ABSTRACT
Solanaceae is represented by herbs, shrubs, trees and climbing plants and has cosmopolitan distribution, with a large number of native species in the Neotropical region. This study aimed to characterize the pollen morphology of ten species of Solanaceae (especially species of Cestrum and Solanum) from Cerrado forest fragments in Brazil, in order to contribute to the palynology, taxonomy and conservation of degraded areas. Pollen grains were acetolysed, measured and photographed using light and scanning electron microscopy. Qualitative data were described, and quantitative data were analyzed statistically according to sample size. Morphologically, the studied pollen grains vary in size (small to medium), amb (subcircular to subtriangular), shape (oblate spheroidal to subprolate), aperture details (very long, long or narrow colpi, rounded or tapered at the polar ends, colpi with margo, sometimes with a fastigium, endoapertures can present costa and median constriction) and ornamentation (psilate, rugulate, striate or microreticulate). We observed rugulate or striate pollen grains without fastigium for Cestrum species, whereas psilate or microreticulate pollen grains with fastigium were observed for Solanum species. Qualitative data on diameters and aperture measurements were also found to be important in characterizing the two genera. The results obtained here confirm Solanaceae as an eurypalynous family.
Keywords:
eurypalynous; multivariate analysis; pollen grains; pollen morphology; Solanales
Introduction
In tropical regions, there is an increasing process of fragmentation of forest habitats, and understanding the transformation of these areas is fundamental for the conservation of regional biodiversity, in addition to assisting in the management of the sustainable use of the remaining biological resources (Necchi 2012Necchi OJr. 2012. Fauna e Flora de Fragmentos Florestais Remanescentes da Região Noroeste do Estado de São Paulo 1st. edn. Ribeirão Preto, Holos Editora.). In order to identify and reinforce the importance and need to preserve and conserve local biodiversity, Ranga et al. (2012)Ranga NT, Rezende AA, Cavasan O, Toniato MTZ, Cielo-Filho R, Stranghetti V. 2012. Caracterização florística de remanescentes de vegetação nativa da região noroeste do Estado de São Paulo. Ribeirão Preto, Holos Editora . conducted a project in fragmented areas of Cerrado in the Northwestern region of the state of São Paulo, Brazil, listing more than 460 plant species, revealing the occurrence of exclusive species and unique characteristics of the analyzed regions. Based on projects similar to these, studies aiming to morphologically characterize native species in these areas of Cerrado contribute with important and useful data in the identification for the preservation of these fragments.
Solanaceae is one of five families of Solanales within the Lamiids group (APG IV 2016APG IV - Angiosperm Phylogeny Group. 2016. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. The Linnean Society of London, Botanical Journal of the Linnean Society 181: 1-20. ) and includes 2,480 species distributed into 102 genera (Stevens 2001Stevens PF. 2001. Angiosperm Phylogeny Website. Version 14, July 2017 (and more or less continuously updated since). http://www.mobot.org/MOBOT/research/APweb/. 20 Jan. 2020.
http://www.mobot.org/MOBOT/research/APwe...
onwards). Solanaceae species are cosmopolitan, but their greatest biodiversity occurs in the western hemisphere, concentrated in the Neotropical region (Olmstead et al. 2008Olmstead RG, Boh L, Migid HA, Santiago-Valentin E, Garcia VF, Collier SM. 2008. A molecular phylogeny of the Solanaceae. Taxon 57: 1158-1181. ; Souza & Lorenzi 2008Souza VC, Lorenzi H. 2008. Botânica Sistemática: Guia ilustrado para identificação das famílias de angiospermas da flora brasileira, baseado em APG II. 2nd. edn. Nova Odessa, Editora Instituto Plantarum.), with the main dispersion centers in Australia and Latin America (Barroso et al. 1991Barroso GM, Peixoto AL, Ichaso CLF, Costa CG, Guimarães EF, Lima HC. 1991. Sistemática de Angiospermas do Brasil. Vol. 3. Viçosa, Universidade Federal de Viçosa.). In Brazil, the family is represented by 504 species, 237 of which are endemic, distributed in its 36 genera, and Solanaceae species can be found in all Brazilian biomes, being widely distributed in the main ecosystems: Atlantic Forest, Amazon and Cerrado (BFG 2015BFG. 2015. Growing knowledge: an overview of seed plant diversity in Brazil. Rodriguésia 66: 1085-1113.; Flora do Brasil 2020 2020Flora do Brasil 2020. 2020. Solanaceae in Flora do Brasil 2020. Jardim Botânico do Rio de Janeiro. http://floradobrasil.jbrj.gov.br/reflora/floradobrasil/FB225. 21 Oct 2020.
http://floradobrasil.jbrj.gov.br/reflora...
).
The family includes species of great economic importance used in food, such as potatoes (Solanum tuberosum) and tomatoes (Solanum lycopersicum), or ornamental (Petunia hybrida). Many species accumulate alkaloids, being characterized as extremely toxic plants, like Atropa belladona Among those native to Brazil, we can highlight Solanum americanum - “maria-pretinha”, an invader of cultures; Solanum lycocarpum, part of the diet of the maned wolf (Chrysocyon brachyurus); and Solanum paniculatum (“jurubeba”), widely used for medicinal purposes; and the ornamental Cestrum spp. “ladies of the night” (Judd et al. 2009Judd WS, Campbell CS, Kellogg EA, Stevens PF, Donoghue MJ. 2009. Sistemática vegetal: um enfoque filogenético. 3rd. edn. Porto Alegre, Artmed Editora.; Souza & Lorenzi 2008Souza VC, Lorenzi H. 2008. Botânica Sistemática: Guia ilustrado para identificação das famílias de angiospermas da flora brasileira, baseado em APG II. 2nd. edn. Nova Odessa, Editora Instituto Plantarum.).
Traditionally, only two subfamilies were recognized in Solanaceae, based on macro-morphological characteristics: Cestroideae, with its straight or slightly bent embryos in small, prismatic to subglobose seeds and typically capsular fruits, and Solanoideae, with its curved embryos contained in flattened discoid seeds and typically berry-like fruits (D'Arcy 1979D’arcy WG. 1979. The classification of the Solanaceae. In: Hawkes JG, Lester RN, Skelding AD. (eds.). The Biology and Taxonomy of the Solanaceae. London, Academic Press. p. 3-48.; 1991D’arcy WG. 1991. The Solanaceae since 1976, with a review of its biogeography. In: Hawkes JG, Lester RN, Nee M, Estrada N. (eds.). Solanaceae III: Taxonomy,Chemistry Evolution. London, Kew, Royal Botanic Gardens. p. 75-138.; Hunziker 1979Hunziker AT. 1979. South American Solanaceae: a synoptic survey. In: Hawkes JG, Lester RN, Skelding AD. (eds.) The Biology and Taxonomy of the Solanaceae . London, Academic Press . p. 49-86.; 2001Hunziker AT. 2001. Genera Solanacearum: The genera of Solanaceae. Illustrated, Arranged According to a New System. Ruggell, Lichtenstein, A.R.G. Gantner Verlag.; Olmstead & Palmer 1992Olmstead RG, Palmer JD. 1992. A chloroplast DNA phylogeny of the Solanaceae: subfamilial relationships and character evolution. Annals of the Missouri Botanical Garden 79: 346-360. ). With the advancement of molecular studies to clarify phylogenetic relationships, new subfamilies have been proposed within Solanaceae. Currently, the family is mainly divided into four subfamilies, viz. Cestroideae, Goetzeoideae, Nicotianoideae and Solanoideae (Martins & Barkman 2005Martins TR, Barkman TJ . 2005. Reconstruction of Solanaceae Phylogeny Using the Nuclear Gene SAMT. Systematic Botany 30: 435-477. ; Olmstead et al. 1999Olmstead RG, Sweere JA, Spangler RE, Bohs L, Palmer JD. 1999. Phylogeny and provisional classification of the Solanaceae based on chloroplast DNA. In: Nee M, Symon D, Lester RN, Jessop J. (eds.) Solanaceae IV: Advances in Biology and Utilization. London, Royal Botanic Gardens, Kew. p. 111-137., 2008Olmstead RG, Boh L, Migid HA, Santiago-Valentin E, Garcia VF, Collier SM. 2008. A molecular phylogeny of the Solanaceae. Taxon 57: 1158-1181. ; Särkinen et al. 2013Särkinen T, Bohs L, Olmstead RG, Knapp S. 2013. A phylogenetic framework for evolutionary study of the nightshades (Solanaceae): a dated 1000-tip tree. BMC Evolutionary Biology 13: 214.), in addition to confirming Cestroideae as a well-supported clade and the ambiguity of characters found in Solanoideae (Jamil et al. 2014Jamil I, Qamarunnisa S, Azhar A, Shinwari ZK, Ali SI, Qaiser M. 2014. Subfamilial relationships within solanaceae as inferred from atpβ-rbcl intergenic spacer. Pakistan Journal of Botany 42: 585-590.).
