataceae (Peltigerales) and transferred to Arctomia (Arctomiaceae, Arctomiales), due to the phylogenetic placement of C. fasciculare that was revealed by DNA sequences (Otálora & Wedin 2013Otálora MAG, Wedin M. 2013. Collema fasciculare belongs in Arctomiaceae. The Lichenologist 5: 295-304. ). No molecular data were generated and analysed for C. leptosporum, C. papuanorum and C. uviforme. Ultimately, considering molecular data and apothecium structure (including ontogeny), Jørgensen (2014Jørgensen PM. 2014. Taxonomy and nomenclature of Collema fasciculare (L.) G. H. Weber. The Lichenologist 46: 594. doi:10.1017/S0024282914000140
https://doi.org/doi:10.1017/S00242829140...
) suggested that C. fasciculare should not be included in Arctomia, but rather in Gabura, another genus of Arctomiaceae. This was confirmed by Magain et al. (2020Magain N, Sprobille T, DiMeglio J, Nelson PR, Miadlikowska J, Sérusiaux E. 2020. Phylogenetic evidence for an expanded circumscription of Gabura (Arctomiaceae). The Lichenologist 52: 3-15. ). The other three species of the C. fasciculare group remained allocated to Arctomia, including Arctomia leptospora.
This present research aimed at investigating the phylogenetic affinities of Arctomia leptospora, in combination with a reanalysis of its morphological features.
Materials and methods
We analyzed 15 specimens of Arctomia leptospora: three new specimens collected in the field, and 12 borrowed from the Universidade Federal de Mato Grosso do Sul herbarium (CGMS), Campo Grande, Brazil. The 15 specimens were all collected in Mato Grosso do Sul state in the west-central region of Brazil. We used only freshly collected material for molecular analyses (Tab. 1).
Information about DNA sequences used in this study. New sequences are indicated in bold. Herb. = Herbarium’s acronym.
The specimens were analyzed with an Olympus SZX7 stereomicroscope and an Olympus CX22LED microscope, and images were captured with a Canon EOS Rebel T3i digital camera.
Preliminary identification was based on descriptions from the literature (Malme 1924Malme GOA. 1924. Die Collematazeen des Regnellschen Herbars. Arkiv för Botanik 19: 1-29.; Degelius 1974Degelius G. 1974. The lichen genus Collema with special reference to the extra-European species. Symbolae Botanicae Upsaliensis 20: 1-215.). To characterize the genus, we used the characters utilized by Otálora et al. (2014Otálora MAG, Jørgensen PM, Wedin M. 2014. A revised generic classification of the jelly lichens, Collemataceae. Fungal Diversity 64: 275-293. ) in the descriptions of genera of Collemataceae. The nomenclature of the pseudo-tissues observed in apothecia was adopted by Degelius (1954Degelius G. 1954. The lichen genus Collema in Europa. Acta Universitatis Upsaliensis, Symbolae Botanicae Upsalienses 13: 1-499., 1974Degelius G. 1974. The lichen genus Collema with special reference to the extra-European species. Symbolae Botanicae Upsaliensis 20: 1-215.) and Kitaura & Marcelli (2013Kitaura MJ, Marcelli MP. 2013. A revision of Leptogium species with spherical-celled hairs (section Mallotium p.p.). The Bryologist 116: 15-27. ).
