ABSTRACT
As primary decomposers of organic matter, mucoralean fungi have an important ecological role in edaphic systems in the Atlantic Forest. However, there is a knowledge gap regarding how communities of Mucorales are structured in soils of Atlantic Forest areas, and whether these communities are influenced by edaphic attributes in this domain. Thus, the current study aimed to understand the influence of edaphic attributes linked to species richness, abundance and composition of Mucorales in dense ombrophilous forest, ‘tabuleiro’ forest, sandbank and mangrove ecosystems located in Pernambuco, Brazil. Altogether, twenty-three taxa, including seven new records, were reported from soil samples from the ecosystems. Species composition was similar among the ecosystems, except for mangrove, while species richness and diversity of Mucorales were highest in dense ombrophilous forest and ‘tabuleiro’. Together the soil variables were responsible for 35.5 % of the variation in species composition, with pH being responsible for 53.32 % and 47.24 % of the variation in richness and abundance of these communities, respectively. These data indicate that pH is the most important attribute in delimiting the structure of mucoralean communities in the study areas, with influence on the composition, richness, and abundance of these fungi.
Keywords:
basal fungal order; diversity; ecology; Mucorales; Mucoromycota; Mucoromycotina; soil; taxonomy
Introduction
Mucorales, a basal fungal order that belongs to the subkingdom Mucoromyceta Doweld, comprises species morphologically characterized by the production of asexual structures, such as sporangia, sporangiola and merosporangia, and by the formation of a sexual spore, the zygospore, in a zygosporangium formed after the fusion of two gametangia (Spatafora et al. 2016Spatafora JW, Chang Y, Benny GL et al. 2016. A phylum-level phylogenetic classification of zygomycete fungi based on genome-scale data. Mycologia 108: 1028-1046.; Tedersoo et al. 2018Tedersoo L, Sáncez-Ramírez S, Kõljalg U, et al. 2018. High-level classification of the Fungi and a tool for evolutionary ecological analyses. Fungal Diversity 90: 135-159. ). These fungi have a worldwide distribution and have been commonly reported in animal dung, stored cereals, fruits, vegetables, and soil, although some species are facultative pathogens of plants, animals, and even other fungi (Hoffmann et al. 2013Hoffmann K, Pawłowska J, Walther G, et al. 2013. The family structure of the Mucorales: a synoptic revision based on comprehensive multigene-genealogies. Persoonia: Molecular Phylogeny and Evolution of Fungi 30: 57-76.; Richardson & Rautemaa-Richardson 2020Richardson MD, Rautemaa-Richardson R. 2020. Biotic environments supporting the persistence of clinically relevant Mucormycetes. Journal of Fungi 6: 2-14. ).
The mucoralean communities in soil play an important role in ecological processes as most of specimens are saprobes, that is, primary decomposers of organic matter and able to degrade mainly simple carbon sources, with some species capable of degrading pectin and hemicelluloses, as well as some lipids and proteins (Ferreira et al. 2013Ferreira JA, Lennartsson PR, Edebo L, Taherzadeh MJ. 2013. Zygomycetes-based biorefinery: Present status and future prospects. Bioresource Technology 135: 523-532.; Lima et al. 2016Lima DX, Santiago ALCMA, Souza-Motta CM. 2016a. Diversity of Mucorales in natural and degraded semi-arid soils. Brazilian Journal of Botany 39: 1127-1133.a). The biochemical properties of some Mucorales spp., such as the production of a large spectrum of enzymes, make these fungi essential for the recycling of nutrients (Richardson 2009Richardson M. 2009. The Ecology of the Zygomycetes and its impact on environmental exposure. Clinical Microbiology and Infection 15: 2-9.; Ziaee et al. 2016Ziaee A, Zia M, Bayat M, Hashemi J. 2016. Identification of Mucorales isolates from soil using morphological and molecular methods. Current Medical Mycology 2: 13-19.).
Studies on how mucoralean communities are structured are extremely relevant since these fungi are pioneers in the ecological succession processes of several substrates (Richardson 2009Richardson M. 2009. The Ecology of the Zygomycetes and its impact on environmental exposure. Clinical Microbiology and Infection 15: 2-9.; Spatafora et al. 2016Spatafora JW, Chang Y, Benny GL et al. 2016. A phylum-level phylogenetic classification of zygomycete fungi based on genome-scale data. Mycologia 108: 1028-1046.). Despite this, ecological data regarding mucoralean communities in soil are still scarce, especially regarding the influence of edaphic attributes on these communities. There have only been three ecological studies focusing on soil mucoralean communities: two in semi-arid regions and one in the Atlantic Forest, all undertaken in Brazil, though none of them used this approach (Santiago et al. 2006Santiago ALCMA, Souza-Motta CM. 2006. Mucorales isolados do solo de mineração de cobre e produção de amilase e inulinase. Acta Botanica Brasilica 20: 641-647. ; Santiago et al. 2013Santiago ALCMA, Santos PJP, Maia LC. 2013. Mucorales from the semiarid of Pernambuco, Brazil. Brazilian Journal of Microbiology 4: 1678-4405.; Lima et al. 2016Lima DX, Santiago ALCMA, Souza-Motta CM. 2016a. Diversity of Mucorales in natural and degraded semi-arid soils. Brazilian Journal of Botany 39: 1127-1133.a; Lima et al. 2018aLima DX, Cordeiro TRL, Souza CAF, Santiago ALCMA, Souza-Motta CM. 2018a. Diversity of basal fungal order Mucorales (Mucoromycota) in a remaining area of the Brazilian Atlantic Rainforest. Nova Hedwigia 107: 459-471.).
The Atlantic Forest domain is known for its large biodiversity and for containing a high number of endemic species of various taxonomic groups, comprising 8 % of global biodiversity (IBGE 2011IBGE-Instituto Brasileiro de Geografia e Estatística. 2011. http://www.ibge.gov.br. 19 Apr. 2019.
http://www.ibge.gov.br...
; Rezende et al. 2018Rezende CL, Scarano FR, Assad ED, et al. 2018. From hotspot to hopespot: An opportunity for the Brazilian Atlantic Forest. Perspectives in Ecology and Conservation 16: 208-214.). This domain has been largely degraded due to the continuous cycle of extraction, agriculture and pasture practices that culminated in the deforestation and fragmentation of forest areas, which are therefore considered as higher priority hotspots for biodiversity conservation (Myers et al. 2000Myers N, Mittermeier RA, Mittermeier CG, Fonseca GAB, Kent K. 2000. Biodiversity hotspots for conservation priorities. Nature 403: 853-845.; Rezende et al. 2018Rezende CL, Scarano FR, Assad ED, et al. 2018. From hotspot to hopespot: An opportunity for the Brazilian Atlantic Forest. Perspectives in Ecology and Conservation 16: 208-214.). However, only 2 % of these areas are legally protected in conservation units such as Biological Reserves and Environmental Protection Areas (IBGE 2011IBGE-Instituto Brasileiro de Geografia e Estatística. 2011. http://www.ibge.gov.br. 19 Apr. 2019.
http://www.ibge.gov.br...
).
Mucorales spp., like other fungi, contribute for the maintenance of ecosystems, including those of the Atlantic Forest, and in this process their communities are influenced by the physical and chemical factors of the soil (Lauber et al. 2008Lauber CL, Strickland MS, Bradford MA, Fierer N. 2008. The influence of soil properties on the structure of bacterial and fungal communities across land-use types. Soil Biology and Biochemistry 40: 2407-2415.; Ziaee et al. 2016Ziaee A, Zia M, Bayat M, Hashemi J. 2016. Identification of Mucorales isolates from soil using morphological and molecular methods. Current Medical Mycology 2: 13-19.; Lima et al. 2018Lima DX, Cordeiro TRL, Souza CAF, Santiago ALCMA, Souza-Motta CM. 2018a. Diversity of basal fungal order Mucorales (Mucoromycota) in a remaining area of the Brazilian Atlantic Rainforest. Nova Hedwigia 107: 459-471.a). However, it is unknown whether soil chemical properties, including pH, as well as other environmental variables, such as vegetation, may influence the structure of the mucoralean communities. In this present study, we addressed the following questions: Do edaphic attributes influence the mucoralean communities? If so, what are the main soil parameters associated?