The pollen morphology of various genera and species of Solanaceae has been described by different types of studies: Pollen morphology of the family in general (Guang-Fang et al. 1985Guang-Fang P, Shu-Ming Z, Su-Qin Z, Dong-Po Z, Yu-Long Z, An-Ming L. 1985. Pollen morphology of chinese Datura and its taxonomic significance. Acta Phytotaxonomica Sinica 23: 29-35.; Bernadello & Luján 1997Bernadello L, Luján MC. 1997. Pollen morphology of tribe Lycieae: Grabowskia, Lycium, Phrodus (Solanaceae). Review of Palaeobotany and Palynology 96: 305-315. ; Khatamsaz & Zangirian 1998Khatamsaz M, Zangirian E. 1998. SEM survey of pollen morphology in iranian species of Hyoscyamus L. (Solanaceae). Iranian Journal of Botany 7: 151-163.; Batista-Franklim & Gonçalves-Esteves 2002Batista-Franklim CPR, Gonçalves-Esteves V. 2002. Morfologia polínica de espécies de Brunfelsia L. (Solanaceae) ocorrentes no Estado do Rio de Janeiro. Brazilian Journal of Botany 25: 137-145. ; Rojas & Laportte 2004Rojas CB, Laportte MC. 2004. Morfologia polínica de Sessea Ruiz y Pavón (Solanaceae: Cestreae). Memoria de la. Fundación. La Salle de Ciencias Naturales, 64: 125-135.; Rodrigues et al. 2016Rodrigues IMC, Falcão BF, Stehmann JR, Bauermann SG. 2016. Pollen morphology in Athenaea Sendtn. and Aureliana Sendtn. (Solanaceae). Palynology 40: 202-215. ; Dhanya & Devipriya 2016Dhanya C, Devipriya V. 2016. Pollen morphological studies on two Solanaceous Genera: Brugmansia Pers. and Datura L. International Journal of Advanced Research 4: 1879-1887. ); Pollen morphology of Cestrum or Solanum (Murry & Eshbaugh 1971Murry LE, Eshbaugh WH. 1971. A palynological study of the Solaninae (Solanaceae). Grana 11: 65-78. ; Silva et al. 2003Silva SN, Carvalho AMV, Santos FAR. 2003. Morfologia polínica de doze espécies de Cestrum L. (Solanaceae) da mata higrófila na Bahia, Brasil. Acta Scientiarum Biological Sciences 25: 439-443. ; Al-Quran 2004Al-Quran S. 2004. Pollen morphology of Solanaceae in Jordan. Pakistan Journal of Biological Sciences 7: 1586-1593. ; Perveen & Qaiser 2007Perveen A, Qaiser M. 2007. Pollen Morphology of family Solanaceae from Pakistan. Pakistan Journal of. Botany 39: 2243-2256.; Batista-Franklim & Gonçalves-Esteves 2008Batista-Franklim CPR, Gonçalves-Esteves V. 2008. Palinologia de Espécies de Solanum L. (Solanaceae A. Juss.) Ocorrentes nas Restingas do Estado do Rio de Janeiro, Brasil. Acta Botanica Brasilica 22: 782-793. ; Lashin 2011Lashin GMA. 2011. Palynology of six species of Solanum (Solanaceae). Life Science Journal 8: 687-697.; Kumar et al. 2015Kumar AVS, Nair MC, Marugan K. 2015. Pollen morphology of selected taxa of the genus Solanum from Southern Western Ghats, Kerala, India. Rheedea 25: 128-145.; Vignoli-Silva et al. 2015Vignoli-Silva M, Batista-Franklim CPR, Correa DSM, Mentz LA, Mendonça CBF, Gonçalves-Esteves V. 2015. Pollen diversity in Cestrum L. (Solanaceae) from extra-Amazonian Brazil. Palynology 39: 76-90. ; Song et al. 2018Song Y, Gu L, Liu J. 2018. Pollen morphology of selected species from the family Solanaceae. Palynology 43: 1-18.) and Pollen flora of some species (Erdtman 1952Erdtman G. 1952. Pollen Morphology and Plant Taxonomy: Angiosperms. Stockholm, Almqvist and Wiksell.; Chung & Huang 1972Chung T, Huang T. 1972. Paleoecological study of Taipei Basin. Taipe Botanical Garden 17: 117-141.; Salgado-Labouriau 1973Salgado-Labouriau ML. 1973. Contribuição à palinologia dos cerrados. Rio de Janeiro, Academia Brasileira de Ciências.; Rao & Ling 1974Rao NA, Ling LF. 1974. Pollen morphology of certain tropical plants. Reinwardtia 9: 153-176. ; Punt & Monna-Brands 1977Punt W, Monna-Brands M. 1977. The Northwest European pollen flora: Solanaceae. Review of Palaeobotany and Palynology 23: 1-30.; Roubik & Moreno 1991Roubik DW, Moreno JE. 1991. Pollen and Spores of Barro Colorado Island. St Louis, Missouri Botanical Garden.; Velásquez & Rangel 1995Velásquez CA, Rangel JO. 1995. Atlas Palinológico de la flora vascular del páramo I. Las famílias más ricas en especies. Caldasia 17: 509-568.; Melhem et al. 2003Melhem TS, Cruz-Barros MAV, Corrêa AS, Makino-Watanabe H, Silvestre-Capelato MSF, Gonçalves-Esteves VL. 2003. Variabilidade polínica em plantas de Campos do Jordão, São Paulo, Brazil. Boletim do Instituto de Botânica de São Paulo 16: 1-104.; Barth & Duarte 2008Barth OM, Duarte SG. 2008. Morfologia Polínica de espécies arbóreas de Solanaceae do Estado de Santa Catarina, Brasil. Hoehnea 35: 379-386. ; Cruz-Barros et al. 2011Cruz-Barros MAV, Silva EL, Gasparino EC, Souza N, Oliveira AC. 2011. Flora Polínica da Reserva do Parque Estadual das Fontes do Ipiranga (São Paulo, Brasil). Família: 136-Solanaceae. Hoehnea 38: 661-685. ; Mercado-Gómez et al. 2013Mercado-Gómez JD, Jiménez-Bulla LC, Sánchez-Montaño LR. 2013. Polen de las Magnoliopsida em el Volcán (Pamplona, Colombia) II: Familias Hypericaceae, Lamiaceae, Lobeliaceae, Polygonaceae, Rhamnaceae, Rosaceae, Rubiaceae, Scrophulariaceae y Solanaceae. Caldasia 35: 409-427.; Silva et al. 2014Silva CI, Fonseca VLI, Groppo M, et al. 2014. Catálogo polínico das plantas usadas por abelhas no Campus da USP de Ribeirão Preto. Ribeirão Preto, SP, Holos., 2016Silva FHM, Santos FAR, Lima LCL. 2016. Flora polínica das caatingas: Estação Biológica de Canudos - Canudos, Bahia, Brasil. Feira de Santana, Micron Bahia.; Lorente et al. 2017Lorente FL, Júnior AAB, Oliveira PE, Pessenda L. 2017. Atlas palinológico: laboratório 14 C-Cena / USP. Piracicaba, Fundação de Estudos Agrários Luiz de Queiroz - FEALQ.). These authors have observed the differences regarding pollen grain aperture and ornamentation of the exine, concluding that the family is eurypalynous.
Given the wide distribution and abundance of Solanaceae species, and even with several studies that have reported the pollen morphology of the family, a better characterization of the pollen grains is necessary in order to extend the existing data in the literature, and seek a better delimitation of species or groups of species. Studies like those of Edmonds (1984)Edmonds JM. 1984. Pollen morphology of Solanum L. section Solanum. Botanical Journal of the Linnean Society 88: 237-251. , Persson et al. (1994)Persson V, Knapp S, Blackmore S. 1994. Pollen morphology and systematic of tribe Juanulloeae A.T. Hunziker (Solanaceae). Review of Palaeobotany and Palynology 83: 1-30. , Knapp et al. (1998)Knapp S, Persson V, Blackmore S. 1998. Pollen morphology and functional dioecy in Solanum (Solanaceae). Plant Systematics and Evolution 210: 113-139. , Stafford & Knapp (2006)Stafford P, Knapp S. 2006. Pollen morphology and systematics of the zygomorphic-flowered nightshades (Solanaceae; Salpiglossideae sensu D’Arcy, 1978 and Cestroideae sensu D’Arcy, 1991, pro parte): a review. Systematics and Biodiversity 4: 173-201. and Du et al. (2017)Du T, Zhao C, Liu J. 2017. The pollen of Solanum L. and its systematic significance. Palynology 42: 1-20. use palynological characters to corroborate the phylogeny of Solanaceae, thus considering pollen morphology of extreme importance in the study of the relationship between species and genera of the family (Song et al. 2018Song Y, Gu L, Liu J. 2018. Pollen morphology of selected species from the family Solanaceae. Palynology 43: 1-18.).
Some studies on the pollen of native species from remaining forest fragments of Cerrado indicate variations in shape, aperture, amb, ornamentation and size of the pollen grains (Souza & Gasparino 2014Souza CN, Gasparino EC. 2014. Pollen morphology of Fridericia Mart. (Bignoniaceae) from Brazilian forest fragments. Brazilian Journal of Botany 37: 83-94. ; Belonsi & Gasparino 2015Belonsi TK, Gasparino EC. 2015. Pollen morphology of Malpighiaceae from Brazilian Forest fragments. Brazilian Journal of Botany 38: 379-393. ; Dutra & Gasparino 2018Dutra FV, Gasparino EC. 2018. Pollen morphology of Rutaceae from Brazilian forest fragments. Palynology 42: 43-54. ; Landi & Gasparino 2018Landi LADC, Gasparino EC. 2018. Palinologia de Amaranthaceae e Araliaceae nativas em fragmentos florestais remanescentes da região noroeste do Estado de São Paulo. Hoehnea 45: 115-125. ; Souza et al. 2019Souza CN, Rezende AA, Gasparino EC. 2019. Pollen morphology of Bignoniaceae from Brazilian forest fragments and its systematic significance. Palynology 43: 333-347. ; Bellonzi et al. 2020Bellonzi TK, Dutra FV, Souza CN, Gasparino EC. 2020. Pollen types of Sapindaceae from Brazilian forest fragments: variations on apertures of the pollen grains. Acta Botanica Brasilica 34: 327-341. ; Dutra et al. 2020Dutra FV, Bellonzi TK, Souza CN, Gasparino EC. 2020. Pollen morphology of Rubiaceae from Cerrado forest fragments: pollen unit, polarity and diversity of the types of apertures. Review of Palaeobotany and Palynology 282: 104297. doi: 10.1016/j.revpalbo.2020.104297
https://doi.org/10.1016/j.revpalbo.2020....
and Soares et al. 2021Soares EL, Landi LADC, Gasparino EC. 2021. Additions to the knowledge of the pollen morphology of some Fabaceae from the cerrado's forest patches of Brazil. Palynology 45: 269-281.); therefore, the aim of this study was to identify morphological characteristics in pollen grains of Solanaceae species, in order to contribute with the palynology, taxonomy and conservation of these species in degraded areas. This analysis also intended to identify which morphological characters of the pollen grains are important in the segregation of native Solanaceae species in Cerrado forest fragments.
Materials and methods
We studied the pollen grains of 10 native species of Solanaceae (Tab. 1) from the remnant forest fragments of Cerrado in the northwest of the State of São Paulo, described by Ranga et al. (2012)Ranga NT, Rezende AA, Cavasan O, Toniato MTZ, Cielo-Filho R, Stranghetti V. 2012. Caracterização florística de remanescentes de vegetação nativa da região noroeste do Estado de São Paulo. Ribeirão Preto, Holos Editora .. This area was chosen to represent the remaining Cerrado forest fragments in the State of São Paulo, and the studied species occur throughout the Brazilian territory, mainly concentrated in the Southeast and South of Brazil (Specieslink 2020Specieslink. 2020. Sistema de Informação Distribuído para Coleções Biológicas. http://splink.cria.org.br./. 12 Jan. 2020.
http://splink.cria.org.br./...
). We analyzed two genera (Cestrum L. and Solanum L.). All Solanaceae species listed in Ranga et al. (2012)Ranga NT, Rezende AA, Cavasan O, Toniato MTZ, Cielo-Filho R, Stranghetti V. 2012. Caracterização florística de remanescentes de vegetação nativa da região noroeste do Estado de São Paulo. Ribeirão Preto, Holos Editora . are new records in the study area. Five of the species analyzed (Cestrum bracteatum Link & Otto, C. nocturnum L., Solanum argenteum Dunal., S. lycocarpum A. St.-Hil. and S. paniculatum L.) are on the IUCN list of threatened species in the category of Least Concern (IUCN 2020IUCN - International Union for Conservation of Nature. 2020. The IUCN Red List of Threatened Species. Version 2020-1. https://www.iucnredlist.org. 19 March 2020.
https://www.iucnredlist.org...
). The pollen material was obtained from samples collected mainly in the remnant forest fragments of the northwest of the State of São Paulo, deposited as dried herbarium specimens in JABU and SJRP herbaria (acronym by Thiers 2019Thiers B. 2019, continuous adapted. Index herbariorum: a global directory of public herbaria and associated staff. New York Botanical Garden’s Virtual herbarium. http://sweetgum.nybg.org/ih/. 03 Feb. 2020.
http://sweetgum.nybg.org/ih/...
, continuous adapted).
Voucher specimens of Solanaceae from Brazilian forest fragments. SJRP = Institution: UNESP, Campus São José Rio Preto; Location: Brazil. São Paulo. São José do Rio Preto and JABU = Institution: UNESP, Campus Jaboticabal; Location: Brazil. São Paulo. São Paulo. * standard specimen.
Pollen grains of all 16 specimens (for each species, when possible, about three specimens were analyzed to confirm the data, considering only one specimen as the standard - Tab. 1) were studied using light microscopy (LM) and scanning electron microscopy (SEM). For the analysis, permanent slides were prepared with pollen material acetolyzed according to Erdtman (1960)Erdtman G. 1960. The acetolysis method. A revised description. Svensk Botanisk Tidskrift 54: 561-564. modified by Melhem et al. (2003)Melhem TS, Cruz-Barros MAV, Corrêa AS, Makino-Watanabe H, Silvestre-Capelato MSF, Gonçalves-Esteves VL. 2003. Variabilidade polínica em plantas de Campos do Jordão, São Paulo, Brazil. Boletim do Instituto de Botânica de São Paulo 16: 1-104.. The pollen grains were measured (pollen diameter, n = 25; aperture and exine thickness, n = 10) seven days after preparation, as established by Salgado-Labouriau et al. (1965)Salgado-Labouriau ML, Vanzolini PE, Melhem TS. 1965. Variation of polar axes and equatorial diameters in pollen grains of two species of Cassia. Grana Palynologica 6: 98-105.. The slides were deposited in the pollen reference collection of the Departamento de Biologia Aplicada à Agropecuária, Unesp, Jaboticabal, SP, Brazil.