DNA extraction was performed with small fragments of the thalli following the methods used by Kitaura et al. (2018Kitaura MJ, Scur MC, Spielmann AA, Lorenz-Lemke AL. 2018. A revision of Leptogium (Collemataceae, lichenized Ascomycota) from Antarctica with a key to species. The Lichenologist 50: 467-485. ). We used the primers ITS1F (Gardes & Bruns 1993Gardes M, Bruns TD. 1993. ITS primers with enhanced specificity for basidiomycetes - application to the identification of mycorrhizae and rusts. Molecular Ecology 2: 113-118. ) and ITS4 (White et al. 1990White T, Bruns T, Lee S, Taylor J. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ. (eds.) PCR Protocols: A Guide to Methods and Applications. New York, Academic Press Inc. p. 315-322.) to amplify the nuITS region, the mrSSU1 and mrSSU3R primers (Zoller et al. 1999Zoller S, Scheidegger C, Sperisen C. 1999. PCR primers for the amplification of mitochondrial small subunit ribosomal DNA of lichen-forming Ascomycetes. The Lichenologist 31: 511-516. ) for the mrSSU region, and the MCM7-709 and MCM7-1348 primers (Schmitt et al. 2009Schmitt I, Crespo A, Divakar PK, et al. 2009. New primers for promising single-copy genes in fungal phylogenetics and systematics. Persoonia 23: 35-40. ) for the MCM7 region. The 25 μL PCR reactions contained 1× PCR Buffer (Promega), 0.2 μM of each primer, 0.2 μM of dNTPs, 2 μM of MgCl2, 1 unit of DNA polymerase (Promega) and 5-20 ng of genomic DNA. PCR reactions were carried out in the Veriti Thermal Cycler (Applied Biosystems) following the conditions described by Kitaura et al. (2018)Kitaura MJ, Scur MC, Spielmann AA, Lorenz-Lemke AL. 2018. A revision of Leptogium (Collemataceae, lichenized Ascomycota) from Antarctica with a key to species. The Lichenologist 50: 467-485. for the mrSSU and nuITS regions and by Otálora & Wedin (2013Otálora MAG, Wedin M. 2013. Collema fasciculare belongs in Arctomiaceae. The Lichenologist 5: 295-304. ) for MCM7. Sequencing was done by Macrogen Inc. (South Korea) and the sequences obtained were deposited in GenBank after assembly (Tab. 1).
Exploratory analyses using the megablast tool (Altschul et al. 1990Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. 1990. Basic local alignment search tool. Journal of Molecular Biology 215: 403-410. ) were performed to compare the new sequences with the reference sequences in GenBank. The blast results revealed that all sequences generated in the present study were closest to the Collemataceae species. Therefore, the sequences used in the Collemataceae phylogeny studies were selected for this study for the mrSSU and MCM7 regions (Wedin et al. 2009Wedin M, Wiklund E, Jørgensen PM, Ekman S. 2009. Slippery when wet: phylogeny and character evolution in the gelatinous cyanobacterial lichens (Peltigerales, Ascomycetes). Molecular Phylogenetics and Evolution 53: 862-871. ; Otálora et al. 2010Otálora MAG, Aragón G, Molina MC, Martínez I, Lutzoni F. 2010a. Disentangling the Collema/Leptogium complex through a molecular phylogenetic study of the Collemataceae (Peltigerales, lichen-forming Ascomycota). Mycologia 102: 279-290. a; Otálora et al. 2013Otálora MAG, Aragón G, Martínez I, Wedin M. 2013. Cardinal characters on a slippery slope - A re-evaluation of phylogeny, character evolution, and evolutionary rates in the jelly lichens (Collemataceae s. str). Molecular and Phylogenetic Evolution 68: 185-198. ; Bjelland et al. 2017Bjelland T, Bendiksby M, Frisch A. 2017. Geographically disjunct phylogenetic lineages in Leptogium hibernicum reveal Leptogium krogiae sp. nov. from East Africa. The Lichenologist 49: 239-251. ; Kitaura et al. 2018Kitaura MJ, Scur MC, Spielmann AA, Lorenz-Lemke AL. 2018. A revision of Leptogium (Collemataceae, lichenized Ascomycota) from Antarctica with a key to species. The Lichenologist 50: 467-485. ; Košuthová et al. 2019Košuthová A, Westberg M, Otálora MAG, Wedin M. 2019. Rostania revised: testing generic delimitations in Collemataceae (Peltigerales, Lecanoromycetes). MycoKeys 47: 17-33. ), and the available related nuITS sequences (Otálora et al. 2008Otálora MAG, Martínez I, Molina MC, Aragón G, Lutzoni F. 2008. Phylogenetic relationships and taxonomy of the Leptogium lichenoides group (Collemataceae, Ascomytoca) in Europe. Taxon 57: 907-921.;Otálora et al. 2010bOtálora MAG, Martínez I, Aragón G, Molina MC. 2010b. Phylogeography and divergence date estimates of a lichen species complex with disjunct distribution pattern. American Journal of Botany 97: 216-223.; Jayalal et al. 2014Jayalal U, Jang SH, Yu NH, Oh SO, HUR JS. 2014. Notes on the lichen genus Leptogium (Collemataceae, Ascomycote) ih South Korea. Mycobiology 42: 120-131.; Magain & Sérusiaux 2014Magain N, Sérusiaux E. 2014. Do photobiont switch and Cephalodia emancipation act as evolutionary drivers in the lichen symbiosis. A case study in the Pannariaceae (Peltigerales). PLOS ONE 9: e89876. doi: 10.1371/journal.pone.0089876
https://doi.org/10.1371/journal.pone.008...