The current study aimed to understand how these chemical attributes influence the richness, abundance and species composition of these fungi in four ecosystems of Atlantic Forest, through ecological indices (diversity and evenness), quantitative and qualitative population data (frequency of occurrence and relative abundance), and similarity among these ecosystems. We also discuss how soil properties are linked to variation in these communities. Therefore, our research helps to provide an understanding of how local mucoralean communities are structured by edaphic attributes, mainly in tropical and subtropical forests.
Materials and methods
Study areas
This study was conducted in two areas: the Saltinho Biological Reserve (8°43′34.73′′ S - 35°10′37.26′′ W) and the Guadalupe Environmental Protection Area (8°46′11.52′′ S - 35°06′27.24′′ W), both located in the ecoregion of the Pernambuco coastal forests in the municipality of Tamandaré. The climate of both areas is characterized as humid (As'), according to Koppen, with an average annual temperature of 25 °C. Rainfall is evenly distributed throughout the year, with no truly dry season and higher precipitation between March and July, while the period of low rainfall occurs from October to December, with an annual precipitation of 1,500 to 2,000 mm. The vegetation in the Saltinho Biological Reserve is predominantly composed of dense ombrophilous forest. The phytogeographic domain that surrounds the Guadalupe Environmental Protection Area is diverse and comprises areas of ‘tabuleiro’ forest, sandbanks and mangroves (IBAMA 2003IBAMA - Instituto Brasileiro do Meio Ambiente e dos Recursos Naturais Renováveis. 2003. Resumo executivo do plano de manejo da Reserva Biológica de Saltinho. Brasília, DF, IBAMA. http://www.icmbio.gov.br/portal/images/stories/imgs-unidades-coservacao/pm_rebio_saltinho_encartes.pdf.
http://www.icmbio.gov.br/portal/images/s...
; Rodrigues et al. 2010Rodrigues RC, Araujo HF, Lyra-Neves RM, Telino-Júnior WR, Botelho CNM. 2010. Caracterização da Avifauna na Área de Proteção Ambiental de Guadalupe, Pernambuco. Ornithologia 2: 47-61.). All the above-mentioned ecosystems belong to the Brazilian Atlantic Forest floristic domain, which is inserted in the Tropical and Subtropical Moist Broadleaf Forest biome (http://ecoregions2017.appspot.com).
The dense ombrophilous forest (DOF) is a well-developed forest with a canopy of 20 to 30 m and species reaching 50 m in height. It includes species of Anacardiaceae, Euphorbiaceae, Lauraceae, Mimosaceae, Moraceae, Myrtaceae and Sapotaceae families (IBAMA 2003IBAMA - Instituto Brasileiro do Meio Ambiente e dos Recursos Naturais Renováveis. 2003. Resumo executivo do plano de manejo da Reserva Biológica de Saltinho. Brasília, DF, IBAMA. http://www.icmbio.gov.br/portal/images/stories/imgs-unidades-coservacao/pm_rebio_saltinho_encartes.pdf.
http://www.icmbio.gov.br/portal/images/s...
). ‘Tabuleiro’ (TAB) is an ecosystem that develops along the edge of the Atlantic Forest, exhibiting plant species shared with the ‘Cerrado’ floristic domain, which is part of the Tropical and Subtropical Savanna biome, in areas of sandy soil near the coast. The vegetation appears in open areas in coastal enclaves (Almeida et al. 2009Almeida JREB, Zickel CS, Pimentel RMM. 2009. Caracterização e espectro biológico da vegetação do litoral arenoso do Rio Grande do Norte. Revista Geografia Norte 23: 66-85.). In addition, it can form a vegetative continuum with sandbank areas, sharing plant species with this ecosystem (Andrade-Lima 1970Andrade-Lima D. 1970. Recursos vegetais de Pernambuco. Boletim Técnico do Instituto de Pesquisas Agronômicas 41: 1-32.; Oliveira-Filho & Carvalho 1993Oliveira-Filho AT, Carvalho DA. 1993. Florística e fisionomia da vegetação no extremo norte do litoral da Paraíba. Revista Brasileira de Botânica 16:115-130). The vegetation of sandbanks (SAN) can vary in physiognomies, from herbaceous to arboreal types, in which plants like Remirea maritima Aubl. and Canavalia rosea (Sw.) DC. are found (Sampaio et al. 2005Sampaio D, Souza VC, Oliveira AA, Paula-Souza J, Rodrigues RR. 2005. Árvores da Restinga: Guia de identificação. São Paulo, Editora Neotrópica.). Mangrove (MAN) ecosystems are exposed to conditions of extreme salinity, and are considered aquatic and terrestrial intermediary systems, with Rhizophora mangle (Rhizophoraceae), Laguncularia racemosa (Combretaceae) and Avicennia spp. (Acanthaceae) included in their composition (Castiglioni & Coelho 2011Castiglioni DS, Coelho PA. 2011. Determinação da maturidade sexual de Ucides cordatus (Crustacea, Brachyura, Ucididae) em duas áreas de manguezal do litoral sul de Pernambuco, Brasil. Iheringia Série Zoologia 101: 138-144.).
Sampling sites
Seven expeditions for soil collection were done monthly from June to December of 2014 at the Saltinho Biological Reserve and the Guadalupe Environmental Protection Area, specifically in areas of DOF (8°43′28.63′′ S - 35°10′45.89′′ W), TAB (8°45′08.65′′ S - 35°07′44.22′′ W), SAN (8°46′29.06′′ S - 35°06′35.21′′ W) and MAN (8°46′24.46′′ S - 35°06′27.22′′ W). At each of the above-mentioned areas, eight quadrants of 100 m2 (10 × 10 m) were spatially dispersed and randomly distributed, with a minimal distance of 200 m among them. In each quadrant, using sterilized spatulas, eight soil sub-samples up to 5 cm deep were collected, placed in clean plastic bags and stored in styrofoam boxes with ice during transport to the Laboratory of the Universidade Federal de Pernambuco (UFPE). In the lab, the eight soil sub-samples collected in each quadrant were mixed to form one composite sample per quadrant, totaling eight composite samples in each area from each collection expedition. Considering the seven collection expeditions, 56 composite samples were analyzed from each area, for a total of 224 samples considering the four areas. The soil samples were stored in the laboratory (UFPE) for isolation of Mucorales and chemical analysis of the soil.
Isolation, purification and identification of Mucorales
Five milligrams of soil from each of 224 composite samples (672 Petri dishes) were sprinkled over Petri dishes containing wheat germ agar culture medium plus chloramphenicol (80 mg.L-1) in triplicate (Benny 2008Benny GL. 2008. The methods used by Dr. R. K. Benjamin, and other mycologists to isolate Zygomycetes. Aliso 26: 37-61.). Colony growth was monitored for 96 hours at 28°C. In order to purify the Mucorales, fragments of the colonies were transferred separately to MEA [malt extract agar, plus chloramphenicol (80 mg.L-1) ] (Benny 2008Benny GL. 2008. The methods used by Dr. R. K. Benjamin, and other mycologists to isolate Zygomycetes. Aliso 26: 37-61.). Taxa were identified by observing their macroscopic (color, appearance and diameter of colonies) and microscopic (microstructures) characteristics, as described by Hesseltine & Ellis (1964Hesseltine CW, Ellis JJ. 1964. The genus Absidia: Gongronella and cylindrical-spored species of Absidia. Mycologia 56: 568-601.), Benny & Benjamin (1975)Benny GL, Benjamin RK. 1975. Observations on Thamnidiaceae (Mucorales). New taxa, new combinations, and notes on selected species. Aliso 8: 301-351., Benny (1982)Benny GL. 1982. Zygomycetes. In: Benny GL. (ed.) Synopsis and classification of living organisms. New York, McGraw-Hill Publishing Company, Inc. p. 184-195., Schipper (1984Schipper MAA. 1984. A revision of the genus Rhizopus I. The Rhizopus stolonifer group and Rhizopus oryzae. Studies in Mycology 25: 1-34.; 1990Schipper MAA. 1990. On certain species of Mucor with a key to all accepted species. Studies in Mycology 25: 1-53.), Zheng & Chen (2001Zheng R, Chen G. 2001. A monograph of Cunninghamella. Mycotaxon 80: 1-75.), Domsch et al. (2007Domsch KH, Gams W, Anderson TH. 2007. Compendium of Soil Fungi. Germany, IHW-Verlag Eching.) and Zheng et al. (2007)Zheng R, Chen G, Huang H, Liu X. 2007. A monograph of Rhizopus. Sydowia 59: 273-372..