A statistical analysis was conducted with the measures taken to obtain the means (x), standard deviation (sx), standard error (s), 95 % confidence intervals (CI), coefficient of variability (V) and range (R) according to Vieira (2011)Vieira S. 2011. Introdução a Bioestatistica. Rio de Janeiro, Elsevier. and Zar (2010)Zar JH. 2010. Biostatistical analysis. New Jersey, Prentice-Hall.. For measures of exine thickness, and length and width of the apertures, only the arithmetic mean was calculated. To compare the values of pollen grain diameters we used the graphs of the MINITAB program which represent the mean and the confidence interval values. A principal component analysis (PCA) and a cluster analysis (hierarchical cluster analysis - HCA) were performed using the programs FITOPAC (Shepherd 1996Shepherd GJ. 1996. Fitopac 1: Manual do usuário. Campinas, Departamento de Botânica, Universidade Estadual de Campinas.) and PC-ORD (McCune & Mefford 2011McCune B, Mefford MJ. 2011. PC-ORD. Multivariate Analysis of Ecological Data (Version 6)’. Oregon, Gleneden Beach, MjM Software.). PCA analysis aims to group the analyzed species according to the metric values of the pollen grains, while in the cluster analysis we want to identify the relationship among the species (similarity data).
We used 14 metric variables for the PCA and HCA: equatorial diameter in polar view (EDPV), polar diameter in equatorial view (PDEV), equatorial diameter in equatorial view (EDEV), length of colpus (CLEG), width of colpus (CWID), margo of colpus (CMAR), length of endoaperture (ELEG), width of endoaperture (EWID), shape (SHAP), exine (EXIN), nexine (NEXI), sexine (SEXI), polar area index (PAI) and width colpus index (WCI). In PCA, the Pearson and Kendall correlation coefficients were considered, and the dendrogram of HCA was produced using the Jaccard distance measure and the Group Average linkage method analyzed the percentage of information (variables) necessary to reach the final number of groups formed.
Pollen terminology follow Punt et al. (2007)Punt W, Hoen PP, Blackmore S, Nilsson S, Le Thomas A. 2007. Glossary of pollen and spore terminology. Review of Paleobotany and Palynology 143: 1-81.; we adopted the polar area index proposed by Faegri & Iversen (1966)Faegri G, Iversen J. 1966. Textbook of modern pollen analysis. Denmark, Scandinavian University Books., colpus width index in accordance with Gasparino et al. (2013)Gasparino EC, Cruz-Barros MAV, Chautems A. 2013. Pollen morphology in Brazilian species of Codonanthe (Mart.) Hanst. and Nematanthus Schrader (Gesneriaceae). Grana 52: 285-274., and pollen description is based on Bellonzi et al. (2020)Bellonzi TK, Dutra FV, Souza CN, Gasparino EC. 2020. Pollen types of Sapindaceae from Brazilian forest fragments: variations on apertures of the pollen grains. Acta Botanica Brasilica 34: 327-341. . Photomicrographs were taken with a Bel Photonics light microscope, for LM photos, and electromicrographs were taken with a JEOL JSM5410.
Results
General description
The pollen grains of the studied Solanaceae species (Tabs. 2, 3, 4; Figs. 1, 2, 3) are monad, isopolar, small to medium, subcircular to subtriangular amb, very small or small polar area, oblate spheroidal to subprolate, 3-colporate with long or very long and narrow colpi, rounded or tapered at the polar ends, colpi with margo, with or without fastigium or ornamented colpus membrane; lalongate endoapertures, sometimes with costa and median constriction. Exine tectate (psilate, rugulate or striate) or semitectate (microreticulate). The sexine is thicker than the nexine.
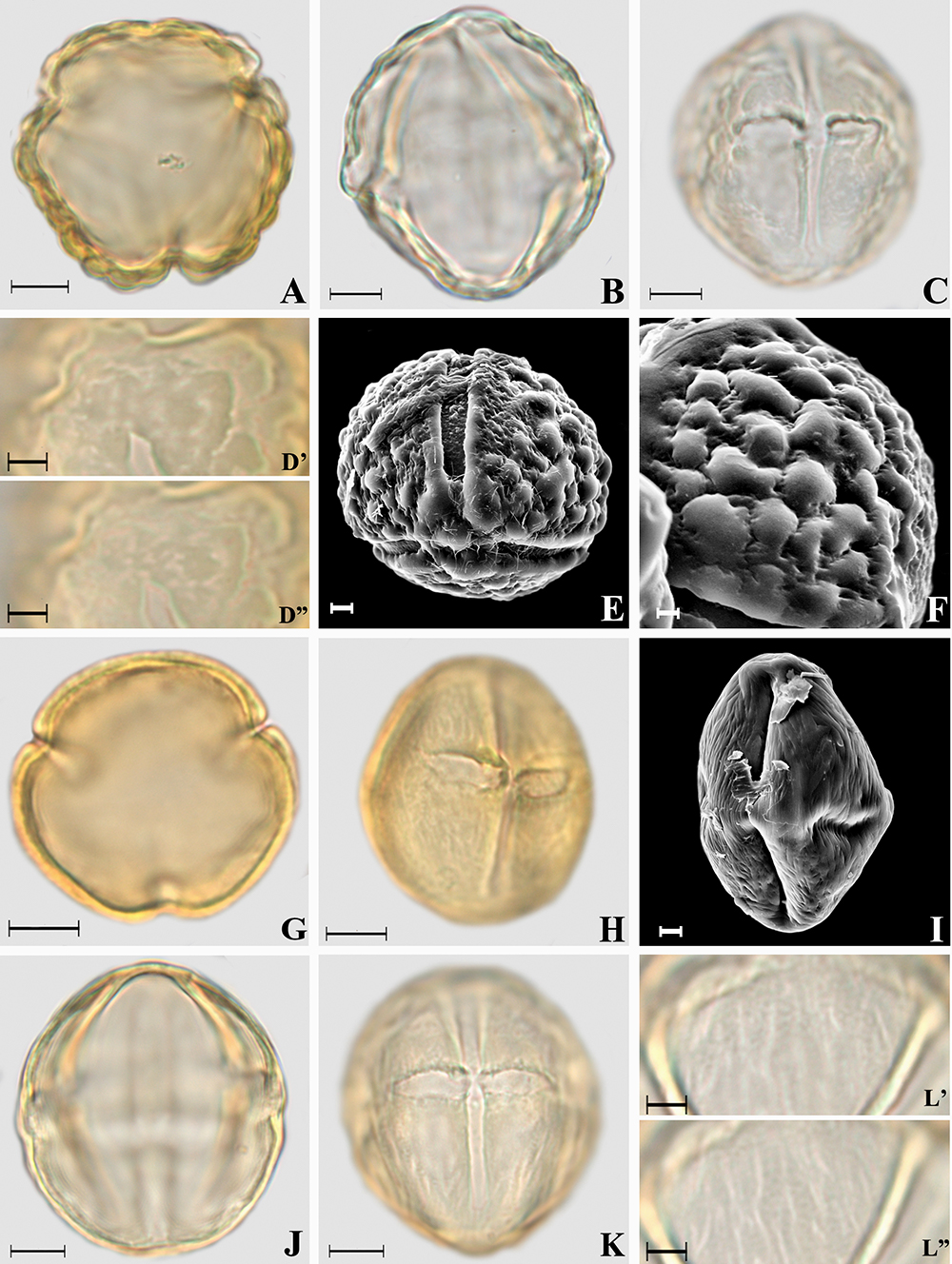
Photomicrographs of Cestrum L. pollen grains. A-F. Cestrum bracteatum. A. Polar view, optical section; B. Equatorial view; C. Equatorial view, colpus and endoaperture; D’-D”. Ornamentation in high and low focus, rugulate exine; E. Polar view, ornamentation in MEV, verrucate exine; F. Ornamentation in SEM details. G-I. Cestrum nocturnum. G. Polar view, optical section; H. Equatorial view, colpus and endoaperture; I. Equatorial view, ornamentation in SEM, striate exine. J-L. Cestrum strigillatum. J. Optical section; K. Equatorial view, colpus and endoaperture; L’-L”. Ornamentation in high and low focus, striate exine. Scale bars: E-F, I = 2 μm; D, L = 5 μm; A-C, G-H, J-K = 10 μm.
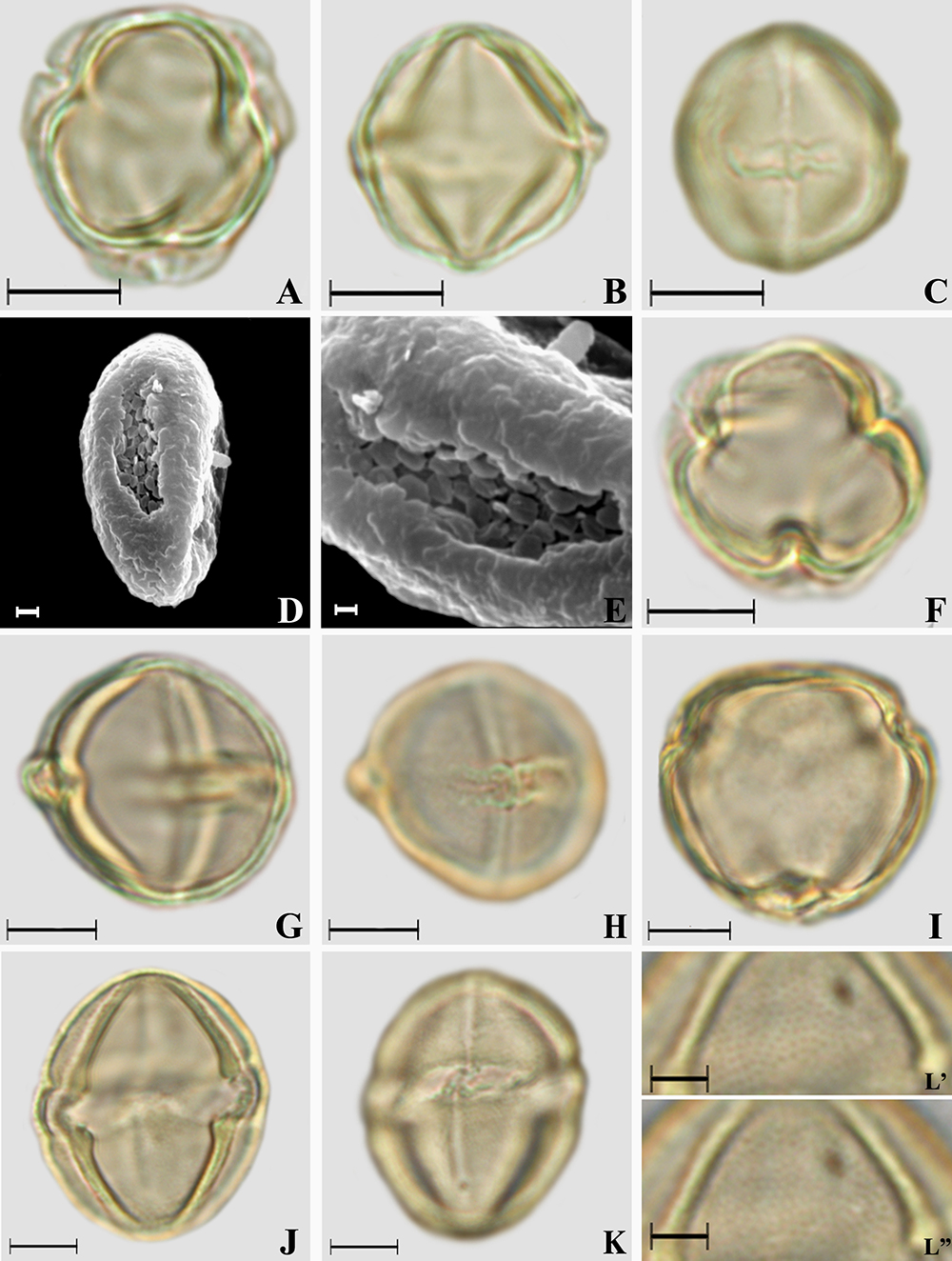
Photomicrographs of Solanum L. pollen grains. A-E. Solanum argenteum. A. Polar view, optical section; B. Equatorial view, optical section; C. Equatorial view, colpus and endoaperture; D. Equatorial view, ornamentation in SEM, granulate exine; E. Ornamentation in SEM, details of the densely verrucate colpus membrane. F-H. Solanum grandiflorum. F. Polar view, optical section; G. Equatorial view, optical section; H. Equatorial view, colpus and endoaperture. I-L. Solanum lycocarpum. I. Polar view, optical section; J. Equatorial view, optical section; K. Equatorial view, colpus and endoaperture; L’-L”. Ornamentation in high and low focus, microreticulate exine. Scale bars: E = 1 μm; D = 2 μm; L = 5 μm; A-C, F-K = 10 μm.