; Kitaura et al. 2018Kitaura MJ, Scur MC, Spielmann AA, Lorenz-Lemke AL. 2018. A revision of Leptogium (Collemataceae, lichenized Ascomycota) from Antarctica with a key to species. The Lichenologist 50: 467-485. ; Marthinsen et al. 2019Marthinsen G, Rui S, Timdal E. 2019. OLICH: A reference library of DNA barcodes for Nordic lichens. Biodiversity Data Journal 7: e36252. doi: 10.3897/BDJ.7.e36252
https://doi.org/10.3897/BDJ.7.e36252...
). Due to the C. fasciculare group´s circumscription history, the sequences of Gabura borbonica and Gabura fascicularis (Arctomiaceae) were also added to the study dataset (Otálora & Wedin 2013Otálora MAG, Wedin M. 2013. Collema fasciculare belongs in Arctomiaceae. The Lichenologist 5: 295-304. ; Magain et al. 2020).
The sequences were aligned separately for each marker using the MAFFT v7.308 (Katoh et al. 2002Katoh K, Misawa K, Kuma K, Miyata T. 2002. Mafft: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Research 30: 3059-3066. ) plugin in Geneious v9.1.2 (Kearse et al. 2012Kearse M, Moir R, Wilson A, et al. 2012. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28: 1647-1649. ) with the auto option. After manual adjustments, the Gblocks webserver was used for the mrSSU alignment to exclude unreliably aligned sites, employing all less stringent options (http://molevol.cmima.csic.es/castresana/Gblocks_server.html).
Substitution models were defined for each region according to jModelTest2 (Guindon & Gascuel 2003Guindon S, Gascuel O. 2003. A simple, fast and accurate method to estimate large phylogenies by maximum-likelihood. Systematic Biology 52: 696-704. ; Darriba et al. 2012Darriba D, Taboada GL, Doallo R, Posada D. 2012. jModelTest 2: more models, new heuristics and parallel computing. Nature Methods 9: 772. doi: 10.1038/nmeth.2109
https://doi.org/doi: 10.1038/nmeth.2109...
), selecting the Akaike information criteria (AIC) that suggested TPM3fu+I+G (mrSSU) and GTR+I+G (MCM7 and nuITS) as the best fitting models. Phylogenetic trees were estimated using Bayesian (BA) and Maximum Likelihood (ML) approaches with the nuITS, mrSSU and MCM7 regions and mrSSU and MCM7 concatenated. The analyses were performed in the CIPRES Science Gateway portal (Miller et al. 2010Miller MA, Pfeiffer W, Schwartz T. 2010. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. http:// www.phylo.org/. 03 Mar. 2020.
http:// www.phylo.org/...
). The BA tree was estimated using the Metropolis-coupled Bayesian Markov chain Monte Carlo algorithm implemented in MrBayes 3.2.2 (Ronquist et al. 2012Ronquist F, Teslenko M, Mark P, et al. 2012. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61: 539-542. ). Two runs of 10 million generations employing four simultaneous chains were executed. Trees were saved every 10,000 generations. The first 25 % of the generated trees were discarded as burn-in, and convergence of the chains was assessed using Tracer v1.7.1 (Rambaut et al. 2018Rambaut A, Drummond AJ, Xie D, Baele G, Suchard MA. 2018. Posterior summarisation in Bayesian phylogenetics using Tracer 1.7. Systematic Biology 1-3. doi:10.1093/sysbio/syy032.
https://doi.org/doi:10.1093/sysbio/syy03...