Soil analyses
Soil pH and other chemical soil analyses were performed from three analytical replicates per each composite soil sample from each quadrant of each area. The analyses were conducted at the ‘Estação Experimental de Cana de Açúcar do Carpina’ of the Universidade Federal Rural de Pernambuco, using standard methods to determine P, Ca, Mg, Na, K, Al3+, H+, S and cation exchange capacity (CEC) levels according to EMBRAPA (1998)Embrapa - Empresa Brasileira de Pesquisa Agropecuaria. 1998. Análises químicas para avaliação da fertilidade do solo. In: Silva FC, Eira PA, Barreto WO, Perez DV, Silva CA. (eds.) Embrapa. Brasília, Empresa Brasileira de Pesquisa Agropecuária. p. 1-40. and Jackson (2005Jackson ML. 2005. Soil chemical analysis: Advanced course. Madison, Libraries Parallel Press.).
Data Analysis
The frequency of occurrence (FO) of the species was estimated according to the following equation: FO = Ji/k x 100, in which: FO = frequency of occurrence of species i; Ji = the number of samples in which species i occurred, and k = the total number of soil samples. According to this formula, the species were classified as follows: very frequent (>10 %), frequent (5-10 %), infrequent (≧1-5< %), and rare (< 1 %) (Hyde & Sarma 2001Hyde KD, Sarma VV. 2001. A review on frequently occurring fungi in mangroves. Fungal Diversity. 8: 1-34.). Species accumulation curves were also calculated per area, allowing the expected and observed richness to be calculated using the Chao 1 and Jackknife 1 estimators, respectively (Clarke & Gorley 2006Clarke KR, Gorley RN. 2006. Primer v6 user manual\tutorial. Plymouth, UK, Primer-E Limited.). The Shannon Wiener diversity index and Pielou’s evenness were estimated using R software (RStudio Team 2009RStudio Team. 2019. RStudio: Integrated Development for R. Boston, USA, RStudio, Inc. URL URL http://www.rstudio.com/ . 10 Apr. 2019.
http://www.rstudio.com/...
). In addition, the richness and abundance (number of colony forming units) were calculated. The relative abundance of each species within the three studied areas was evaluated according to the following equation: RA = (Ni/N) x 100, where RA = relative abundance of the species i; Ni=number of CFU of the species i; N = total number of CFU of fungi in all samples in each area). According to this formula, each taxon can be classified as one of the following: RA < 0.5 % = rare; 0.5 ≤ RA < 1.5 % = occasional; 1.5 ≤ Ra < 3.0 % = common; or RA > 3.0 % = abundant (Silva & Cavalcanti 2010). An analysis of indicator species was performed to assess the statistical significance of relationships among Mucorales species abundance in each area inventoried. The indicator value was calculated and the significance was obtained using the Monte Carlo test (500 random replications) according to Dufrêne & Legendre (1997Dufrêne M, Legendre P. 1997. Species assemblages and indicator species: the need for a flexible asymmetrical approach. Ecological Monographs 67: 345-366.), being considered indicator species when values were greater or equal to 25 % with p<0.05. The richness, abundance, Shannon diversity and Pielou’s evenness values were submitted to analysis of variance (One-way ANOVA) using Past software and means were compared by the Tukey’s pairwise test (P≤0.05) (Hammer et al. 2001Hammer Ø, Harper DAT, Ryan, PD. 2001. PAST: paleontological statistics software package for education and data analysis. Palaeontologia Electronica 4: 1-9.). The soil chemical attributes were also submitted to analysis of variance and the means compared by the Tukey test (p≤0.05). NMDS (Non-Metric Multidimensional Scaling) was carried out to determine the composition of Mucorales species in the study areas. This analysis was based on a matrix of communities using Euclidean distance. In addition, Analysis of Similarity (ANOSIM) was used to detect significant differences among the groups identified in the NMDS. The influence of soil variables on the Mucorales community was evaluated using principal component analysis (PCA) and the existence of a relationship among Mucorales species richness, abundance and the soil variables was tested through multiple models. Before this test, multicollinearity among the ten soil variables was evaluated through the variance inflation factor (VIF), and only variables with a factor less than 5 were included in the linear model. To test the independence of the quadrants sampled within each area, we evaluated eight linear models, four to each variable (richness and abundance). Two standard linear models as follow: i) all soil predictor variables without quadrants as fixed effects and without random effects; ii) all soil predictor variables and quadrants as fixed effects without random effects; in addition to two mixed models; iii) all soil predictor variables as fixed effects and quadrants as random effects; iv) and a null model with only quadrants as random effects. These last two were adjusted by the Restricted Maximum Likelihood (REML) to avoid bias in the variance estimates of the models. The model selection was performed using the likelihood ratio test according to Zuur et al. (2009Zuur A, Ieno E, Walker N, Saveliev A, Smith G. 2009. Mixed effects models and extensions in ecology with R. German, Springer Science & Business Media.). All analyses were performed using R software and the RStudio interface with an alpha level of 0.05 (RStudio Team 2009RStudio Team. 2019. RStudio: Integrated Development for R. Boston, USA, RStudio, Inc. URL URL http://www.rstudio.com/ . 10 Apr. 2019.
http://www.rstudio.com/...
).
Results
Overall, twenty-three species of Mucorales were isolated from the soils of Atlantic Forest ecosystems distributed among Absidia, Backusella, Cunninghamella, Gongronella, Lichtheimia, Mucor, Rhizopus and Syncephalastrum. The DOF and TAB areas had the greatest number of Mucorales colony-forming units per gram of soil (Tab. 1).
Number of colony forming units of Mucorales per gram of soil (CFU.g-1) in the soil of dense ombrophilous forest (DOF), ‘tabuleiro’ forest (TAB), sandbanks (SAN) and mangroves (MAN) from the Saltinho Biological Reserve and Guadalupe Environmental Protection Area. Values followed by the same lowercase letter do not differ in the Tukey test (p = 0.05).
Gongronella butleri was very frequent (FO = 16.6 %) in DOF, followed by G. brasiliensis (FO = 7.14 %), R. microsporus (FO = 5.35 %) and M. irregularis (FO = 5.35 %). In TAB, four taxa were frequent: G. butleri (FO = 8.92 %), C. elegans, (FO = 5.95 %), M. indicus (FO = 5.95 %) and S. racemosum (FO = 5.95 %). Rhizopus microsporus (FO = 16.6 %) and G. butleri (FO = 5.35 %) were very frequent in SAN areas. According to the relative abundance of each taxon, most species of Mucorales were rare. Gongronella butleri was occasional in all ecosystems, except in MAN, while R. microsporus was occasional in SAN. Only two species were isolated from MAN: R. stolonifer (FO = 1.19 %, RA = 0.26 %) and S. racemosum (FO = 2.90 %, RA = 0.66 %), both of which were infrequent and rare (Tab. 2). Nine species were defined as indicators for specific ecosystems and could be described as characteristic for these areas: three for DOF and six for TAB (Tab. 2).
The average abundance (N), richness (S), Shannon Wiener index (H’) and Pielou’s evenness index (J’) of the Mucorales communities were greatest in the soil of DOF and TAB areas, with no significant difference between these two areas (Fig. 1). SAN exhibited intermediate values while MAN exhibited the lowest values with fewer species and individuals than the other ecosystems with significant differences between extreme values (e.g. TAB vs MAN) (Fig. 1). The species accumulation curve indicated a sufficient sampling performance. The Chao 1 estimator indicated the same values for all areas (Fig. 2). However, Jackknife 1 revealed that richness may have been higher than what was observed in DOF (20.94 ± 1.7) (Fig. 2).
Average Richness (S), Abundance (N), Shannon Wiener index (H’) and Pielou’s Evenness index (J’) of Mucorales communities from dense ombrophilous forest (DOF), ‘tabuleiro’ forest (TAB), sandbanks (SAN) and mangroves (MAN) from the Saltinho Biological Reserve and the Guadalupe Environmental Protection Area. Asterisks (*) indicate significantly higher values of the evaluated attribute based on one-way ANOVA. Median (central dot), quartile (box), maximum and minimum (error bars) are shown.
Chao 1 and Jackknife 1 Richness estimators of Mucorales from dense ombrophilous forest (DOF), ‘tabuleiro’ forest (TAB), sandbanks (SAN) and mangroves (MAN) from Saltinho Biological Reserve and Guadalupe Environmental Protection Area.