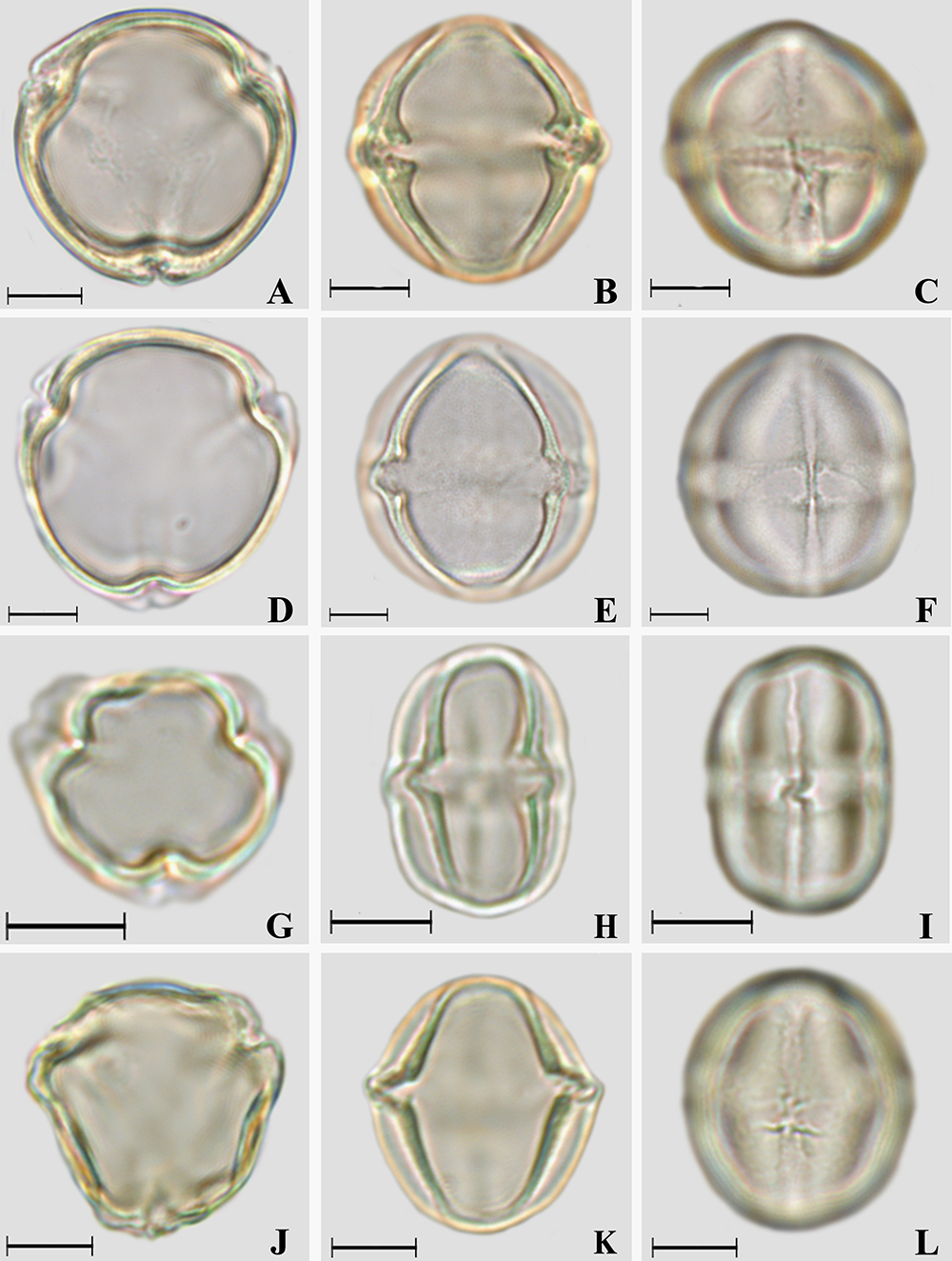
Photomicrographs of Solanum L. pollen grains. A-C. Solanum mauritianum. A. Polar view, optical section; B. Equatorial view, optical section; C. Equatorial view, colpus and endoaperture. D-F. Solanum paniculatum. D. Polar view, optical section; E. Equatorial view, optical section; F. Equatorial view, colpus and endoaperture. G-I. Solanum pseudoquina. G. Polar view, optical section; H. Equatorial view, optical section; I. Equatorial view, colpus and endoaperture. J-L. Solanum ramulosum. J. Polar view, optical section; K. Equatorial view, optical section; L. Equatorial view, colpus and endoaperture. Scale bars: 10 μm.
Genera description
Cestrum (Fig. 1)
Studied species: Cestrum bracteatum (Fig. 1A-F); Cestrum nocturnum (Fig. 1G-I); Cestrum strigillatum (Fig. 1J-L).
Amb and shape: subcircular to subtriangular amb or only subtriangular (Cestrum strigillatum) amb, very small (C. strigillatum) or small polar area, oblate spheroidal (C. bracteatum) to prolate spheroidal (Tab. 2).
Aperture: 3-colporate, long or very long (Cestrum strigillatum) and narrow colpi, rounded (C. strigillatum) or tapered at the polar ends (C. bracteatum and C. nocturnumFig. 1D-E, G), with margo and with ornamented colpus membrane only in C. bracteatum (Fig. 1E), without fastigium (all species). Endoaperture - lalongate (Fig. 1C, H, K -Tabs. 3, 4), without costa and with median constriction (Fig. 1C, H).
Exine: Tectate and rugulate in Cestrum bracteatum (presenting verrucate ornamentation in SEM, Figs. 1E-F); tectate and striate in C. nocturnum and C. strigillatum (Fig. 1H-I, K-L, Tab. 2). The sexine is thicker than the nexine (Tab. 4).
Solanum (Fig. 2-3)
Studied species: Solanum argenteum (Fig. 2A-E), Solanum grandiflorum (Fig. 2F-H), Solanum lycocarpum (Fig. 2I-L), Solanum mauritianum (Fig. 3A-C), Solanum paniculatum (Fig. 3D-F), Solanum pseudoquina (Fig. 3G-I) and Solanum ramulosum (Fig. 3JL).
Size: small (Solanum argenteum and S. pseudoquina), small to medium (S. grandiflorum, S lycocarpum and S. ramulosum) and medium (S. mauritianum and S. paniculatum - Tabs. 2, 3).
Amb and shape: subcircular and subtriangular (S. argenteum and S. ramulosum) amb, very small (S. argenteum) or small polar area, prolate spheroidal to subprolate (Tab. 2).
Aperture: 3-colporate, long or very long (Solanum argenteum) and narrow colpi, rounded (S. mauritianum and S. paniculatum) or tapered at the polar ends, with margo; with or without ornamented colpus membrane (in S. argenteum, the colpus membrane is verrucate under SEM, Figs. 2D-E); with fastigium (all species). Endoaperture - lalongate (Figs. 2C, H, K, 3C, F, I, J - Tab. 4) endoaperture with costa (in S. lycocarpum, S. mauritianum and S. paniculatum - Figs. 2J, 3B, E) or not and with median constriction (only in S. paniculatum - Fig. 3F).
Exine: Semitectate and microreticulate (Solanum lycocarpum, S. mauritianum and S. paniculatum - Figs. 2L, 3B-C, E-F); tectate and psilate (other species), sometimes psilate and slightly granulate in S. argenteum (Figs. 2D-E). The sexine is thicker than the nexine (Tab. 4).
Artificial Key to the Solanaceae taxa studied
1. Pollen grains without fastigium and rugulate or striate ornamentation ............................ 2
1’. Pollen grains with fastigium and psilate or microreticulate ornamentation .................... 4
2. Rugulate ornamentation..................................................................... Cestrum bracteatum
2’. Striate ornamentation ...................................................................................................... 3
3. Very long colpus, rounded at the polar ends ..................................... Cestrum strigillatum
3’. Long colpus, tapered at the polar ends .............................................. Cestrum nocturnum
4. Microreticulate ornamentation, endoaperture with costa ................................................. 5
4’. Psilate ornamentation, endoaperture without costa ........................................................ 7
5. Endoaperture with median constriction ……………………..……. Solanum paniculatum
5’. Endoaperture without median constriction ………………….………………………… 6
6. Colpus tapered at the polar ends …………………………………...Solanum lycocarpum
6’. Colpus rounded at the polar ends …………………….………… Solanum mauritianum
7. Pollen grains with subcircular amb …………………………………………………….. 8
7’. Pollen grains with subtriangular amb …………………………………………………. 9
8. Prolate spheroidal shape …………………………………………Solanum grandiflorum
8’. Subprolate shape ………………………….……………………. Solanum pseudoquina
9. Very long colpus, very small polar area and subprolate shape …..…Solanum argenteum
9’. Long colpus, small polar area and prolate spheroidal shape ……… Solanum ramulosum
Analysis of quantitative data
For the quantitative analysis of pollen grain size, we used the polar and equatorial diameters in equatorial view (Fig. 4). When we observe the mean and confidence interval of the pollen grain diameters, most species present the same order of increasing values for both diameters, with the exception of species Cestrum bracteatum and C. nocturnum, which interchange the values of equatorial diameter (Fig. 4B).
Representation of the confidence interval of the mean in 95 % of the Solanaceae pollen grains. A. Polar diameter in equatorial view. B. Equatorial diameter in equatorial view. The highest and lowest boundaries show the confidence interval; the average circle shows the arithmetic mean. C. brac= Cestrum bracteatum; C. noc= Cestrum nocturnum; C. str= Cestrum strigillatum; S. arg= Solanum argenteum; S. gra= Solanum grandiflorum; S. lyc= Solanum lycocarpum; S. mau= Solanum mauritianum; S. pan= Solanum paniculatum; S. pse= Solanum pseudoquina; S. ram= Solanum ramulosum. The values are in µm.
We found that Solanum argenteum and S. pseudoquina are separated from the other species by lower polar and equatorial diameters. Regarding polar diameter, Cestrum strigillatum has a larger diameter than all other species. When we consider the equatorial diameter, C. bracteatum and C. strigillatum are separated from the other species because they have similar and larger diameters than the others. The other species are in a continuous group of intermediate diameter values (Fig. 4A-B).
When we compare the diameter values, both equatorial and polar, it is evident that Cestrum species differ from Solanum species because they present the highest mean values of the diameters.
PCA is an exploratory analysis of quantitative data valued for Solanaceae pollen grains. This analysis summarizes in its two axes (Fig. 5) 94.28 % of total data variability. The first axis of the PCA explained 70.83 % of the total variability according to the metric variables. This component explained the variance based mainly on endoaperture width (EWID), diameters (EDEV and EDPV) and colpus length (CLEG) (Tab. 5). The species of the genus Cestrum are grouped on the negative side of axis 1, with the highest diameter values (especially equatorial diameter in equatorial view and equatorial diameter in polar view) and the species of the genus Solanum, mainly on the positive side, presenting the lowest values. The second component explains 23.45 % of the variability of the species studied, the values of colpus index (WCI), endoaperture length (ELEG) and colpus width (CWID). Cestrum nocturnum was separated from all other species because it has a high colpus index (WCI).
Principal component analysis performed with the pollen metrical variables from Solanaceae species. EDPV = equatorial diameter in polar view; PDEV = polar diameter in equatorial view; EDEV = equatorial diameter in equatorial view; CLEG = length of colpus; CWID = width of colpus; CMAR = margo of colpus; ELEG = length of endoaperture; EWID = width of endoaperture; SHAP = shape; EXIN = exine; NEXI = nexine; SEXI = sexine; PAI = polar area index; WCI = width colpus index. Species abbreviations in figure 4.
Pearson and Kendall correlation coefficients for pollen grain metric variables of the first and the second axis of principal component analysis (PCA) in Solanaceae species.