). The ML tree was built in RaxML 8 (Stamatakis 2014Stamatakis A. 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30: 1312-1313. ) and implemented in Geneious using the GTRGAMMA model, 1000 bootstrap pseudoreplicates, and the remaining parameters set as default. Staurolemma omphalarioides (Anzi) P.M. Jørg. & Henssen and Pannaria rubiginosa (Thunb.) Delise were used as outgroups, following Otálora et al. (2013Otálora MAG, Aragón G, Martínez I, Wedin M. 2013. Cardinal characters on a slippery slope - A re-evaluation of phylogeny, character evolution, and evolutionary rates in the jelly lichens (Collemataceae s. str). Molecular and Phylogenetic Evolution 68: 185-198. ) and Košuthová et al. (2019Košuthová A, Westberg M, Otálora MAG, Wedin M. 2019. Rostania revised: testing generic delimitations in Collemataceae (Peltigerales, Lecanoromycetes). MycoKeys 47: 17-33. ). The program FigTree v1.4.4 (http://tree.bio.ed.ac.uk/software/figtree/) was used to edit the trees.
Results
Phylogenetic analyses
We generated DNA sequences of the three freshly-collected specimens of Arctomia leptospora for the nuITS, mrSSU and MCM7 regions (Tab. 1).
For the phylogenetic analyses, the alignments of the mrSSU region (731 base pairs) and MCM7 (565 base pairs) were analyzed separately and concatenated, resulting in a final dataset with a total of 35 species of Collemataceae, and 3 species of Arctomiaceae, covering all the primary clades of Collemataceae. The nuITS alignment (424 base pairs) was composed of 13 species of Collemataceae and 3 of Arctomiaceae (Tab. 1).
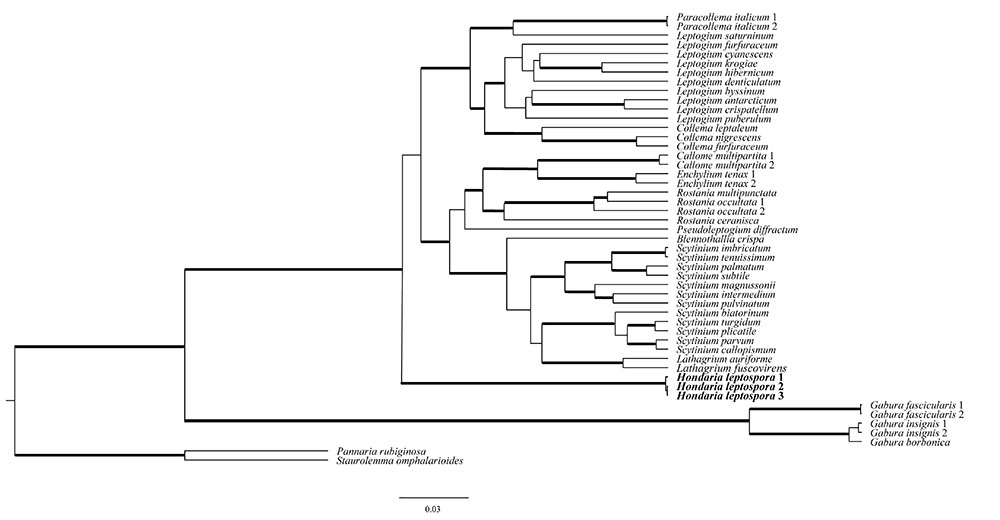
The trees of the concatenated dataset of the MCM7 and mrSSU regions (Fig. 1), nuITS (Fig. 2) and, mrSSU and MCM7 single region analyses (Supplementary material 1) showed that the Gabura species (Arctomiaceae) is a separate group; and Arctomia leptospora and the remaining Collemataceae form a well-supported group.
Phylogenetic relationships of Hondaria, other Collemataceae and Gabura (Arctomiaceae) based on Bayesian analysis of a 2-locus data set (mrSSU and MCM7). Thickened branches indicate support branches (posterior probabilities ≥ 0.90 and bootstrap ≥ 70 %).
Phylogenetic relationships of Hondaria; other Collemataceae and Gabura (Arctomiaceae) based on Bayesian analysis of the nuITS region. Thickened branches indicate support branches (posterior probabilities ≥ 0.90 and bootstrap ≥ 70 %).
Arctomia leptospora is not included in any of the known genera of Collemataceae; and, therefore, it is assigned here to the new genus Hondaria, with the single species Hondaria leptospora.