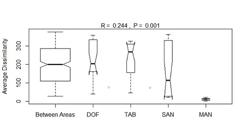
The highest similarity indices of the Mucorales community were observed between DOF and SAN areas (54.13 %), followed by SAN and TAB (50 %), and DOF and TAB (47.45 %) areas. The MAN community exhibited low similarities with all other areas (Tab. 3). This result was corroborated by the ANOSIM (Analysis of Similarity), which showed that MAN differs significantly from DOF, TAB and SAN areas. However, there were no significant differences in species composition among DOF, TAB and SAN areas (Fig. 3).
Similarity of Mucorales composition between dense ombrophilous forest (DOF), ‘tabuleiro’ forest (TAB), sandbanks (SAN) and mangroves (MAN) from the Saltinho Biological Reserve and the Guadalupe Environmental Protection Area.
Analysis of Similarity (ANOSIM) results among communities of Mucorales of dense ombrophilous forest (DOF), ‘tabuleiro’ forest (TAB), sandbanks (SAN) and mangroves (MAN) from the Saltinho Biological Reserve and the Guadalupe Environmental Protection area. Average dissimilarity calculated using Bray-Curtis distance.
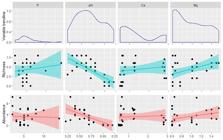
Principal component analysis (PCA) revealed low percentages of explanation, with the sum of the first two dimensions explaining 35.5 % of the variation (Fig. 4). Thus, the contribution of chemical-physical attributes to the composition of soil varied between 7 % and 8 % (Fig. 4). In general, the concentration of edaphic attributes analyzed differed among areas, with some exceptions between TAB-SAN (Mg, Na, K and S) and MAN-SAN (P and Al3+ + H+) (Tab. 4). At DOF, there were high concentration of phosphorus, magnesium, potassium, soil acidity (Al3+ + H+) and cation exchange capacity, while at MAN, high levels of sodium and sulfur were found. Soil data revealed three groups of communities: (i) DOF; (ii) TAB + SAN; (iii) MAN. After colinearity tests, only P, pH, Ca and Mg were selected for multiple models. Comparing the models, we did not obtain significant differences among them, which indicates that the dependence among the quadrant samples does not significantly affect these variables (Tab. 5). Thus, we selected the simplest model (model i) to test the influence of soil variables on the abundance and richness of Mucorales. According to these regressions, only pH significantly affected the richness and abundance of the Mucorales communities (Multiple regression - Richness; F(4.23)=8.71, p < 0.01; Multiple regression - Abundance; F(4.22)=6.82, p < 0.01), being responsible for 53.32 % and 47.24 % of the variation in richness and abundance found in the Mucorales communities, respectively (Fig. 5).
Two-dimensional Projection of Principal Component Analysis (PCA) of seven soil samples in each different vegetational type at the Saltinho Biological Reserve and the Guadalupe Environmental Protection Area. The colored dots represent soil samples and larger are the samples centroids. Green arrows show evaluated soil nutrients and their contribution to the variation of principal components. Legend: “Al” Aluminum, “Ca” calcium, “CEC” cation exchange capacity, “H” hydrogen, “K” potassium, “H” hydrogen, “Mg” magnesium, “Na” sodium, “P” phosphorus, “S” sulfur.
Likelihood ratio test for eight linear models performed to evaluate the influence of soil variables on the richness and abundance of Mucorales in four forest types from the Saltinho Biological Reserve and the Guadalupe Environmental Protection Area. Legend: DF - Degrees of Freedom; AIC - Akaike information criterion.
Multiple regression with P, pH, Ca and Mg showing a negative effect of pH on the richness and abundance of Mucorales communities in the Saltinho Biological Reserve and the Guadalupe Environmental Protection Area.
Discussion
This manuscript reports ecological data regarding the communities of Mucorales in DOF, TAB, SAN and MAN ecosystems located in Pernambuco, Brazil. Most of the mucoralean species isolated in the present study have already been reported in other inventories of Atlantic Forest soil (Eicker 1969Eicker A. 1969. Microfungi from surface soil of forest communities in Zululand. Transactions of the British Mycological Society 53: 381-392.; Varghese 1972Varghese G. 1972. Soil microflora of plantations natural Rain Forest of West Malaysia. Mycopathologia et Mycologia Applicata 48: 43-61.; Ogbonna & Pugh 1982Ogbonna CIC, Pugh GJF. 1982. Nigerian soil fungi. Nova Hedwigia 36:795-808.; Rambelli et al. 1984Rambelli A, Persiani AM, Maggi O, Onofri S, Riess S, Dowgiallo G, Zucconi L. 1984. Comparative studies on microfungi in tropical ecosystems. Further mycological studies in South Western Ivory Coast forest. Plant Biosystems 118: 201-243. ; Bettuci & Roquebert 1995Bettuci L, Roquebert MF. 1995. Microfungi from tropical rain Forest litter and soil, a preliminary study. Nova Hedwigia 61: 111-118.; Schoenlein-Crusius et al. 1996Schoenlein-Crusius IH, Trufem SFB, Malatinsky SMM, Ninomiya A, Antunes MFR. 1996. Mucorales (Zygomycotina) from soil affected by excrement of birds in the "Parque Estadual das Fontes do Ipiranga", São Paulo, Brazil. Brazilian Journal of Botany 19: 7-10.; Schoenlein-Crusius & Milanez 1997Schoenlein-Crusius IH, Milanez AI. 1997. Mucorales (Zygomycotina) da Mata Atlântica da reserva biológica do Alto da Serra de Paranapiacaba, Santo André, SP. Acta Botanica Brasilica 11: 95-101.; Schoenlein-Crusius & Milanez 1998Schoenlein-Crusius IH, Milanez AI. 1998. Fungos microscópicos da Mata Atlântica de Paranapiacaba, São Paulo, Brasil. Brazilian Journal of Botany 21: 73-79.; Schoenlein-Crusius et al. 2006Schoenlein-Crusius IH, Milanez AI, Trufem SFB, Pires-Zottarelli CLA, Grandi RAP. 2006. Microscopic fungi in the Atlantic Rainforest in Cubatão, São Paulo, Brazil. Brazilian Journal of Microbiology 37: 267-275.; Maia et al. 2006Maia LC, Cavalcanti MA, Gibertoni T, Goto BT, Melo AMM, Baseia IG, Silvério ML. 2006. Fungos. In: Grillo AS, Oliveira MA, Porto K, Almeida-Cortez JS, Tabarelli M. (eds.) Diversidade biológica e conservação da Floresta Atlântica ao norte do Rio São Francisco. Brasília, Ministério do Meio Ambiente. p. 74-106.; Lima et al. 2018Lima DX, Cordeiro TRL, Souza CAF, Santiago ALCMA, Souza-Motta CM. 2018a. Diversity of basal fungal order Mucorales (Mucoromycota) in a remaining area of the Brazilian Atlantic Rainforest. Nova Hedwigia 107: 459-471.a). However, seven new records for this domain are reported herein: Absidia sp., Cunninghamella bertholletiae, C. elegans, Gongronella brasiliensis, Lichtheimia ramosa, Mucor indicus, and M. irregularis, which represent an addition of almost 10 % of the currently known species in the Brazilian Atlantic Forest for which 66 species of Mucorales are currently recorded (Flora do Brasil 2020Flora do Brasil 2020 em Construção. 2020. http://floradobrasil.jbrj.gov.br/. 10 Apr. 2019.
http://floradobrasil.jbrj.gov.br/...
). Although DOF, TAB, SAN and MAN are part of the same domain, the Mucorales communities in these ecosystems varied in relation to richness and abundance of species.
