ABSTRACT
Leaf venation has not been widely used in taxonomic integrative works, although some potential in delimiting taxa has been reported. Hyptidendron, a neotropical genus with 20 species, seemed to present some variation in leaf venation patterns, which we sought to further investigate. A number of different herbaria were consulted, and herborized leaves were diaphanized for 20 species of Hyptidendron and a set of unidentified material. The taxa were classified according to their venation patterns. Hyptidendron possesses pinnate semicraspedodromous venation with reticulate irregular tertiary, quaternary and quinternary veins. Freely Ending Veinlets show some variation between species but without clear taxonomic importance. Otherwise, perimarginal veins were greatly informative, being present only in the unidentified material. Together with differences from other species of the genus in leaf shape, margins shape, calyx indumentum and petiole size, we hypothesize the unidentified material as a new species: Hyptidendron cerradoense, described here. We provide a full description, illustration, a distribution map, a preliminary conservation assessment and comments on both the taxonomy and ecology of the new species. Our study supports the importance of leaf venation for taxonomic studies, even in smaller genera.
Keywords:
Cerrado; clearing technique; Hyptidendron; Lamiaceae; leaf venation; neotropical flora; taxonomy
Introduction
Leaf venation has not been widely used as a source of taxonomic information, although different studies report the usage of it for recognizing taxa in different ranks (Marinho et al. 2016Marinho LC, Fiaschi P, Gahagen B, Santos FAR, Amorim AM. 2016. Tovomita (Clusiaceae) from the Brazilian Atlantic Forest: Taxonomy and Utility of Leaf Venation Characters at the Species Level. Systematic Botany 41: 758-774.;; Sun et al. 2018Sun X, Xue J, Lei Z, et al. 2018. Taxonomic and phylogenetic significance of leaf venation characteristics in Dioscorea plants. Archives of Biological Sciences 70: 397-407.; Buot 2020Buot IEJ. 2020. Leaf Architecture as a Promising Tool in Confirming Identity of Confusing Plant Taxa. Journal of Nature Studies 19: 134-143. ). For Hyptidinae, a mostly neotropical subtribe of Lamiaceae with ca. 400 species (Harley & Pastore 2012Harley RM, Pastore JFB. 2012. A generic revision and new combinations in the Hyptidinae (Lamiaceae), based on molecular and morphological evidence. Phytotaxa 58: 1-55.), some works showed that differences in leaf venation can be informative within the group (Rudall 1980Rudall PJ. 1980. Leaf anatomy of the subtribe Hyptidinae (Labiatae). Botanical Journal of the Linnean Society 80: 319-340.; Silva-Luz et al. 2012Silva-Luz CL, Gomes CG, Pirani JR, Harley RM. 2012. Flora da Serra do Cipó, Minas Gerais: Lamiaceae. Boletim de Botânica da Universidade de São Paulo 30: 109-155.), suggesting that this character should be used in new systematic studies.
Hyptidendron, one of the 19 genera of Hyptidinae, is endemic to South America, occurring in Bolivia, Colombia, Ecuador, Guyana, Peru, Venezuela, and especially in Brazil, where all the 20 known species occur (Harley 1988Harley RM. 1988. Revision of generic limits in Hyptis Jacq. (Labiatae) and its allies. Botanical Journal of the Linnean Society 98: 87-95.; Harley & Antar 2017Harley RM, Antar GM. 2017. Hyptidendron albidum (Lamiaceae, Hyptidinae), a remarkable new species from northern Minas Gerais state, Brazil. Phytotaxa 308: 97-103.; Antar et al. 2019Antar GM, Harley RM, Pastore JFB, Sano PT. 2019. Novelties in Hyptidendron (Hyptidinae - Lamiaceae): a new species and a rediscovery. Brittonia 71: 64-72. ; Antar et al. 2021Antar GM, Harley RM, Pastore JFB, Gonella PM, Sano PT. 2021. Hyptidendron pulcherrimum (Hyptidinae - Lamiaceae) a new narrowly endemic species from Minas Gerais, Brazil. Adansonia 43: 1-8.). The last taxonomic revision of the genus, when it was still part of Hyptis, was made by Epling (1949Epling C. 1949. Revisión del género Hyptis (Labiatae). Revista del Museo de La Plata, Sección Botánica 7: 153-497.), which did not encompass any mention of the leaf venation as a relevant taxonomic character.
During the preparation of a taxonomic revision of Hyptidendron, a promising leaf venation variation was detected among species studied under the stereomicroscope, leading to further investigation, which is reported here. Together with these results, we provide the description of a new species, Hyptidendron cerradoense Antar & Harley, recognized by the unique leaf venation pattern in the genus.