Hondaria M.J. Kitaura & A.P. Lorenz, gen. nov.
MycoBank number: MB 835521
Type species: Hondaria leptospora (Malme) M.J. Kitaura, M.C. Scur & A.P. Lorenz
Etymology - The generic name is a tribute to Dr. Neli Kika Honda, who has dedicated her scientific career to study the chemistry of the lichens from Mato Grosso do Sul since 1992 (Oliva et al. 1992Oliva MLV, Mendes CR, Bueno NR, Honda NK, Sampaio MU, Sampaio CAM. 1992. Cysteine proteinase inhibitors in lichen (Collema leptosporum Malme). Brazilian Journal of Medical and Biological Research 25: 999-1002.).
Thallus foliose, medium-sized (3-5 cm across), homoiomerous, black to dark olive brown when dry (Fig. 3A, B, C); lobes irregular in outline, irregularly branched, 1.5-3.0 mm wide, plane; lobe surface plane and with longitudinal ridges when dry, not swollen; cortex with amorphous layer without euparaplectenchymatous cells (Fig. 3D); isidia granular, laminal, marginal, and on the margin of apothecia, simple to grouped; tomentum not observed. Apothecia usually present, frequent, laminal, pedicellate, ornamented by isidia (Fig. 3B); disc plane to slightly concave, reddish brown; proper exciple euparaplectenchymatous (Degelius 1954Degelius G. 1954. The lichen genus Collema in Europa. Acta Universitatis Upsaliensis, Symbolae Botanicae Upsalienses 13: 1-499., Fig. 3C and E). Asci 150-170 × 12.5-17.5 µm. Ascospores (100-)120-175(-200) × 2-4(-5) µm, acicular, straight to curved, transversally 5-8-septate. Pycnidia not observed.
Hondaria leptospora. A. Specimen JBP 04. B. Detail of the ornamented apothecia with granular isidia on the margin. C. Diametral section of an apothecium. D. Detail of amorphous cortex. E. Detail of euparaplectenchymatous tissue. (hm = hymenium, ep = proper exciple, ct = cortex, hp = hyphae, arrow = cyanobacteria).
Hondaria leptospora (Malme) M.J. Kitaura, M.C. Scur & A.P. Lorenz, comb. nov.
MycoBank number: MB 835522
( Collema leptosporum Malme, Ark. Bot. 19(8): 6 1924. Type - Brazil, Mato Grosso do Sul state, Corumbá municipality, Malme s/n (LD, S, UPS - syntypes).
( Arctomia leptospora (Malme) Otálora & Wedin, Lichenologist 45(3): 302 2013.
Etymology - The epithet leptospora refers to thin ascospores of the species, 3-4(-5) µm thick.
Description - see description of the genus and more details in Malme (1924Malme GOA. 1924. Die Collematazeen des Regnellschen Herbars. Arkiv för Botanik 19: 1-29.) and Degelius (1974Degelius G. 1974. The lichen genus Collema with special reference to the extra-European species. Symbolae Botanicae Upsaliensis 20: 1-215.).
Known distribution - On cortex in west-central Brazil near the border region with Bolivia, and Paraguay.
Material examined - Brazil, Mato Grosso do Sul state, Aquidauana municipality, Vila Palmeiras, 17 Nov 1993, N.K. Honda & Devincenzi 061H, 095DH (CGMS); IDEM, Campo Grande municipality, campus of Universidade Federal de Mato Grosso do Sul (UFMS), 24 Jul 2017, J.B. Paula & M.J. Kitaura 02, 03, 04 (CGMS); IDEM, campus of UFMS, 19 Jan 2011, A.L. Simal & P.H.R. Medeiros 22 (CGMS 42162); IDEM, campus of UFMS, 29 Feb 1989, I. Riquelme 061 (CGMS); IDEM, Vila da Base Aérea, on cortex, 25 Feb 1989, I. Riquelme 094, 273, 288 (CGMS); IDEM, Jardim Itatiaia, 13 Mar 1991, I. Riquelme 282, 283 (CGMS); IDEM, Jaraguari municipality, furnas do Dionísio, 13 Nov 2015, C.M. Bernardo 813 (CGMS); IDEM, 20°08’54.3” S, 54°34’14.9” W 435 m. alt., 12 Sep 2015, C.M. Bernardo, A.A. Spielmann, M.J. Kitaura et al. 762 (CGMS); IDEM, 20°08’54.1” S, 54°34’15.1” W, 425 m alt., 22 Nov 2011, A.L. Simal, L.S. Canêz & A.A. Spielmann 92 (CGMS).