Some genera were found predominantly or exclusively in DOF, such as Absidia, Backusella and Mucor. Absidia species are described as common in tropical forests soils, whereas B. constricta is the only species of this genus reported in DOF (Pfenning & Abreu 2006Pfenning LH, Abreu LM. 2006. Diversity of microfungi in tropical soils. In: Moreira FM, Siqueira JO, Brussaard L. (eds.) Soil Biodiversity in Amazonian and Other Brazilian Ecosystems. Oxfordshire, CABI Publishing. p. 184-205.; Lima et al. 2016Lima DX, Voigt K, Souza CA, Oliveira RJ, Souza-Motta CM, Santiago ALCMA. 2016b. Description of Backusella constricta sp. nov. (Mucorales, ex Zygomycota) from the Brazilian Atlantic Rainforest, including a key to species of Backusella. Phytotaxa 289: 59-68.b; Lima et al. 2018aLima DX, Cordeiro TRL, Souza CAF, Santiago ALCMA, Souza-Motta CM. 2018a. Diversity of basal fungal order Mucorales (Mucoromycota) in a remaining area of the Brazilian Atlantic Rainforest. Nova Hedwigia 107: 459-471.). Although several Mucor species have been previously reported in the Atlantic Forest (Flora do Brasil 2020Flora do Brasil 2020 em Construção. 2020. http://floradobrasil.jbrj.gov.br/. 10 Apr. 2019.
http://floradobrasil.jbrj.gov.br/...
), M. irregularis isolated during this survey was recorded for the first time in South America (Lima et al. 2018bLima DX, Souza CAF, Oliveira RJV, Bezerra JL, Santiago ALCMA, Souza-Motta CM. 2018b. Mucor irregularis, a first record for South America. Mycotaxon 133: 429-438.). Cunninghamella species have been commonly found in the soil of the Atlantic Forest and soils of subtropical regions (Domsch et al. 2007Domsch KH, Gams W, Anderson TH. 2007. Compendium of Soil Fungi. Germany, IHW-Verlag Eching.; Lima et al. 2018aLima DX, Cordeiro TRL, Souza CAF, Santiago ALCMA, Souza-Motta CM. 2018a. Diversity of basal fungal order Mucorales (Mucoromycota) in a remaining area of the Brazilian Atlantic Rainforest. Nova Hedwigia 107: 459-471.). Although S. racemosum is a common soil species, this is the second report of it in Brazilian Atlantic Forest soils (Domsch et al. 2007Domsch KH, Gams W, Anderson TH. 2007. Compendium of Soil Fungi. Germany, IHW-Verlag Eching.; Lima et al. 2018aLima DX, Cordeiro TRL, Souza CAF, Santiago ALCMA, Souza-Motta CM. 2018a. Diversity of basal fungal order Mucorales (Mucoromycota) in a remaining area of the Brazilian Atlantic Rainforest. Nova Hedwigia 107: 459-471.). Rhizopus and Lichtheimia are ubiquitous, but they are more common in soils from the Brazilian semi-arid areas than in Brazilian Atlantic Forest soils (Santiago et al. 2013Santiago ALCMA, Santos PJP, Maia LC. 2013. Mucorales from the semiarid of Pernambuco, Brazil. Brazilian Journal of Microbiology 4: 1678-4405.; Lima et al 2016aLima DX, Santiago ALCMA, Souza-Motta CM. 2016a. Diversity of Mucorales in natural and degraded semi-arid soils. Brazilian Journal of Botany 39: 1127-1133.; Lima et al. 2018aLima DX, Cordeiro TRL, Souza CAF, Santiago ALCMA, Souza-Motta CM. 2018a. Diversity of basal fungal order Mucorales (Mucoromycota) in a remaining area of the Brazilian Atlantic Rainforest. Nova Hedwigia 107: 459-471.). Among the isolated species, some are indicators or characteristic of these ecosystems, such as C. phaeospora, G. brasiliensis and M. irregularis, found in DOF, and C. echinulata, C. elegans, L. brasiliensis, L. ramosa, M. indicus and M. luteus observed in TAB (Dufrêne & Legendre 1997Dufrêne M, Legendre P. 1997. Species assemblages and indicator species: the need for a flexible asymmetrical approach. Ecological Monographs 67: 345-366.). These indicator species might be useful to define the habitat preference of these species (Bouffaud et al. 2016Bouffaud ML, Creamer RE, Stone D, Plassart P, Tuinen D, Lemanceau P, Wipf D, Redecker D. 2016. Indicator species and co-occurrence in communities of arbuscular mycorrhizal fungi at the European scale. Soil Biology and Biochemistry 103: 464-470.).
Although Mucorales species were present in the soil of all the ecosystems studied, the frequency of occurrence and the relative abundance of the majority of species were low. Other studies have also shown that the majority of mucoralean species in soil is infrequent and abundance is relatively low (Lima et al. 2016Lima DX, Santiago ALCMA, Souza-Motta CM. 2016a. Diversity of Mucorales in natural and degraded semi-arid soils. Brazilian Journal of Botany 39: 1127-1133.a; Oliveira et al. 2013Oliveira LG, Cavalcanti MAQ, Fernandes MJS, Lima DMM. 2013. Diversity of filamentous fungi isolated from the soil in the semiarid area, Pernambuco. Journal of Arid Environments 95: 49-54.). However, G. butleri was occasional, very frequent or frequent in most ecosystems with the exception of MAN. This species was reported as frequent in tropical forests and with a greater distribution in Atlantic Forest ecosystems (Lima et al. 2018aLima DX, Cordeiro TRL, Souza CAF, Santiago ALCMA, Souza-Motta CM. 2018a. Diversity of basal fungal order Mucorales (Mucoromycota) in a remaining area of the Brazilian Atlantic Rainforest. Nova Hedwigia 107: 459-471.). It is considered generalist in terms of habitat use due to its broad geographic distribution (Persiani et al. 1998Persiani AM, Maggi O, Casado MA, Pineda FD. 1998. Diversity and variability in soil fungi from a disturbed tropical rain forest. Mycologia 90: 206-214.). This result was expected, as species of Mucorales commonly have few taxa with a high frequency of occurrence and a clear dominance over other species (Richardson 2009Richardson M. 2009. The Ecology of the Zygomycetes and its impact on environmental exposure. Clinical Microbiology and Infection 15: 2-9.; Lima et al. 2016aLima DX, Santiago ALCMA, Souza-Motta CM. 2016a. Diversity of Mucorales in natural and degraded semi-arid soils. Brazilian Journal of Botany 39: 1127-1133.; Lima et al. 2018aLima DX, Cordeiro TRL, Souza CAF, Santiago ALCMA, Souza-Motta CM. 2018a. Diversity of basal fungal order Mucorales (Mucoromycota) in a remaining area of the Brazilian Atlantic Rainforest. Nova Hedwigia 107: 459-471.).
Fungi may be sensitive to changes in the vegetation type (Heinemeyer et al. 2004Heinemeyer A, Ridgway KP, Edwards EJ, Benham DG, Young JPW, Fitter AH. 2004. Impact of soil warming and shading on colonization and community structure of arbuscular mycorrhizal fungi in roots of a native grassland community. Global Change Biology 10: 52-64.), but the distribution of a species in a given soil is also influenced by abiotic factors such as temperature, pH, salinity, amount of organic matter and nutrients (Cruz et al. 2017Cruz R, Ramos SMS, Fonseca JC, Motta CMDS, Moreira KA. 2017. Anthropization Effects on the Filamentous Fungal Community of the Brazilian Catimbau National Park. Revista Brasileira de Ciência do Solo 41: 1-13.). The soil variables analyzed together in the present study were responsible for 35.5 % of variation in the species composition of the Mucorales community. Of all test variables, Na and S exerted greater influence on the species composition of communities in MAN, whereas Al3+ + H+ exerted greater influence on the species composition in DOF. Additionally, differently from what has been reported by Lauber et al. (2008Lauber CL, Strickland MS, Bradford MA, Fierer N. 2008. The influence of soil properties on the structure of bacterial and fungal communities across land-use types. Soil Biology and Biochemistry 40: 2407-2415.) and Rousk et al. (2010Rousk J, Bååth E, Brookes PC, et al. 2010. Soil bacterial and fungal communities across a pH gradient in an arable soil. The Multidisciplinary Journal of Microbial Ecology 4: 1340-1351.) in other fungal communities, we also found a direct influence of pH on the composition of Mucorales in our soil samples, mainly in TAB and SAN.