The HCA based on the Jaccard linkage method produces a dendrogram with a linkage value of 10.00 %. When considering the percentage of similarity (based in pollen grain metric variables - Tab. 5), two groups were recognized, representing the two genera analyzed (with approximately 35 % of similarity - Fig. 6). With about 35 % of similarity, the Cestrum species were grouped for presenting larger diameter values. Cestrum bracteatum and C. strigillatum (approximately 80 % of similarity) form a group for presenting the highest values of endoaperture length (ELEG). The other species comprise the Solanum group, with about 53 % of similarity. The species Solanum argenteum and S. pseudoquina are closely related with approximately 68 % of similarity for presenting the smallest diameter values among all species. In addition, species S. grandiflorum, S. lycocarpum and S. ramulosum were 94 % similar, for presenting intermediate diameter values within Solanum. The group formed by S. grandiflorum and S. lycocarpum presents similar values for exine (EXIN) and lower values for endoaperture length (ELEG). The species S. mauritianum and S. paniculatum were grouped for presenting the highest diameter values within the genera and the highest values for colpus length (CLEG). Qualitative data were also optimized in Figure 6.
Dendrogram of the hierarchical cluster analysis (HCA) with the pollen metrical variables from Solanaceae species and optimization of qualitative pollen data. The triangle corresponds to the species of Cestrum and the squares correspond to Solanum. Species abbreviations in Figure 4.
Discussion
Several studies, including Erdtman (1952)Erdtman G. 1952. Pollen Morphology and Plant Taxonomy: Angiosperms. Stockholm, Almqvist and Wiksell., Salgado-Labouriau (1973)Salgado-Labouriau ML. 1973. Contribuição à palinologia dos cerrados. Rio de Janeiro, Academia Brasileira de Ciências., Roubik & Moreno (1991)Roubik DW, Moreno JE. 1991. Pollen and Spores of Barro Colorado Island. St Louis, Missouri Botanical Garden., Velásquez & Rangel (1995)Velásquez CA, Rangel JO. 1995. Atlas Palinológico de la flora vascular del páramo I. Las famílias más ricas en especies. Caldasia 17: 509-568., Melhem et al. (2003)Melhem TS, Cruz-Barros MAV, Corrêa AS, Makino-Watanabe H, Silvestre-Capelato MSF, Gonçalves-Esteves VL. 2003. Variabilidade polínica em plantas de Campos do Jordão, São Paulo, Brazil. Boletim do Instituto de Botânica de São Paulo 16: 1-104., Silva et al. (2003)Silva SN, Carvalho AMV, Santos FAR. 2003. Morfologia polínica de doze espécies de Cestrum L. (Solanaceae) da mata higrófila na Bahia, Brasil. Acta Scientiarum Biological Sciences 25: 439-443. , Barth & Duarte (2008)Barth OM, Duarte SG. 2008. Morfologia Polínica de espécies arbóreas de Solanaceae do Estado de Santa Catarina, Brasil. Hoehnea 35: 379-386. , Cruz-Barros et al. (2011)Cruz-Barros MAV, Silva EL, Gasparino EC, Souza N, Oliveira AC. 2011. Flora Polínica da Reserva do Parque Estadual das Fontes do Ipiranga (São Paulo, Brasil). Família: 136-Solanaceae. Hoehnea 38: 661-685. , Mercado-Gómez et al. (2013)Mercado-Gómez JD, Jiménez-Bulla LC, Sánchez-Montaño LR. 2013. Polen de las Magnoliopsida em el Volcán (Pamplona, Colombia) II: Familias Hypericaceae, Lamiaceae, Lobeliaceae, Polygonaceae, Rhamnaceae, Rosaceae, Rubiaceae, Scrophulariaceae y Solanaceae. Caldasia 35: 409-427., Vignoli-Silva et al. (2015)Vignoli-Silva M, Batista-Franklim CPR, Correa DSM, Mentz LA, Mendonça CBF, Gonçalves-Esteves V. 2015. Pollen diversity in Cestrum L. (Solanaceae) from extra-Amazonian Brazil. Palynology 39: 76-90. , Silva et al. (2016)Silva FHM, Santos FAR, Lima LCL. 2016. Flora polínica das caatingas: Estação Biológica de Canudos - Canudos, Bahia, Brasil. Feira de Santana, Micron Bahia. and Song et al. (2018)Song Y, Gu L, Liu J. 2018. Pollen morphology of selected species from the family Solanaceae. Palynology 43: 1-18., describe Cestrum pollen grains as: monad; isopolar or occasionally heteropolar; mostly medium sized; suboblate to prolate shape, circular to triangular amb; 3(4)-colporate or occasionaly syncolporate with margo, displaying equatorial constriction or not, very long or long colpus, narrow, sunken; with or without colpus membrane; distinctly lalongate endoaperture, fastigiated or not, or occasionally zonorate forming ring, with or without constriction; scabrate, striate, striate-rugulate, perfurate-scabrate, rugulate, rugulate-areolate rugulate-striate, or verrucate ornamentation and sexine slightly thicker than the nexine.
Cestrum bracteatum pollen grains have been previously studied by Velásquez & Rangel (1995)Velásquez CA, Rangel JO. 1995. Atlas Palinológico de la flora vascular del páramo I. Las famílias más ricas en especies. Caldasia 17: 509-568., Silva et al. (2003)Silva SN, Carvalho AMV, Santos FAR. 2003. Morfologia polínica de doze espécies de Cestrum L. (Solanaceae) da mata higrófila na Bahia, Brasil. Acta Scientiarum Biological Sciences 25: 439-443. , Cruz-Barros et al. (2011)Cruz-Barros MAV, Silva EL, Gasparino EC, Souza N, Oliveira AC. 2011. Flora Polínica da Reserva do Parque Estadual das Fontes do Ipiranga (São Paulo, Brasil). Família: 136-Solanaceae. Hoehnea 38: 661-685. , Mercado-Gómez et al. (2013)Mercado-Gómez JD, Jiménez-Bulla LC, Sánchez-Montaño LR. 2013. Polen de las Magnoliopsida em el Volcán (Pamplona, Colombia) II: Familias Hypericaceae, Lamiaceae, Lobeliaceae, Polygonaceae, Rhamnaceae, Rosaceae, Rubiaceae, Scrophulariaceae y Solanaceae. Caldasia 35: 409-427. and Vignoli-Silva et al. (2015)Vignoli-Silva M, Batista-Franklim CPR, Correa DSM, Mentz LA, Mendonça CBF, Gonçalves-Esteves V. 2015. Pollen diversity in Cestrum L. (Solanaceae) from extra-Amazonian Brazil. Palynology 39: 76-90. . These studies disagree in terms of colpus structures (presence or absence of fastigium, operculum and constriction) and exine ornamentation (varying among psilate, striate, rugulate-striate and rugulate in the mesocolpus and psilate in the apocolpus, respectively). In our studies, the pollen grains of this species do not present fastigium or operculum, as previously noted, with the presence of the median constriction in the endoaperture; under LM the exine is regulate; however under SEM it is possible to observe verruca in the ornamentation, which is not seen under LM.
Roubik & Moreno (1991)Roubik DW, Moreno JE. 1991. Pollen and Spores of Barro Colorado Island. St Louis, Missouri Botanical Garden. analyzed the pollen grains of Cestrum nocturnum and described them as 3-colporate and syncolporate, long colpi and lalongate endoaperture, like a ring. We observed 3-colporate pollen grains and lalongate endoaperture without a ring. Roubik & Moreno (1991)Roubik DW, Moreno JE. 1991. Pollen and Spores of Barro Colorado Island. St Louis, Missouri Botanical Garden. also described rugulate pollen grains, while in our analysis exine ornamentation is striate, confirmed by scanning electron microscopy (SEM), in some cases it is difficult to define the ornamentation of Cestrum pollen grains only under LM.
Cestrum strigillatum pollen grains have been analyzed by Barth & Duarte (2008)Barth OM, Duarte SG. 2008. Morfologia Polínica de espécies arbóreas de Solanaceae do Estado de Santa Catarina, Brasil. Hoehnea 35: 379-386. and Vignoli-Silva et al. (2015)Vignoli-Silva M, Batista-Franklim CPR, Correa DSM, Mentz LA, Mendonça CBF, Gonçalves-Esteves V. 2015. Pollen diversity in Cestrum L. (Solanaceae) from extra-Amazonian Brazil. Palynology 39: 76-90. . Vignoli-Silva et al. (2015)Vignoli-Silva M, Batista-Franklim CPR, Correa DSM, Mentz LA, Mendonça CBF, Gonçalves-Esteves V. 2015. Pollen diversity in Cestrum L. (Solanaceae) from extra-Amazonian Brazil. Palynology 39: 76-90. described the presence of fastigium and constriction in the endoaperture, and rugulate-striate exine (conspicuous in the mesocolpus and psilate in the apocolpium); for Barth & Duarte (2008)Barth OM, Duarte SG. 2008. Morfologia Polínica de espécies arbóreas de Solanaceae do Estado de Santa Catarina, Brasil. Hoehnea 35: 379-386. , pollen ornamentation is striate. For this species we did not observe fastigium and we can see endoaperture with constriction and striate ornamentation in the pollen grains.
Solanum species had their pollen grains previously studied by Erdtman (1952)Erdtman G. 1952. Pollen Morphology and Plant Taxonomy: Angiosperms. Stockholm, Almqvist and Wiksell., Murry & Eshbaugh (1971)Murry LE, Eshbaugh WH. 1971. A palynological study of the Solaninae (Solanaceae). Grana 11: 65-78. , Chung & Huang (1972)Chung T, Huang T. 1972. Paleoecological study of Taipei Basin. Taipe Botanical Garden 17: 117-141., Salgado-Labouriau (1973)Salgado-Labouriau ML. 1973. Contribuição à palinologia dos cerrados. Rio de Janeiro, Academia Brasileira de Ciências., Rao & Ling (1974)Rao NA, Ling LF. 1974. Pollen morphology of certain tropical plants. Reinwardtia 9: 153-176. , Punt & Monna-Brands (1977)Punt W, Monna-Brands M. 1977. The Northwest European pollen flora: Solanaceae. Review of Palaeobotany and Palynology 23: 1-30., Edmonds (1984)Edmonds JM. 1984. Pollen morphology of Solanum L. section Solanum. Botanical Journal of the Linnean Society 88: 237-251. , Roubik & Moreno (1991)Roubik DW, Moreno JE. 1991. Pollen and Spores of Barro Colorado Island. St Louis, Missouri Botanical Garden., Velásquez & Rangel (1995)Velásquez CA, Rangel JO. 1995. Atlas Palinológico de la flora vascular del páramo I. Las famílias más ricas en especies. Caldasia 17: 509-568., Knapp et al. (1998)Knapp S, Persson V, Blackmore S. 1998. Pollen morphology and functional dioecy in Solanum (Solanaceae). Plant Systematics and Evolution 210: 113-139. , Melhem et al. (2003)Melhem TS, Cruz-Barros MAV, Corrêa AS, Makino-Watanabe H, Silvestre-Capelato MSF, Gonçalves-Esteves VL. 2003. Variabilidade polínica em plantas de Campos do Jordão, São Paulo, Brazil. Boletim do Instituto de Botânica de São Paulo 16: 1-104., Al-Quran (2004Al-Quran S. 2004. Pollen morphology of Solanaceae in Jordan. Pakistan Journal of Biological Sciences 7: 1586-1593. ), Perveen & Qaiser (2007)Perveen A, Qaiser M. 2007. Pollen Morphology of family Solanaceae from Pakistan. Pakistan Journal of. Botany 39: 2243-2256., Batista-Franklim & Gonçalves-Esteves (2008)Batista-Franklim CPR, Gonçalves-Esteves V. 2008. Palinologia de Espécies de Solanum L. (Solanaceae A. Juss.) Ocorrentes nas Restingas do Estado do Rio de Janeiro, Brasil. Acta Botanica Brasilica 22: 782-793. , Barth & Duarte (2008)Barth OM, Duarte SG. 2008. Morfologia Polínica de espécies arbóreas de Solanaceae do Estado de Santa Catarina, Brasil. Hoehnea 35: 379-386. , Cruz-Barros et al. (2011)Cruz-Barros MAV, Silva EL, Gasparino EC, Souza N, Oliveira AC. 2011. Flora Polínica da Reserva do Parque Estadual das Fontes do Ipiranga (São Paulo, Brasil). Família: 136-Solanaceae. Hoehnea 38: 661-685. , Lashin (2011)Lashin GMA. 2011. Palynology of six species of Solanum (Solanaceae). Life Science Journal 8: 687-697., Silva et al. (2014)Silva CI, Fonseca VLI, Groppo M, et al. 2014. Catálogo polínico das plantas usadas por abelhas no Campus da USP de Ribeirão Preto. Ribeirão Preto, SP, Holos., Kumar et al. (2015)Kumar AVS, Nair MC, Marugan K. 2015. Pollen morphology of selected taxa of the genus Solanum from Southern Western Ghats, Kerala, India. Rheedea 25: 128-145., Silva et al. (2016)Silva FHM, Santos FAR, Lima LCL. 2016. Flora polínica das caatingas: Estação Biológica de Canudos - Canudos, Bahia, Brasil. Feira de Santana, Micron Bahia., Du et al. (2017)Du T, Zhao C, Liu J. 2017. The pollen of Solanum L. and its systematic significance. Palynology 42: 1-20. and Lorente et al. (2017)Lorente FL, Júnior AAB, Oliveira PE, Pessenda L. 2017. Atlas palinológico: laboratório 14 C-Cena / USP. Piracicaba, Fundação de Estudos Agrários Luiz de Queiroz - FEALQ.. The authors, in general, describe pollen grains as: monad; isopolar; small to medium size; oblate-spheroidal to subprolate shape; circular to triangular and lobate amb; mostly 3-colporate, occasionally 4-colporate, sometimes 2-6 colporate or pantocolporate, syncolporate or parasyncolporate, colpus very long, long or short, with or without margo, fastigium, operculum, granulate or tuberculate colpus membrane and medium constriction; lalongate endoaperture, sometimes elliptic, with or without medium constriction, occasionally forming constinuous ring, with or without margo, costa and fastigium; granulate, granulate-perforate, granulate-punctate, granulate-punctate-fossulate; granulate-verrucate-punctate, echinate, microechinate, microreticulate, psilate, spinulose, spinulose-perforate, spinulose-punctate, reticulate, rugulate-ganulate, scabrate, verrucate, verrucate-punctate, with or without ornamented operculum and aspis; sexine thicker or thinner than nexine or as thick as nexine.