Notes - The species is characterized by the presence of granular isidia (Fig. 3A, B), a euparaplectenchymatous proper exciple (Fig. 3C and E), and transversely septate ascospores with (100-)120-175(-200) × 2-4(-5) µm (Degelius 1974Degelius G. 1974. The lichen genus Collema with special reference to the extra-European species. Symbolae Botanicae Upsaliensis 20: 1-215.).
Discussion
Our phylogenetic analysis showed that Arctomia leptospora is not a member of Arctomia, Gabura (Arctomiales) or Collema. Instead, the three sequenced specimens belong to a novel clade of the Collemataceae, and is here recognized as a new genus, Hondaria. Consequently, the previously recognized Collema fasciculare group is composed of species from two different families and orders of Ascomycota that show some level of phenotypic convergence. Such notable convergence has already been reported for Arctomiaceae vs. Collemataceae (Otálora & Wedin 2013Otálora MAG, Wedin M. 2013. Collema fasciculare belongs in Arctomiaceae. The Lichenologist 5: 295-304. ), and Arctomiaceae vs. Massalongiaceae (Ertz et al. 2017Ertz D, Poulsen RS, Charrier M, Søchting U. 2017. Taxonomy and phylogeny of the genus Steinera (Arctomiales, Arctomiaceae) in the subantarctic islands of Crozet and Kerguelen. Phytotaxa 324: 201-238. , Magain et al. 2020Magain N, Sprobille T, DiMeglio J, Nelson PR, Miadlikowska J, Sérusiaux E. 2020. Phylogenetic evidence for an expanded circumscription of Gabura (Arctomiaceae). The Lichenologist 52: 3-15. ).
In addition to phylogeny, our study revealed that certain anatomical characteristics can also be used to separate Hondaria leptospora from Arctomia and Gabura. The apothecia of Gabura fasciculare have a thin proper exciple composed by euthyplectenchymatous cells and a thin thalline exciple (Degelius 1954Degelius G. 1954. The lichen genus Collema in Europa. Acta Universitatis Upsaliensis, Symbolae Botanicae Upsalienses 13: 1-499.; Otálora & Wedin 2013Otálora MAG, Wedin M. 2013. Collema fasciculare belongs in Arctomiaceae. The Lichenologist 5: 295-304. ), whereas H. leptospora has a thick paraplectenchymatous proper exciple and a thin cortex of thalline exciple (Figs. 3C and E).
Anatomically, Arctomia papuanorum and A. uviforme are more similar to H. leptospora than to G. fasciculare since they also have a thick paraplectenchymatous proper exciple. These two species might therefore also belong in the genus Hondaria; however, formal combinations are not proposed here, since DNA sequences are not yet available for these taxa. Hondaria leptospora produces the longest and thinnest ascospores of the previously defined C. fasciculare group (Degelius 1974Degelius G. 1974. The lichen genus Collema with special reference to the extra-European species. Symbolae Botanicae Upsaliensis 20: 1-215.; Otálora & Wedin 2013Otálora MAG, Wedin M. 2013. Collema fasciculare belongs in Arctomiaceae. The Lichenologist 5: 295-304. ; see Tab. 2).
These results highlight that much remains to be investigated about lichenized fungi in South America and elsewhere. Furthermore, for jelly lichens it appears essential to integrate molecular, morphological, and anatomical data to accurately assess their phylogenetic relationships and classification.
Acknowledgements
We thank the Universidade Federal de Mato Grosso do Sul, especially the curator of the CGMS herbarium for the loan of specimens, and we also thank the reviewers of the manuscript. M.J. Kitaura is grateful to CAPES for the post-doctoral support, and M.C. Scur and A.C. Piovezan-Borges are grateful to CAPES and FUNDECT for PhD scholarships. This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001.
References
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. 1990. Basic local alignment search tool. Journal of Molecular Biology 215: 403-410.