Some surveys have indicated that the structure of soil fungal communities is more influenced by nutrients than pH (Lauber et al. 2008Lauber CL, Strickland MS, Bradford MA, Fierer N. 2008. The influence of soil properties on the structure of bacterial and fungal communities across land-use types. Soil Biology and Biochemistry 40: 2407-2415.; Rousk et al. 2010Rousk J, Bååth E, Brookes PC, et al. 2010. Soil bacterial and fungal communities across a pH gradient in an arable soil. The Multidisciplinary Journal of Microbial Ecology 4: 1340-1351.). However, we found that pH was responsible for 53.32 % and 47.24 % of the variation in richness and abundance found in the Mucorales communities, respectively. This became clear after observing that the richness and abundance of Mucorales decreased when pH increased (Fig. 5). SAN, for example, seemed more affected by pH variation, since it had a higher pH than the other areas, and the means of richness and abundance were lower than DOF and TAB (Tab. 4, Fig. 1). According to Glassman et al. (2017Glassman SI, Wang IJ, Bruns TD. 2017. Environmental filtering by pH and soil nutrients drives community assembly in fungi at fine spatial scales. Molecular ecology 26(24): 6960-6973.), the pH is a strong factor of shaping fungal communities due to the fact that it affects the availability of all soil nutrients, and an acidic pH is most favorable to the development of Mucorales (Richardson & Rautemaa-Richardson 2020Richardson MD, Rautemaa-Richardson R. 2020. Biotic environments supporting the persistence of clinically relevant Mucormycetes. Journal of Fungi 6: 2-14. ). On the other hand, the variation of other edaphic factors of ecosystems, climatic conditions, as well as fungal physiology and competition for organic matter resulting from niche overlap may also alter the microbial structure in the rhizosphere (Bills et al. 2004Bills GF, Christensen M, Powell M, Thom G. 2004. Saprobic soil fungi. In: Mueller GM, Bills GF, Foster MS. (eds.) Biodiversity of Fungi: Inventory and Monitoring Methods. San Diego, Elsevier Academic Press. p. 271-302.; Pandey & Palni 2007Pandey A, Palni LMS. 2007. The rhizosphere effect in trees of the Indian Central Himalaya with special reference to altitude. Applied Ecology and Environmental Research 5: 93-102.).
The community of Mucorales in DOF showed a high number of CFU.g-1 of soil. This fact may be explained by the low average soil pH value of this area, in addition to the high pool nutrient availability and potential acidity (H+ + Al3+) in these soils (Tabarelli et al. 2005Tabarelli M, Pinto LP, Silva JMC, Hirota MM, Bedê LC. 2005. Desafios e oportunidades para a conservação da biodiversidade na Mata Atlântica brasileira. Megadiversidade 1: 132-138.; Cruz et al. 2013Cruz R, Lima JS, Fonseca JC, et al. 2013. Diversity of filamentous fungi of area from Brazilian Caatinga and high-level tannase production using mango (Mangifera indica L.) and surinam cherry (Eugenia uniflora L.) leaves under SSF. Advances in Microbiology 3: 52-60.), determining factors for the increased abundance and diversity of fungi in an ecosystem (Heijden et al. 2008Heijden MG, Bardgett RD, Straalen NM. 2008. The unseen majority: soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecology Letters 11: 296-310.; Lauber et al. 2008Lauber CL, Strickland MS, Bradford MA, Fierer N. 2008. The influence of soil properties on the structure of bacterial and fungal communities across land-use types. Soil Biology and Biochemistry 40: 2407-2415.). The richness and diversity of Mucorales in DOF were also high, as well as in TAB. However, according to Jackknife 1, the estimated richness was attained for all study areas, except for DOF, which indicates that the richness obtained was lower than expected.
Although the mucoralean diversity in TAB was greater than in SAN, both ecosystems are similar in Mucorales species composition. This result was expected, since TAB and SAN naturally form a vegetation continuum that is difficult to define, sharing various plant species (Tavares 1964Tavares S. 1964. Contribuição ao estudo da cobertura vegetal dos tabuleiros do Nordeste. Boletim de Recursos Naturais 2: 13-25.; Andrade-Lima 1970Andrade-Lima D. 1970. Recursos vegetais de Pernambuco. Boletim Técnico do Instituto de Pesquisas Agronômicas 41: 1-32.), moreover, both ecosystems share soil characteristics, such as Mg, Na, K and S. In addition, both TAB and SAN exhibit sandy soils, with open vegetation and intense solar radiation (Tavares 1964Tavares S. 1964. Contribuição ao estudo da cobertura vegetal dos tabuleiros do Nordeste. Boletim de Recursos Naturais 2: 13-25.; Andrade-Lima 1970Andrade-Lima D. 1970. Recursos vegetais de Pernambuco. Boletim Técnico do Instituto de Pesquisas Agronômicas 41: 1-32.), which may explain the higher occurrence of the thermotolerant genera Lichtheimia and Rhizopus in these areas, since the high incidence of solar radiation favors the sporulation and germination of these fungal sporangiospores (Abdullah & Al-Bader 1990Abdullah SK, Al-Bader SM. 1990. On the thermophilic and thermotolerant mycoflora of Iraqi soils. Sydowia 42: 1-7.; Lima et al. 2016aLima DX, Santiago ALCMA, Souza-Motta CM. 2016a. Diversity of Mucorales in natural and degraded semi-arid soils. Brazilian Journal of Botany 39: 1127-1133.).
Although MAN exhibits a soil pH favorable to the growth of Mucorales, it is considered an extreme ecosystem, with low soil oxygenation and high concentration of salinity and sulfides (Hossain & Nuruddin 2016Hossain MD, Nuruddin AA. 2016. Soil and mangrove: a review. Journal of Environmental Science and Technology 9: 198-207.; Doi et al. 2018Doi SA, Pinto AB, Canali MC, Polezel DR, Merguizo RAC, Oliveira AJFC. 2018. Density and Diversity of Filamentous Fungi in the Water and Sediment of Araçá Bay in São Sebastião, São Paulo, Brazil. Biota Neotropica 18: e20170416. http://dx.doi.org/10.1590/1676-0611-BN-2017-0416
http://dx.doi.org/10.1590/1676-0611-BN-2...
), which was corroborated by our soil analysis (Tab. 4, Fig. 4) and seemed determinant for the lower Mucorales richness, abundance and diversity in this area. The lower species richness found in MAN soils explains the low similarity in species composition among MAN and the other areas (Tab. 3). However, species composition among DOF, TAB and SAN areas were quite similar, which suggests homogeneous communities among these areas. Due to the peculiar characteristics of MAN soil, such as high salinity, poor aeration and high temperatures, this ecosystem is a receptive habitat for thermophilic/thermo-tolerant and halophilic/halotolerant fungi (Doi et al. 2018Doi SA, Pinto AB, Canali MC, Polezel DR, Merguizo RAC, Oliveira AJFC. 2018. Density and Diversity of Filamentous Fungi in the Water and Sediment of Araçá Bay in São Sebastião, São Paulo, Brazil. Biota Neotropica 18: e20170416. http://dx.doi.org/10.1590/1676-0611-BN-2017-0416
http://dx.doi.org/10.1590/1676-0611-BN-2...
; Jaitly & Rai 1982Jaitly AK, Rai JN. 1982. Thermophilic and thermotolerant fungi isolated from mangrove swamps. Mycologia 74: 1021-1022.). Only R. stolonifer and S. racemosum were isolated from MAN soils in this study and both are hereby reported for the first time in mangrove sediments in Brazil. Species of these genera have been reported in mangrove ecosystems in India (Senthilkumaran et al. 2016Senthilkumaran R, Sivakumar T, Ravikumar M. 2016. Occurrence, Distribution, Hydrocarbon degradation studies of fungi isolated from South East coast of Tamil Nadu, India. International Journal of Current Research in Biology and Medicine 1: 19-34.) and on substrates with high saline concentration, such as marine algae in the Red Sea, Egypt (Abdel-Gawad et al. 2014Abdel-Gawad KM, Hifney AF, Issa AA, Gomaa M. 2014. Spatio-temporal, environmental factors, and host identity shape culturable-epibiotic fungi of seaweeds in the Red Sea, Egypt. Hydrobiologia 740: 37-49.). According Abdel-Gawad et al. (2014)Abdel-Gawad KM, Hifney AF, Issa AA, Gomaa M. 2014. Spatio-temporal, environmental factors, and host identity shape culturable-epibiotic fungi of seaweeds in the Red Sea, Egypt. Hydrobiologia 740: 37-49., the abundance of Mucorales increases with increased temperature and pH, and decreases with increased salinity. Therefore, the presence of these species in MAN may indicate that they are halotolerant.