Roubik & Moreno (1991)Roubik DW, Moreno JE. 1991. Pollen and Spores of Barro Colorado Island. St Louis, Missouri Botanical Garden. and Melhem et al. (2003)Melhem TS, Cruz-Barros MAV, Corrêa AS, Makino-Watanabe H, Silvestre-Capelato MSF, Gonçalves-Esteves VL. 2003. Variabilidade polínica em plantas de Campos do Jordão, São Paulo, Brazil. Boletim do Instituto de Botânica de São Paulo 16: 1-104. studied Solanum argenteum and described 3-colporate pollen grains without fastigium, lalongate endoaperture and psilate sexine; Batista-Franklim & Gonçalves-Esteves (2008)Batista-Franklim CPR, Gonçalves-Esteves V. 2008. Palinologia de Espécies de Solanum L. (Solanaceae A. Juss.) Ocorrentes nas Restingas do Estado do Rio de Janeiro, Brasil. Acta Botanica Brasilica 22: 782-793. also observed 3-colporate pollen grains, but they described fastigium in the lalongate endoaperture (with constriction), scabrate and granulate sexine in SEM. Our study portrayed the pollen grains with margo in the colpus, endoaperture with median constriction and psilate exine ornamentation; in addition, we observed a higher diameter value than those mentioned by the authors. In SEM, it is possible to observe granules in the ornamentation of the pollen grains of this species, as well as an ornamented membrane densely verrucate in the colpus, different from the characteristics of the genus Solanum presented by Du et al. (2017)Du T, Zhao C, Liu J. 2017. The pollen of Solanum L. and its systematic significance. Palynology 42: 1-20., since the authors cite granulate or tuberculate ornamentation distributed on the surface of the colpus.
The pollen grains of Solanum grandiflorum were described by Salgado-Labouriau (1973)Salgado-Labouriau ML. 1973. Contribuição à palinologia dos cerrados. Rio de Janeiro, Academia Brasileira de Ciências. as 3-colporate with long colpi and prominent margo, lalongate endoaperture with margo and occasionally constricted, psilate or granulate ornamentation. For this species, the characteristics found by Salgado-Labouriau (1973)Salgado-Labouriau ML. 1973. Contribuição à palinologia dos cerrados. Rio de Janeiro, Academia Brasileira de Ciências. are very similar to those observed by us. We describe the endoaperture without costa for the pollen grains, following the definition proposed by Punt et al. (2007)Punt W, Hoen PP, Blackmore S, Nilsson S, Le Thomas A. 2007. Glossary of pollen and spore terminology. Review of Paleobotany and Palynology 143: 1-81., since the term margo refers to a differentiated area in the ectocolpus (Iversen & Troels-Smith 1950Iversen J, Troels-Smith J. 1950. Pollenmorphologische Definitonen und Typen. Danmarks Geologiske Undersøgelse, Ser 43: 1-54.).
The species Solanum lycocarpum has been analyzed by several authors (Salgado-Labouriau 1973Salgado-Labouriau ML. 1973. Contribuição à palinologia dos cerrados. Rio de Janeiro, Academia Brasileira de Ciências.; Cruz-Barros et al. 2011Cruz-Barros MAV, Silva EL, Gasparino EC, Souza N, Oliveira AC. 2011. Flora Polínica da Reserva do Parque Estadual das Fontes do Ipiranga (São Paulo, Brasil). Família: 136-Solanaceae. Hoehnea 38: 661-685. ; Du et al. 2017Du T, Zhao C, Liu J. 2017. The pollen of Solanum L. and its systematic significance. Palynology 42: 1-20.; Lorente et al. 2017Lorente FL, Júnior AAB, Oliveira PE, Pessenda L. 2017. Atlas palinológico: laboratório 14 C-Cena / USP. Piracicaba, Fundação de Estudos Agrários Luiz de Queiroz - FEALQ.), which describe the pollen grains as 3-colporate, long colpi with margo, lalongate endoaperture with margo and constriction (Cruz-Barros et al. 2011Cruz-Barros MAV, Silva EL, Gasparino EC, Souza N, Oliveira AC. 2011. Flora Polínica da Reserva do Parque Estadual das Fontes do Ipiranga (São Paulo, Brasil). Família: 136-Solanaceae. Hoehnea 38: 661-685. ; Salgado-Labouriau 1973Salgado-Labouriau ML. 1973. Contribuição à palinologia dos cerrados. Rio de Janeiro, Academia Brasileira de Ciências.) and with conspicuous fastigium (Cruz-Barros et al. 2011Cruz-Barros MAV, Silva EL, Gasparino EC, Souza N, Oliveira AC. 2011. Flora Polínica da Reserva do Parque Estadual das Fontes do Ipiranga (São Paulo, Brasil). Família: 136-Solanaceae. Hoehnea 38: 661-685. ); nevertheless, the authors report differences in exine ornamentation: psilate (Salgado-Labouriau 1973Salgado-Labouriau ML. 1973. Contribuição à palinologia dos cerrados. Rio de Janeiro, Academia Brasileira de Ciências.), rugulate (Cruz-Barros et al. 2011Cruz-Barros MAV, Silva EL, Gasparino EC, Souza N, Oliveira AC. 2011. Flora Polínica da Reserva do Parque Estadual das Fontes do Ipiranga (São Paulo, Brasil). Família: 136-Solanaceae. Hoehnea 38: 661-685. ), granulate (Du et al. 2017Du T, Zhao C, Liu J. 2017. The pollen of Solanum L. and its systematic significance. Palynology 42: 1-20.) and microreticulate (Lorente et al. 2017Lorente FL, Júnior AAB, Oliveira PE, Pessenda L. 2017. Atlas palinológico: laboratório 14 C-Cena / USP. Piracicaba, Fundação de Estudos Agrários Luiz de Queiroz - FEALQ.). We observed margo in the colpi and endoaperture with costa, ornamented membrane, granulate in the colpus, endoaperture without constriction, and psilate ornamentation for S. lycocarpum.
Batista-Franklim & Gonçalves-Esteves (2008)Batista-Franklim CPR, Gonçalves-Esteves V. 2008. Palinologia de Espécies de Solanum L. (Solanaceae A. Juss.) Ocorrentes nas Restingas do Estado do Rio de Janeiro, Brasil. Acta Botanica Brasilica 22: 782-793. , Cruz-Barros et al. (2011)Cruz-Barros MAV, Silva EL, Gasparino EC, Souza N, Oliveira AC. 2011. Flora Polínica da Reserva do Parque Estadual das Fontes do Ipiranga (São Paulo, Brasil). Família: 136-Solanaceae. Hoehnea 38: 661-685. , Silva et al. (2014)Silva CI, Fonseca VLI, Groppo M, et al. 2014. Catálogo polínico das plantas usadas por abelhas no Campus da USP de Ribeirão Preto. Ribeirão Preto, SP, Holos., Kumar et al. (2015)Kumar AVS, Nair MC, Marugan K. 2015. Pollen morphology of selected taxa of the genus Solanum from Southern Western Ghats, Kerala, India. Rheedea 25: 128-145. and Lorente et al. (2017)Lorente FL, Júnior AAB, Oliveira PE, Pessenda L. 2017. Atlas palinológico: laboratório 14 C-Cena / USP. Piracicaba, Fundação de Estudos Agrários Luiz de Queiroz - FEALQ. have studied the species Solanum mauritianum and described 3-colporate pollen grains with margo (ornamented by Lorente et al. 2017Lorente FL, Júnior AAB, Oliveira PE, Pessenda L. 2017. Atlas palinológico: laboratório 14 C-Cena / USP. Piracicaba, Fundação de Estudos Agrários Luiz de Queiroz - FEALQ.), ornamented membrane in the colpus and median constriction in the endoaperture (Cruz-Barros et al. 2011Cruz-Barros MAV, Silva EL, Gasparino EC, Souza N, Oliveira AC. 2011. Flora Polínica da Reserva do Parque Estadual das Fontes do Ipiranga (São Paulo, Brasil). Família: 136-Solanaceae. Hoehnea 38: 661-685. ), lalongate endoaperture with fastigium, without constriction (Batista-Franklim & Gonçalves-Esteves 2008Batista-Franklim CPR, Gonçalves-Esteves V. 2008. Palinologia de Espécies de Solanum L. (Solanaceae A. Juss.) Ocorrentes nas Restingas do Estado do Rio de Janeiro, Brasil. Acta Botanica Brasilica 22: 782-793. ), or with constriction and operculum in the endoaperture (Kumar et al. 2015Kumar AVS, Nair MC, Marugan K. 2015. Pollen morphology of selected taxa of the genus Solanum from Southern Western Ghats, Kerala, India. Rheedea 25: 128-145.) or forming a continuous ring (Silva et al. 2014Silva CI, Fonseca VLI, Groppo M, et al. 2014. Catálogo polínico das plantas usadas por abelhas no Campus da USP de Ribeirão Preto. Ribeirão Preto, SP, Holos.) and granulate, microreticulate and spinulose ornamentation. Our results for S. mauritianum described margo present in the colpus and endoaperture with costa (without constriction) and microreticulate ornamentation, data similar to those of Cruz-Barros et al. (2011)Cruz-Barros MAV, Silva EL, Gasparino EC, Souza N, Oliveira AC. 2011. Flora Polínica da Reserva do Parque Estadual das Fontes do Ipiranga (São Paulo, Brasil). Família: 136-Solanaceae. Hoehnea 38: 661-685. .