- Bendiksby M, Mazzoni S, Jørgensen RH, Holien H. 2014. Combining genetic analyses of archived specimens with distribution modelling to explain the anomalous distribution of the rare lichen Staurolemma omphalarioides: long-distance dispersal or vicariance?. Journal of Biogeography 41: 2020-2031.
- Bjelland T, Bendiksby M, Frisch A. 2017. Geographically disjunct phylogenetic lineages in Leptogium hibernicum reveal Leptogium krogiae sp. nov. from East Africa. The Lichenologist 49: 239-251.
- Darriba D, Taboada GL, Doallo R, Posada D. 2012. jModelTest 2: more models, new heuristics and parallel computing. Nature Methods 9: 772. doi: 10.1038/nmeth.2109
» https://doi.org/doi: 10.1038/nmeth.2109 - Degelius G. 1954. The lichen genus Collema in Europa. Acta Universitatis Upsaliensis, Symbolae Botanicae Upsalienses 13: 1-499.
- Degelius G. 1974. The lichen genus Collema with special reference to the extra-European species. Symbolae Botanicae Upsaliensis 20: 1-215.
- Ekman S, Jørgensen PM. 2002. Towards a molecular phylogeny for the lichen family Pannariaceae (Lecanorales, Ascomycota). Canadian Journal of Botany 80: 625-634.
- Ertz D, Poulsen RS, Charrier M, Søchting U. 2017. Taxonomy and phylogeny of the genus Steinera (Arctomiales, Arctomiaceae) in the subantarctic islands of Crozet and Kerguelen. Phytotaxa 324: 201-238.
- Gardes M, Bruns TD. 1993. ITS primers with enhanced specificity for basidiomycetes - application to the identification of mycorrhizae and rusts. Molecular Ecology 2: 113-118.
- Guindon S, Gascuel O. 2003. A simple, fast and accurate method to estimate large phylogenies by maximum-likelihood. Systematic Biology 52: 696-704.
- Jayalal U, Jang SH, Yu NH, Oh SO, HUR JS. 2014. Notes on the lichen genus Leptogium (Collemataceae, Ascomycote) ih South Korea. Mycobiology 42: 120-131.
- Jørgensen PM. 2014. Taxonomy and nomenclature of Collema fasciculare (L.) G. H. Weber. The Lichenologist 46: 594. doi:10.1017/S0024282914000140
» https://doi.org/doi:10.1017/S0024282914000140 - Katoh K, Misawa K, Kuma K, Miyata T. 2002. Mafft: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Research 30: 3059-3066.
- Kearse M, Moir R, Wilson A, et al 2012. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28: 1647-1649.
- Kitaura MJ, Marcelli MP. 2013. A revision of Leptogium species with spherical-celled hairs (section Mallotium p.p.). The Bryologist 116: 15-27.
- Kitaura MJ, Scur MC, Spielmann AA, Lorenz-Lemke AL. 2018. A revision of Leptogium (Collemataceae, lichenized Ascomycota) from Antarctica with a key to species. The Lichenologist 50: 467-485.
- Košuthová A, Westberg M, Otálora MAG, Wedin M. 2019. Rostania revised: testing generic delimitations in Collemataceae (Peltigerales, Lecanoromycetes). MycoKeys 47: 17-33.
- Magain N, Sérusiaux E. 2014. Do photobiont switch and Cephalodia emancipation act as evolutionary drivers in the lichen symbiosis. A case study in the Pannariaceae (Peltigerales). PLOS ONE 9: e89876. doi: 10.1371/journal.pone.0089876
» https://doi.org/10.1371/journal.pone.0089876 - Magain N, Sprobille T, DiMeglio J, Nelson PR, Miadlikowska J, Sérusiaux E. 2020. Phylogenetic evidence for an expanded circumscription of Gabura (Arctomiaceae). The Lichenologist 52: 3-15.
- Malme GOA. 1897. Die Flechten der ersten Regnellschen Expedition. I. Einleitung. Die Gattung Pyxine (Fr.) Nyl. Bihang till Kongl. Svenska Vetenskaps-Akademiens Handlingar 23 III: 1-52.
- Malme GOA. 1924. Die Collematazeen des Regnellschen Herbars. Arkiv för Botanik 19: 1-29.