The present study highlights the ecology of Mucorales in the Brazilian Atlantic Forest, increasing knowledge of the diversity, richness, frequency of occurrence and relative abundance of Mucorales in the soil of this domain. Gongronella butleri is common in Atlantic Forest ecosystems, except in mangrove ecosystems, which are not suitable for the establishment of most Mucorales species. The present study is a pioneering survey on Mucorales communities in ecosystems of ‘tabuleiro’ forest and Brazilian mangrove. Although the chemical variables analyzed in the soil, such as Al3+ + H+, Na and S, influence Mucorales composition, pH was the key edaphic attribute that influenced the composition, richness and abundance of mucoralean communities. Future research may identify the influence of other variables, such as the availability of organic matter and soil temperature, on the structure of these communities.
Acknowledgements
This study was financed by the project ‘URM Herbarium: characterization and availability of the collection as a source of microbiological resources’ (FACEPE - APQ - 0143-2.12/15) and ‘Diversity of Mucoromycotina in the different ecosystems of the Atlantic Forest of Pernambuco’ (FACEPE - First Projects Program PPP/FACEPE/CNPq - APQ - 0842-2.12/14). We thank the Fundação de Amparo à Ciência e Tecnologia do Estado de Pernambuco for the scholarships awarded to Diogo Xavier Lima. We also thank Roger Fagner Ribeiro Melo for his revision of the manuscript.
References
- Abdel-Gawad KM, Hifney AF, Issa AA, Gomaa M. 2014. Spatio-temporal, environmental factors, and host identity shape culturable-epibiotic fungi of seaweeds in the Red Sea, Egypt. Hydrobiologia 740: 37-49.
- Abdullah SK, Al-Bader SM. 1990. On the thermophilic and thermotolerant mycoflora of Iraqi soils. Sydowia 42: 1-7.
- Almeida JREB, Zickel CS, Pimentel RMM. 2009. Caracterização e espectro biológico da vegetação do litoral arenoso do Rio Grande do Norte. Revista Geografia Norte 23: 66-85.
- Andrade-Lima D. 1970. Recursos vegetais de Pernambuco. Boletim Técnico do Instituto de Pesquisas Agronômicas 41: 1-32.
- Benny GL, Benjamin RK. 1975. Observations on Thamnidiaceae (Mucorales). New taxa, new combinations, and notes on selected species. Aliso 8: 301-351.
- Benny GL. 1982. Zygomycetes. In: Benny GL. (ed.) Synopsis and classification of living organisms. New York, McGraw-Hill Publishing Company, Inc. p. 184-195.
- Benny GL. 2008. The methods used by Dr. R. K. Benjamin, and other mycologists to isolate Zygomycetes. Aliso 26: 37-61.
- Bettuci L, Roquebert MF. 1995. Microfungi from tropical rain Forest litter and soil, a preliminary study. Nova Hedwigia 61: 111-118.
- Bills GF, Christensen M, Powell M, Thom G. 2004. Saprobic soil fungi. In: Mueller GM, Bills GF, Foster MS. (eds.) Biodiversity of Fungi: Inventory and Monitoring Methods. San Diego, Elsevier Academic Press. p. 271-302.
- Bouffaud ML, Creamer RE, Stone D, Plassart P, Tuinen D, Lemanceau P, Wipf D, Redecker D. 2016. Indicator species and co-occurrence in communities of arbuscular mycorrhizal fungi at the European scale. Soil Biology and Biochemistry 103: 464-470.
- Castiglioni DS, Coelho PA. 2011. Determinação da maturidade sexual de Ucides cordatus (Crustacea, Brachyura, Ucididae) em duas áreas de manguezal do litoral sul de Pernambuco, Brasil. Iheringia Série Zoologia 101: 138-144.
- Clarke KR, Gorley RN. 2006. Primer v6 user manual\tutorial. Plymouth, UK, Primer-E Limited.
- Cruz R, Lima JS, Fonseca JC, et al 2013. Diversity of filamentous fungi of area from Brazilian Caatinga and high-level tannase production using mango (Mangifera indica L.) and surinam cherry (Eugenia uniflora L.) leaves under SSF. Advances in Microbiology 3: 52-60.
- Cruz R, Ramos SMS, Fonseca JC, Motta CMDS, Moreira KA. 2017. Anthropization Effects on the Filamentous Fungal Community of the Brazilian Catimbau National Park. Revista Brasileira de Ciência do Solo 41: 1-13.
- Doi SA, Pinto AB, Canali MC, Polezel DR, Merguizo RAC, Oliveira AJFC. 2018. Density and Diversity of Filamentous Fungi in the Water and Sediment of Araçá Bay in São Sebastião, São Paulo, Brazil. Biota Neotropica 18: e20170416. http://dx.doi.org/10.1590/1676-0611-BN-2017-0416
» http://dx.doi.org/10.1590/1676-0611-BN-2017-0416 - Domsch KH, Gams W, Anderson TH. 2007. Compendium of Soil Fungi. Germany, IHW-Verlag Eching.
- Dufrêne M, Legendre P. 1997. Species assemblages and indicator species: the need for a flexible asymmetrical approach. Ecological Monographs 67: 345-366.
- Eicker A. 1969. Microfungi from surface soil of forest communities in Zululand. Transactions of the British Mycological Society 53: 381-392.
- Embrapa - Empresa Brasileira de Pesquisa Agropecuaria. 1998. Análises químicas para avaliação da fertilidade do solo. In: Silva FC, Eira PA, Barreto WO, Perez DV, Silva CA. (eds.) Embrapa. Brasília, Empresa Brasileira de Pesquisa Agropecuária. p. 1-40.
- Ferreira JA, Lennartsson PR, Edebo L, Taherzadeh MJ. 2013. Zygomycetes-based biorefinery: Present status and future prospects. Bioresource Technology 135: 523-532.
- Flora do Brasil 2020 em Construção. 2020. http://floradobrasil.jbrj.gov.br/ 10 Apr. 2019.
» http://floradobrasil.jbrj.gov.br/ - Glassman SI, Wang IJ, Bruns TD. 2017. Environmental filtering by pH and soil nutrients drives community assembly in fungi at fine spatial scales. Molecular ecology 26(24): 6960-6973.
- Hammer Ø, Harper DAT, Ryan, PD. 2001. PAST: paleontological statistics software package for education and data analysis. Palaeontologia Electronica 4: 1-9.
- Heijden MG, Bardgett RD, Straalen NM. 2008. The unseen majority: soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecology Letters 11: 296-310.
- Heinemeyer A, Ridgway KP, Edwards EJ, Benham DG, Young JPW, Fitter AH. 2004. Impact of soil warming and shading on colonization and community structure of arbuscular mycorrhizal fungi in roots of a native grassland community. Global Change Biology 10: 52-64.
- Hesseltine CW, Ellis JJ. 1964. The genus Absidia: Gongronella and cylindrical-spored species of Absidia Mycologia 56: 568-601.
- Hoffmann K, Pawłowska J, Walther G, et al 2013. The family structure of the Mucorales: a synoptic revision based on comprehensive multigene-genealogies. Persoonia: Molecular Phylogeny and Evolution of Fungi 30: 57-76.
- Hossain MD, Nuruddin AA. 2016. Soil and mangrove: a review. Journal of Environmental Science and Technology 9: 198-207.
- Hyde KD, Sarma VV. 2001. A review on frequently occurring fungi in mangroves. Fungal Diversity. 8: 1-34.
- IBAMA - Instituto Brasileiro do Meio Ambiente e dos Recursos Naturais Renováveis. 2003. Resumo executivo do plano de manejo da Reserva Biológica de Saltinho. Brasília, DF, IBAMA. http://www.icmbio.gov.br/portal/images/stories/imgs-unidades-coservacao/pm_rebio_saltinho_encartes.pdf
» http://www.icmbio.gov.br/portal/images/stories/imgs-unidades-coservacao/pm_rebio_saltinho_encartes.pdf - IBGE-Instituto Brasileiro de Geografia e Estatística. 2011. http://www.ibge.gov.br 19 Apr. 2019.
» http://www.ibge.gov.br - Jackson ML. 2005. Soil chemical analysis: Advanced course. Madison, Libraries Parallel Press.
- Jaitly AK, Rai JN. 1982. Thermophilic and thermotolerant fungi isolated from mangrove swamps. Mycologia 74: 1021-1022.