Some studies have depicted the pollen grains of Solanum paniculatum: Batista-Franklim & Gonçalves-Esteves (2008)Batista-Franklim CPR, Gonçalves-Esteves V. 2008. Palinologia de Espécies de Solanum L. (Solanaceae A. Juss.) Ocorrentes nas Restingas do Estado do Rio de Janeiro, Brasil. Acta Botanica Brasilica 22: 782-793. described 3-colporate pollen grains with fastigium, colpus with thin margo, lalongate endoaperture, without constriction, and granulate exine. Cruz-Barros et al. (2011)Cruz-Barros MAV, Silva EL, Gasparino EC, Souza N, Oliveira AC. 2011. Flora Polínica da Reserva do Parque Estadual das Fontes do Ipiranga (São Paulo, Brasil). Família: 136-Solanaceae. Hoehnea 38: 661-685. observed ornamented membranes in the colpus and microreticulate exine, while Silva et al. (2016)Silva FHM, Santos FAR, Lima LCL. 2016. Flora polínica das caatingas: Estação Biológica de Canudos - Canudos, Bahia, Brasil. Feira de Santana, Micron Bahia. reported costa in the endoaperture and psilate ornamentation with granulum when observed in SEM. Other morphological characteristics, such as the presence of a vestibulum and endocingulum (Silva et al. 2014Silva CI, Fonseca VLI, Groppo M, et al. 2014. Catálogo polínico das plantas usadas por abelhas no Campus da USP de Ribeirão Preto. Ribeirão Preto, SP, Holos.), and scabrate ornamentation (Lorente et al. 2017Lorente FL, Júnior AAB, Oliveira PE, Pessenda L. 2017. Atlas palinológico: laboratório 14 C-Cena / USP. Piracicaba, Fundação de Estudos Agrários Luiz de Queiroz - FEALQ.) have also been described for the pollen grains of S. paniculatum. In our study we observed 3-colporate pollen grains with margo and ornamented membrane in the colpus, lalongate endoaperture and microreticulate ornamentation, corroborating the data of Cruz-Barros et al. (2011)Cruz-Barros MAV, Silva EL, Gasparino EC, Souza N, Oliveira AC. 2011. Flora Polínica da Reserva do Parque Estadual das Fontes do Ipiranga (São Paulo, Brasil). Família: 136-Solanaceae. Hoehnea 38: 661-685. . Analyzing the definitions of the palynological glossaries (especially Punt et al. 2007Punt W, Hoen PP, Blackmore S, Nilsson S, Le Thomas A. 2007. Glossary of pollen and spore terminology. Review of Paleobotany and Palynology 143: 1-81.) we did not observe structures like vestibulum and endocingulum for the pollen grains of this species.
Solanum pseudoquina pollen grains have been previously studied by Melhem et al. (2003)Melhem TS, Cruz-Barros MAV, Corrêa AS, Makino-Watanabe H, Silvestre-Capelato MSF, Gonçalves-Esteves VL. 2003. Variabilidade polínica em plantas de Campos do Jordão, São Paulo, Brazil. Boletim do Instituto de Botânica de São Paulo 16: 1-104., Batista-Franklim & Gonçalves-Esteves (2008)Batista-Franklim CPR, Gonçalves-Esteves V. 2008. Palinologia de Espécies de Solanum L. (Solanaceae A. Juss.) Ocorrentes nas Restingas do Estado do Rio de Janeiro, Brasil. Acta Botanica Brasilica 22: 782-793. and Cruz-Barros et al. (2011)Cruz-Barros MAV, Silva EL, Gasparino EC, Souza N, Oliveira AC. 2011. Flora Polínica da Reserva do Parque Estadual das Fontes do Ipiranga (São Paulo, Brasil). Família: 136-Solanaceae. Hoehnea 38: 661-685. , who have described 3-colporate pollen grains, with fastigium, lalongate endoaperture and slightly granulate ornamentation (Melhem et al. 2003Melhem TS, Cruz-Barros MAV, Corrêa AS, Makino-Watanabe H, Silvestre-Capelato MSF, Gonçalves-Esteves VL. 2003. Variabilidade polínica em plantas de Campos do Jordão, São Paulo, Brazil. Boletim do Instituto de Botânica de São Paulo 16: 1-104.), scabrate in LM and rugulate in SEM (Batista-Franklim & Gonçalves-Esteves 2008Batista-Franklim CPR, Gonçalves-Esteves V. 2008. Palinologia de Espécies de Solanum L. (Solanaceae A. Juss.) Ocorrentes nas Restingas do Estado do Rio de Janeiro, Brasil. Acta Botanica Brasilica 22: 782-793. ) and scabrate (Cruz-Barros et al. 2011Cruz-Barros MAV, Silva EL, Gasparino EC, Souza N, Oliveira AC. 2011. Flora Polínica da Reserva do Parque Estadual das Fontes do Ipiranga (São Paulo, Brasil). Família: 136-Solanaceae. Hoehnea 38: 661-685. ). We observed fastigium, margo in the colpus, lalongate endoaperture without costa and psilate ornamentation for S. pseudoquina.
Du et al. (2017)Du T, Zhao C, Liu J. 2017. The pollen of Solanum L. and its systematic significance. Palynology 42: 1-20. presented the only study for Solanum ramulosum pollen grains, and the authors described small pollen grains and granulate ornamentation. We observed for this species variation between small and medium pollen grains and slightly granulate or psilate ornamentation, corroborating the data found by Du et al. (2017)Du T, Zhao C, Liu J. 2017. The pollen of Solanum L. and its systematic significance. Palynology 42: 1-20..
The qualitative data on pollen grains are similar among the species of the same genera; however, they allow the differentiation between Cestrum and Solanum, mainly due to the presence or absence of fastigium (which was confirmed by the quantitative data of the pollen grains). Exine ornamentation patterns can vary among the species of the studied genera: rugulate or striate exine was observed for Cestrum species and microreticulate or psilate exine (sometimes finely granulate) for Solanum pollen grains (see the artificial pollen key). Nonetheless, previous studies have shown that ornamentation patterns in Solanaceae pollen grains can be very variable.
As observed by the quantitative data (PCA and cluster analysis), the measurements of the pollen grain diameters, as well as the length and width of the apertures, helped to distinguish among the studied genera. Although there are other studies on Solanaceae palynology, it is the first time that the importance of these detailed quantitative data in the separation of Cestrum and Solanum is reported. Among the Solanum species, it is interesting to highlight the qualitative similarity of S. grandiflorum and S. ramulosum (artificial pollen key); nonetheless, we can differentiate the pollen grains of these species by higher endoaperture values (in S. ramulosum) and higher exine values (in S. grandiflorum).
When we compare the quantitative data of Solanaceae pollen grains with recent studies conducted in the same area (forest fragments from Cerrado), we can verify that the values of diameters combined with aperture measurements are also important for species segregation in Fridericia (Souza & Gasparino 2014Souza CN, Gasparino EC. 2014. Pollen morphology of Fridericia Mart. (Bignoniaceae) from Brazilian forest fragments. Brazilian Journal of Botany 37: 83-94. ), Malpighiaceae (Belonsi & Gasparino 2015Belonsi TK, Gasparino EC. 2015. Pollen morphology of Malpighiaceae from Brazilian Forest fragments. Brazilian Journal of Botany 38: 379-393. ) and Sapindaceae (Bellonzi et al. 2020Bellonzi TK, Dutra FV, Souza CN, Gasparino EC. 2020. Pollen types of Sapindaceae from Brazilian forest fragments: variations on apertures of the pollen grains. Acta Botanica Brasilica 34: 327-341. ). For Bignoniaceae (Souza et al. 2019Souza CN, Rezende AA, Gasparino EC. 2019. Pollen morphology of Bignoniaceae from Brazilian forest fragments and its systematic significance. Palynology 43: 333-347. ) and Rubiaceae (Dutra et al. 2020Dutra FV, Bellonzi TK, Souza CN, Gasparino EC. 2020. Pollen morphology of Rubiaceae from Cerrado forest fragments: pollen unit, polarity and diversity of the types of apertures. Review of Palaeobotany and Palynology 282: 104297. doi: 10.1016/j.revpalbo.2020.104297
https://doi.org/10.1016/j.revpalbo.2020....
) species, the pollen grain diameters associated with other metric data help the recognition of some taxa. The same is true when considering the measurements of apertures for the studies with Rutaceae (Dutra & Gasparino 2018Dutra FV, Gasparino EC. 2018. Pollen morphology of Rutaceae from Brazilian forest fragments. Palynology 42: 43-54. ) and Caesalpinioideae (Soares et al. 2021Soares EL, Landi LADC, Gasparino EC. 2021. Additions to the knowledge of the pollen morphology of some Fabaceae from the cerrado's forest patches of Brazil. Palynology 45: 269-281.).
Therefore, we can conclude that: 1) The main characteristics that differentiate the genera in this study is the absence of fastigium and rugulate or striate exine ornamentation for pollen grains of Cestrum, and pollen grains with fastigium with microreticulate or psilate exine ornamentation for Solanum species; 2) Among the quantitative data, the values of pollen grain diameter allow the grouping of the species, with the Cestrum species having the highest values and the Solanum species, the lowest values. In addition, the width of the endoaperture and the length of the colpus are always greater in the Cestrum species when compared to the pollen grains of Solanum; 3) The morphological variation found in this study, besides being important in the characterization of the genera, confirm Solanaceae as an eurypalynous family.
Acknowledgements
The authors are grateful to the JABU and SJRP herbaria for the support in the collection of plant materials and the Laboratório de Microscopia Eletrônica, FCAV for the analysis in SEM.
References
- Al-Quran S. 2004. Pollen morphology of Solanaceae in Jordan. Pakistan Journal of Biological Sciences 7: 1586-1593.
- APG IV - Angiosperm Phylogeny Group. 2016. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. The Linnean Society of London, Botanical Journal of the Linnean Society 181: 1-20.
- Barroso GM, Peixoto AL, Ichaso CLF, Costa CG, Guimarães EF, Lima HC. 1991. Sistemática de Angiospermas do Brasil. Vol. 3. Viçosa, Universidade Federal de Viçosa.
- Barth OM, Duarte SG. 2008. Morfologia Polínica de espécies arbóreas de Solanaceae do Estado de Santa Catarina, Brasil. Hoehnea 35: 379-386.
- Batista-Franklim CPR, Gonçalves-Esteves V. 2002. Morfologia polínica de espécies de Brunfelsia L. (Solanaceae) ocorrentes no Estado do Rio de Janeiro. Brazilian Journal of Botany 25: 137-145.
- Batista-Franklim CPR, Gonçalves-Esteves V. 2008. Palinologia de Espécies de Solanum L. (Solanaceae A. Juss.) Ocorrentes nas Restingas do Estado do Rio de Janeiro, Brasil. Acta Botanica Brasilica 22: 782-793.
- Bellonzi TK, Dutra FV, Souza CN, Gasparino EC. 2020. Pollen types of Sapindaceae from Brazilian forest fragments: variations on apertures of the pollen grains. Acta Botanica Brasilica 34: 327-341.
- Belonsi TK, Gasparino EC. 2015. Pollen morphology of Malpighiaceae from Brazilian Forest fragments. Brazilian Journal of Botany 38: 379-393.
- Bernadello L, Luján MC. 1997. Pollen morphology of tribe Lycieae: Grabowskia, Lycium, Phrodus (Solanaceae). Review of Palaeobotany and Palynology 96: 305-315.
- BFG. 2015. Growing knowledge: an overview of seed plant diversity in Brazil. Rodriguésia 66: 1085-1113.
- Chung T, Huang T. 1972. Paleoecological study of Taipei Basin. Taipe Botanical Garden 17: 117-141.
- Cruz-Barros MAV, Silva EL, Gasparino EC, Souza N, Oliveira AC. 2011. Flora Polínica da Reserva do Parque Estadual das Fontes do Ipiranga (São Paulo, Brasil). Família: 136-Solanaceae. Hoehnea 38: 661-685.
- D’arcy WG. 1979. The classification of the Solanaceae. In: Hawkes JG, Lester RN, Skelding AD. (eds.). The Biology and Taxonomy of the Solanaceae. London, Academic Press. p. 3-48.
- D’arcy WG. 1991. The Solanaceae since 1976, with a review of its biogeography. In: Hawkes JG, Lester RN, Nee M, Estrada N. (eds.). Solanaceae III: Taxonomy,Chemistry Evolution. London, Kew, Royal Botanic Gardens. p. 75-138.
- Dhanya C, Devipriya V. 2016. Pollen morphological studies on two Solanaceous Genera: Brugmansia Pers. and Datura L. International Journal of Advanced Research 4: 1879-1887.
- Du T, Zhao C, Liu J. 2017. The pollen of Solanum L. and its systematic significance. Palynology 42: 1-20.
- Dutra FV, Gasparino EC. 2018. Pollen morphology of Rutaceae from Brazilian forest fragments. Palynology 42: 43-54.