- Marthinsen G, Rui S, Timdal E. 2019. OLICH: A reference library of DNA barcodes for Nordic lichens. Biodiversity Data Journal 7: e36252. doi: 10.3897/BDJ.7.e36252
» https://doi.org/10.3897/BDJ.7.e36252 - Miller MA, Pfeiffer W, Schwartz T. 2010. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. http:// www.phylo.org/ 03 Mar. 2020.
» http:// www.phylo.org/ - Oliva MLV, Mendes CR, Bueno NR, Honda NK, Sampaio MU, Sampaio CAM. 1992. Cysteine proteinase inhibitors in lichen (Collema leptosporum Malme). Brazilian Journal of Medical and Biological Research 25: 999-1002.
- Otálora MAG, Martínez I, Molina MC, Aragón G, Lutzoni F. 2008. Phylogenetic relationships and taxonomy of the Leptogium lichenoides group (Collemataceae, Ascomytoca) in Europe. Taxon 57: 907-921.
- Otálora MAG, Aragón G, Molina MC, Martínez I, Lutzoni F. 2010a. Disentangling the Collema/Leptogium complex through a molecular phylogenetic study of the Collemataceae (Peltigerales, lichen-forming Ascomycota). Mycologia 102: 279-290.
- Otálora MAG, Martínez I, Aragón G, Molina MC. 2010b. Phylogeography and divergence date estimates of a lichen species complex with disjunct distribution pattern. American Journal of Botany 97: 216-223.
- Otálora MAG, Aragón G, Martínez I, Wedin M. 2013. Cardinal characters on a slippery slope - A re-evaluation of phylogeny, character evolution, and evolutionary rates in the jelly lichens (Collemataceae s. str). Molecular and Phylogenetic Evolution 68: 185-198.
- Otálora MAG, Wedin M. 2013. Collema fasciculare belongs in Arctomiaceae. The Lichenologist 5: 295-304.
- Otálora MAG, Jørgensen PM, Wedin M. 2014. A revised generic classification of the jelly lichens, Collemataceae. Fungal Diversity 64: 275-293.
- Pott A, Pott VJ. 1994. Plantas do Pantanal. Corumbá, EMBRAPA/CPAP.
- Prado SRT, Gorin PAJ, Stuelp PM, Honda NK, Iacomini M. 1999. An unusual juxtaposition of polysaccharide components of Collema leptosporum. Carbohydrate Polymers 40: 271-276.
- Rambaut A, Drummond AJ, Xie D, Baele G, Suchard MA. 2018. Posterior summarisation in Bayesian phylogenetics using Tracer 1.7. Systematic Biology 1-3. doi:10.1093/sysbio/syy032.
» https://doi.org/doi:10.1093/sysbio/syy032 - Ronquist F, Teslenko M, Mark P, et al. 2012. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61: 539-542.
- Schmitt I, Crespo A, Divakar PK, et al. 2009. New primers for promising single-copy genes in fungal phylogenetics and systematics. Persoonia 23: 35-40.
- Spielmann AA, Cânez LS. 2012. Breve histórico sobre a taxonomia de liquens no Estado de Mato Grosso do Sul, Brasil. Glalia 4: 53-60.
- Stamatakis A. 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30: 1312-1313.
- Wedin M, Wiklund E, Jørgensen PM, Ekman S. 2009. Slippery when wet: phylogeny and character evolution in the gelatinous cyanobacterial lichens (Peltigerales, Ascomycetes). Molecular Phylogenetics and Evolution 53: 862-871.
- White T, Bruns T, Lee S, Taylor J. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ. (eds.) PCR Protocols: A Guide to Methods and Applications. New York, Academic Press Inc. p. 315-322.
- Wiklund E, Wedin M. 2003. The phylogenetic relationships of the cyanobacterial lichens in the Lecanorales suborder Peltigerineae. Cladistics 19: 419-431.
- Zoller S, Scheidegger C, Sperisen C. 1999. PCR primers for the amplification of mitochondrial small subunit ribosomal DNA of lichen-forming Ascomycetes. The Lichenologist 31: 511-516.
Publication Dates
-
Publication in this collection
22 Mar 2021 -
Date of issue
Oct-Dec 2020
History
-
Received
12 Mar 2020 -
Accepted
26 June 2020