- Lauber CL, Strickland MS, Bradford MA, Fierer N. 2008. The influence of soil properties on the structure of bacterial and fungal communities across land-use types. Soil Biology and Biochemistry 40: 2407-2415.
- Lima DX, Santiago ALCMA, Souza-Motta CM. 2016a. Diversity of Mucorales in natural and degraded semi-arid soils. Brazilian Journal of Botany 39: 1127-1133.
- Lima DX, Voigt K, Souza CA, Oliveira RJ, Souza-Motta CM, Santiago ALCMA. 2016b. Description of Backusella constricta sp. nov. (Mucorales, ex Zygomycota) from the Brazilian Atlantic Rainforest, including a key to species of Backusella Phytotaxa 289: 59-68.
- Lima DX, Cordeiro TRL, Souza CAF, Santiago ALCMA, Souza-Motta CM. 2018a. Diversity of basal fungal order Mucorales (Mucoromycota) in a remaining area of the Brazilian Atlantic Rainforest. Nova Hedwigia 107: 459-471.
- Lima DX, Souza CAF, Oliveira RJV, Bezerra JL, Santiago ALCMA, Souza-Motta CM. 2018b. Mucor irregularis, a first record for South America. Mycotaxon 133: 429-438.
- Maia LC, Cavalcanti MA, Gibertoni T, Goto BT, Melo AMM, Baseia IG, Silvério ML. 2006. Fungos. In: Grillo AS, Oliveira MA, Porto K, Almeida-Cortez JS, Tabarelli M. (eds.) Diversidade biológica e conservação da Floresta Atlântica ao norte do Rio São Francisco. Brasília, Ministério do Meio Ambiente. p. 74-106.
- Myers N, Mittermeier RA, Mittermeier CG, Fonseca GAB, Kent K. 2000. Biodiversity hotspots for conservation priorities. Nature 403: 853-845.
- Ogbonna CIC, Pugh GJF. 1982. Nigerian soil fungi. Nova Hedwigia 36:795-808.
- Oliveira LG, Cavalcanti MAQ, Fernandes MJS, Lima DMM. 2013. Diversity of filamentous fungi isolated from the soil in the semiarid area, Pernambuco. Journal of Arid Environments 95: 49-54.
- Oliveira-Filho AT, Carvalho DA. 1993. Florística e fisionomia da vegetação no extremo norte do litoral da Paraíba. Revista Brasileira de Botânica 16:115-130
- Pandey A, Palni LMS. 2007. The rhizosphere effect in trees of the Indian Central Himalaya with special reference to altitude. Applied Ecology and Environmental Research 5: 93-102.
- Persiani AM, Maggi O, Casado MA, Pineda FD. 1998. Diversity and variability in soil fungi from a disturbed tropical rain forest. Mycologia 90: 206-214.
- Pfenning LH, Abreu LM. 2006. Diversity of microfungi in tropical soils. In: Moreira FM, Siqueira JO, Brussaard L. (eds.) Soil Biodiversity in Amazonian and Other Brazilian Ecosystems. Oxfordshire, CABI Publishing. p. 184-205.
- Rambelli A, Persiani AM, Maggi O, Onofri S, Riess S, Dowgiallo G, Zucconi L. 1984. Comparative studies on microfungi in tropical ecosystems. Further mycological studies in South Western Ivory Coast forest. Plant Biosystems 118: 201-243.
- Rezende CL, Scarano FR, Assad ED, et al 2018. From hotspot to hopespot: An opportunity for the Brazilian Atlantic Forest. Perspectives in Ecology and Conservation 16: 208-214.
- Richardson M. 2009. The Ecology of the Zygomycetes and its impact on environmental exposure. Clinical Microbiology and Infection 15: 2-9.
- Richardson MD, Rautemaa-Richardson R. 2020. Biotic environments supporting the persistence of clinically relevant Mucormycetes. Journal of Fungi 6: 2-14.
- Rodrigues RC, Araujo HF, Lyra-Neves RM, Telino-Júnior WR, Botelho CNM. 2010. Caracterização da Avifauna na Área de Proteção Ambiental de Guadalupe, Pernambuco. Ornithologia 2: 47-61.
- Rousk J, Bååth E, Brookes PC, et al 2010. Soil bacterial and fungal communities across a pH gradient in an arable soil. The Multidisciplinary Journal of Microbial Ecology 4: 1340-1351.
- RStudio Team. 2019. RStudio: Integrated Development for R. Boston, USA, RStudio, Inc. URL URL http://www.rstudio.com/ 10 Apr. 2019.
» http://www.rstudio.com/ - Sampaio D, Souza VC, Oliveira AA, Paula-Souza J, Rodrigues RR. 2005. Árvores da Restinga: Guia de identificação. São Paulo, Editora Neotrópica.
- Santiago ALCMA, Souza-Motta CM. 2006. Mucorales isolados do solo de mineração de cobre e produção de amilase e inulinase. Acta Botanica Brasilica 20: 641-647.
- Santiago ALCMA, Santos PJP, Maia LC. 2013. Mucorales from the semiarid of Pernambuco, Brazil. Brazilian Journal of Microbiology 4: 1678-4405.
- Schipper MAA. 1984. A revision of the genus Rhizopus I. The Rhizopus stolonifer group and Rhizopus oryzae Studies in Mycology 25: 1-34.
- Schipper MAA. 1990. On certain species of Mucor with a key to all accepted species. Studies in Mycology 25: 1-53.
- Schoenlein-Crusius IH, Trufem SFB, Malatinsky SMM, Ninomiya A, Antunes MFR. 1996. Mucorales (Zygomycotina) from soil affected by excrement of birds in the "Parque Estadual das Fontes do Ipiranga", São Paulo, Brazil. Brazilian Journal of Botany 19: 7-10.
- Schoenlein-Crusius IH, Milanez AI. 1997. Mucorales (Zygomycotina) da Mata Atlântica da reserva biológica do Alto da Serra de Paranapiacaba, Santo André, SP. Acta Botanica Brasilica 11: 95-101.
- Schoenlein-Crusius IH, Milanez AI. 1998. Fungos microscópicos da Mata Atlântica de Paranapiacaba, São Paulo, Brasil. Brazilian Journal of Botany 21: 73-79.
- Schoenlein-Crusius IH, Milanez AI, Trufem SFB, Pires-Zottarelli CLA, Grandi RAP. 2006. Microscopic fungi in the Atlantic Rainforest in Cubatão, São Paulo, Brazil. Brazilian Journal of Microbiology 37: 267-275.
- Senthilkumaran R, Sivakumar T, Ravikumar M. 2016. Occurrence, Distribution, Hydrocarbon degradation studies of fungi isolated from South East coast of Tamil Nadu, India. International Journal of Current Research in Biology and Medicine 1: 19-34.
- Spatafora JW, Chang Y, Benny GL et al 2016. A phylum-level phylogenetic classification of zygomycete fungi based on genome-scale data. Mycologia 108: 1028-1046.
- Tabarelli M, Pinto LP, Silva JMC, Hirota MM, Bedê LC. 2005. Desafios e oportunidades para a conservação da biodiversidade na Mata Atlântica brasileira. Megadiversidade 1: 132-138.
- Tavares S. 1964. Contribuição ao estudo da cobertura vegetal dos tabuleiros do Nordeste. Boletim de Recursos Naturais 2: 13-25.
- Tedersoo L, Sáncez-Ramírez S, Kõljalg U, et al 2018. High-level classification of the Fungi and a tool for evolutionary ecological analyses. Fungal Diversity 90: 135-159.
- Varghese G. 1972. Soil microflora of plantations natural Rain Forest of West Malaysia. Mycopathologia et Mycologia Applicata 48: 43-61.
- Zheng R, Chen G. 2001. A monograph of Cunninghamella Mycotaxon 80: 1-75.
- Zheng R, Chen G, Huang H, Liu X. 2007. A monograph of Rhizopus Sydowia 59: 273-372.
- Ziaee A, Zia M, Bayat M, Hashemi J. 2016. Identification of Mucorales isolates from soil using morphological and molecular methods. Current Medical Mycology 2: 13-19.
- Zuur A, Ieno E, Walker N, Saveliev A, Smith G. 2009. Mixed effects models and extensions in ecology with R. German, Springer Science & Business Media.
Publication Dates
-
Publication in this collection
22 Mar 2021 -
Date of issue
Oct-Dec 2020
History
-
Received
25 Mar 2020 -
Accepted
12 Oct 2020