- Dutra FV, Bellonzi TK, Souza CN, Gasparino EC. 2020. Pollen morphology of Rubiaceae from Cerrado forest fragments: pollen unit, polarity and diversity of the types of apertures. Review of Palaeobotany and Palynology 282: 104297. doi: 10.1016/j.revpalbo.2020.104297
» https://doi.org/10.1016/j.revpalbo.2020.104297 - Edmonds JM. 1984. Pollen morphology of Solanum L. section Solanum Botanical Journal of the Linnean Society 88: 237-251.
- Erdtman G. 1952. Pollen Morphology and Plant Taxonomy: Angiosperms. Stockholm, Almqvist and Wiksell.
- Erdtman G. 1960. The acetolysis method. A revised description. Svensk Botanisk Tidskrift 54: 561-564.
- Faegri G, Iversen J. 1966. Textbook of modern pollen analysis. Denmark, Scandinavian University Books.
- Flora do Brasil 2020. 2020. Solanaceae in Flora do Brasil 2020. Jardim Botânico do Rio de Janeiro. http://floradobrasil.jbrj.gov.br/reflora/floradobrasil/FB225 21 Oct 2020.
» http://floradobrasil.jbrj.gov.br/reflora/floradobrasil/FB225 - Gasparino EC, Cruz-Barros MAV, Chautems A. 2013. Pollen morphology in Brazilian species of Codonanthe (Mart.) Hanst. and Nematanthus Schrader (Gesneriaceae). Grana 52: 285-274.
- Guang-Fang P, Shu-Ming Z, Su-Qin Z, Dong-Po Z, Yu-Long Z, An-Ming L. 1985. Pollen morphology of chinese Datura and its taxonomic significance. Acta Phytotaxonomica Sinica 23: 29-35.
- Hunziker AT. 1979. South American Solanaceae: a synoptic survey. In: Hawkes JG, Lester RN, Skelding AD. (eds.) The Biology and Taxonomy of the Solanaceae . London, Academic Press . p. 49-86.
- Hunziker AT. 2001. Genera Solanacearum: The genera of Solanaceae. Illustrated, Arranged According to a New System. Ruggell, Lichtenstein, A.R.G. Gantner Verlag.
- IUCN - International Union for Conservation of Nature. 2020. The IUCN Red List of Threatened Species. Version 2020-1. https://www.iucnredlist.org 19 March 2020.
» https://www.iucnredlist.org - Iversen J, Troels-Smith J. 1950. Pollenmorphologische Definitonen und Typen. Danmarks Geologiske Undersøgelse, Ser 43: 1-54.
- Jamil I, Qamarunnisa S, Azhar A, Shinwari ZK, Ali SI, Qaiser M. 2014. Subfamilial relationships within solanaceae as inferred from atpβ-rbcl intergenic spacer. Pakistan Journal of Botany 42: 585-590.
- Judd WS, Campbell CS, Kellogg EA, Stevens PF, Donoghue MJ. 2009. Sistemática vegetal: um enfoque filogenético. 3rd. edn. Porto Alegre, Artmed Editora.
- Khatamsaz M, Zangirian E. 1998. SEM survey of pollen morphology in iranian species of Hyoscyamus L. (Solanaceae). Iranian Journal of Botany 7: 151-163.
- Knapp S, Persson V, Blackmore S. 1998. Pollen morphology and functional dioecy in Solanum (Solanaceae). Plant Systematics and Evolution 210: 113-139.
- Kumar AVS, Nair MC, Marugan K. 2015. Pollen morphology of selected taxa of the genus Solanum from Southern Western Ghats, Kerala, India. Rheedea 25: 128-145.
- Landi LADC, Gasparino EC. 2018. Palinologia de Amaranthaceae e Araliaceae nativas em fragmentos florestais remanescentes da região noroeste do Estado de São Paulo. Hoehnea 45: 115-125.
- Lashin GMA. 2011. Palynology of six species of Solanum (Solanaceae). Life Science Journal 8: 687-697.
- Lorente FL, Júnior AAB, Oliveira PE, Pessenda L. 2017. Atlas palinológico: laboratório 14 C-Cena / USP. Piracicaba, Fundação de Estudos Agrários Luiz de Queiroz - FEALQ.
- Martins TR, Barkman TJ . 2005. Reconstruction of Solanaceae Phylogeny Using the Nuclear Gene SAMT. Systematic Botany 30: 435-477.
- McCune B, Mefford MJ. 2011. PC-ORD. Multivariate Analysis of Ecological Data (Version 6)’. Oregon, Gleneden Beach, MjM Software.
- Melhem TS, Cruz-Barros MAV, Corrêa AS, Makino-Watanabe H, Silvestre-Capelato MSF, Gonçalves-Esteves VL. 2003. Variabilidade polínica em plantas de Campos do Jordão, São Paulo, Brazil. Boletim do Instituto de Botânica de São Paulo 16: 1-104.
- Mercado-Gómez JD, Jiménez-Bulla LC, Sánchez-Montaño LR. 2013. Polen de las Magnoliopsida em el Volcán (Pamplona, Colombia) II: Familias Hypericaceae, Lamiaceae, Lobeliaceae, Polygonaceae, Rhamnaceae, Rosaceae, Rubiaceae, Scrophulariaceae y Solanaceae. Caldasia 35: 409-427.
- Murry LE, Eshbaugh WH. 1971. A palynological study of the Solaninae (Solanaceae). Grana 11: 65-78.
- Necchi OJr. 2012. Fauna e Flora de Fragmentos Florestais Remanescentes da Região Noroeste do Estado de São Paulo 1st. edn. Ribeirão Preto, Holos Editora.
- Olmstead RG, Palmer JD. 1992. A chloroplast DNA phylogeny of the Solanaceae: subfamilial relationships and character evolution. Annals of the Missouri Botanical Garden 79: 346-360.
- Olmstead RG, Sweere JA, Spangler RE, Bohs L, Palmer JD. 1999. Phylogeny and provisional classification of the Solanaceae based on chloroplast DNA. In: Nee M, Symon D, Lester RN, Jessop J. (eds.) Solanaceae IV: Advances in Biology and Utilization. London, Royal Botanic Gardens, Kew. p. 111-137.
- Olmstead RG, Boh L, Migid HA, Santiago-Valentin E, Garcia VF, Collier SM. 2008. A molecular phylogeny of the Solanaceae. Taxon 57: 1158-1181.
- Persson V, Knapp S, Blackmore S. 1994. Pollen morphology and systematic of tribe Juanulloeae A.T. Hunziker (Solanaceae). Review of Palaeobotany and Palynology 83: 1-30.
- Perveen A, Qaiser M. 2007. Pollen Morphology of family Solanaceae from Pakistan. Pakistan Journal of. Botany 39: 2243-2256.
- Punt W, Monna-Brands M. 1977. The Northwest European pollen flora: Solanaceae. Review of Palaeobotany and Palynology 23: 1-30.
- Punt W, Hoen PP, Blackmore S, Nilsson S, Le Thomas A. 2007. Glossary of pollen and spore terminology. Review of Paleobotany and Palynology 143: 1-81.
- Ranga NT, Rezende AA, Cavasan O, Toniato MTZ, Cielo-Filho R, Stranghetti V. 2012. Caracterização florística de remanescentes de vegetação nativa da região noroeste do Estado de São Paulo. Ribeirão Preto, Holos Editora .
- Rao NA, Ling LF. 1974. Pollen morphology of certain tropical plants. Reinwardtia 9: 153-176.
- Rodrigues IMC, Falcão BF, Stehmann JR, Bauermann SG. 2016. Pollen morphology in Athenaea Sendtn. and Aureliana Sendtn. (Solanaceae). Palynology 40: 202-215.
- Rojas CB, Laportte MC. 2004. Morfologia polínica de Sessea Ruiz y Pavón (Solanaceae: Cestreae). Memoria de la. Fundación. La Salle de Ciencias Naturales, 64: 125-135.
- Roubik DW, Moreno JE. 1991. Pollen and Spores of Barro Colorado Island. St Louis, Missouri Botanical Garden.
- Salgado-Labouriau ML. 1973. Contribuição à palinologia dos cerrados. Rio de Janeiro, Academia Brasileira de Ciências.
- Salgado-Labouriau ML, Vanzolini PE, Melhem TS. 1965. Variation of polar axes and equatorial diameters in pollen grains of two species of Cassia Grana Palynologica 6: 98-105.
- Särkinen T, Bohs L, Olmstead RG, Knapp S. 2013. A phylogenetic framework for evolutionary study of the nightshades (Solanaceae): a dated 1000-tip tree. BMC Evolutionary Biology 13: 214.
- Shepherd GJ. 1996. Fitopac 1: Manual do usuário. Campinas, Departamento de Botânica, Universidade Estadual de Campinas.
- Silva SN, Carvalho AMV, Santos FAR. 2003. Morfologia polínica de doze espécies de Cestrum L. (Solanaceae) da mata higrófila na Bahia, Brasil. Acta Scientiarum Biological Sciences 25: 439-443.
- Silva CI, Fonseca VLI, Groppo M, et al 2014. Catálogo polínico das plantas usadas por abelhas no Campus da USP de Ribeirão Preto. Ribeirão Preto, SP, Holos.
- Silva FHM, Santos FAR, Lima LCL. 2016. Flora polínica das caatingas: Estação Biológica de Canudos - Canudos, Bahia, Brasil. Feira de Santana, Micron Bahia.
- Soares EL, Landi LADC, Gasparino EC. 2021. Additions to the knowledge of the pollen morphology of some Fabaceae from the cerrado's forest patches of Brazil. Palynology 45: 269-281.
- Song Y, Gu L, Liu J. 2018. Pollen morphology of selected species from the family Solanaceae. Palynology 43: 1-18.
- Souza VC, Lorenzi H. 2008. Botânica Sistemática: Guia ilustrado para identificação das famílias de angiospermas da flora brasileira, baseado em APG II. 2nd. edn. Nova Odessa, Editora Instituto Plantarum.
- Souza CN, Gasparino EC. 2014. Pollen morphology of Fridericia Mart. (Bignoniaceae) from Brazilian forest fragments. Brazilian Journal of Botany 37: 83-94.
- Souza CN, Rezende AA, Gasparino EC. 2019. Pollen morphology of Bignoniaceae from Brazilian forest fragments and its systematic significance. Palynology 43: 333-347.
- Specieslink. 2020. Sistema de Informação Distribuído para Coleções Biológicas. http://splink.cria.org.br./ 12 Jan. 2020.
» http://splink.cria.org.br./ - Stafford P, Knapp S. 2006. Pollen morphology and systematics of the zygomorphic-flowered nightshades (Solanaceae; Salpiglossideae sensu D’Arcy, 1978 and Cestroideae sensu D’Arcy, 1991, pro parte): a review. Systematics and Biodiversity 4: 173-201.
- Stevens PF. 2001. Angiosperm Phylogeny Website. Version 14, July 2017 (and more or less continuously updated since). http://www.mobot.org/MOBOT/research/APweb/ 20 Jan. 2020.
» http://www.mobot.org/MOBOT/research/APweb/ - Thiers B. 2019, continuous adapted. Index herbariorum: a global directory of public herbaria and associated staff. New York Botanical Garden’s Virtual herbarium. http://sweetgum.nybg.org/ih/ 03 Feb. 2020.
» http://sweetgum.nybg.org/ih/ - Velásquez CA, Rangel JO. 1995. Atlas Palinológico de la flora vascular del páramo I. Las famílias más ricas en especies. Caldasia 17: 509-568.
- Vieira S. 2011. Introdução a Bioestatistica. Rio de Janeiro, Elsevier.
- Vignoli-Silva M, Batista-Franklim CPR, Correa DSM, Mentz LA, Mendonça CBF, Gonçalves-Esteves V. 2015. Pollen diversity in Cestrum L. (Solanaceae) from extra-Amazonian Brazil. Palynology 39: 76-90.
- Zar JH. 2010. Biostatistical analysis. New Jersey, Prentice-Hall.
Publication Dates
-
Publication in this collection
04 Feb 2022 -
Date of issue
Oct-Dec 2021
History
-
Received
09 June 2020 -
Accepted
16 Mar 2021