Materials and methods
The morphological description and diagnosis were drawn up after examining and analysing specimens of Hyptidendron from the following herbaria: ALCB, BHCB, BHZB, BM, BRBA, CEN, CESJ, CGMS, COR, CTBS, DIAM, ESA, ESAL, G, HDJF, HEPH, HRB, HRCB, HUEFS, HUFSJ, HXBH, IBGE, K, MBM, MBML, NX, NY, P, PAMG, R, RB, SAMES, SP, SPF, SPSC, SPSF, UB, UEC, UFG, UFMT, UFOP, UPCB, US, VIES (acronyms according to Thiers 2022Thiers B. 2022, continuously updated. Index Herbariorum: a global directory of public herbaria and associated staff. New York Garden’s Virtual Herbarium. http://sweetgum.nybg.org/ih/ herbarium. php?irn=174420. 15 Jan. 2022.
http://sweetgum.nybg.org/ih/ herbarium. ...
, continuously updated). A × 10 to × 60 magnification stereomicroscope was used to analyse morphological features of the specimens. Terminology follows Harris & Harris (2001Harris JG, Harris MW. 2001. Plant identification terminology: an illustrated glossary. 2nd. edn. Spring Lake, Spring Lake Publishing. ) for general morphology and Hickey (1973Hickey LJ. 1973. Classification of the architecture of dicotyledonous leaves. American Journal of Botany 60: 17-33.) for leaf shape, as well as Antar et al. (2021Antar GM, Harley RM, Pastore JFB, Gonella PM, Sano PT. 2021. Hyptidendron pulcherrimum (Hyptidinae - Lamiaceae) a new narrowly endemic species from Minas Gerais, Brazil. Adansonia 43: 1-8.) for specific terms.
IUCN (2012)IUCN. 2012. IUCN Red List categories and criteria: Version 3.1. 2nd. edn. Cambridge, IUCN. criteria and subsequent guidelines (IUCN 2019IUCN Standards and Petitions Committee. 2019. Guidelines for using the IUCN Red List categories and criteria. Version 13. IUCN Standards and Petitions Subcommittee. http://www. iucnredlist.org/documents/RedListGuidelines.pdf.
http://www. iucnredlist.org/documents/Re...
) alongside the GeoCAT tool (Bachman et al. 2011Bachman S, Moat J, Hill AW, de la Torre J, Scott B. 2011. Supporting Red List threat assessments with GeoCAT: Geospatial conservation assessment tool. ZooKeys 150: 117-126.) were used to infer a preliminary conservation status based on criterion B, measuring the Extent of Occurrence (EOO) and Area of Occupancy (AOO). GeoCAT was applied with the IUCN default cell width of 2 km² for the AOO analysis. The distribution map was produced in QGIS version 3.0.1 (QGIS Development Team 2018QGIS Development Team. 2018. QGIS Geographic information system. [s.l.], Open Source Geospatial Foundation Project. ). In cases where herbarium specimens lacked geo-reference data, the geographic coordinates were approximated using the locality description of the specimen label and Google Earth GIS software (http://earth.google.com/).
A list of the sampled material for leaf venation analyses is described in table 1. At least two leaves per specimen were used for each of the 21 species sampled, but whenever possible more specimens were used. Only cauline leaves were selected meaning mature leaves, not representing bracts and from ± the middle of the stem. For the description and classification of venation patterns, the leaves were cleared, adapting the method proposed by Strittmatter (1973Strittmatter CGD. 1973. Nueva técnica de diafanización. Boletín de la Sociedad Argentina de Botánica 15:126-129.). The herborized material was rehydrated by boiling in distilled water, and then transferred to 5 % potassium hydroxide for twenty minutes. The leaves were then immersed in 20 % sodium hypochlorite until clarification. Complete clarification was obtained by subjecting the material to 5 % chloral hydrate. The cleared leaves were dehydrated and then stained with 1 % safranin in 100 % ethanol and butyl acetate (1:1). The leaves were stretched onto glass plates and mounted with Canada balsam. We followed Ellis et al. (2009Ellis B, Daly DC, Hickey LJ, et al. 2009. Manual of leaf architecture. 1st edn. New York, The New York Botanical Garden.) for venation pattern terminology. The following vein characters were analysed: 1) Primary Vein Framework; 2) Major Secondary Vein Framework; 3) Perimarginal veins; 4) Intercoastal Tertiary Vein Fabric; 5) Quaternary Vein Fabric; 6) Quinternary Vein Fabric; and 7) Freely Ending Veinlets (FEVs). For the FEVs, where more than one type was detected, we categorized it according to the most common type.
List of the species, vouchers and herbaria where deposited and variable venation characters in Hyptidendron. FEVs = Freely Ending Veinlets.
Results
Leaf venation
Hyptidendron possesses primary pinnate venation (Fig. 1). Primary veins can be straight or rarely curved as in H. claussenii. Primary veins are usually prominent in the abaxial surface, and slightly impressed or slightly prominent on the adaxial surface. Secondary veins are semicraspedodromous (Fig. 1). Tertiary, Quaternary and Quinternary veins are reticulate irregular (Fig. 2). Veins are usually prominent and conspicuous (sometimes prominulous) in the abaxial surface and usually plane and inconspicuous in the adaxial surface. FEVs are variable between species. However, no unique species patterns could be found, nor do the most morphologically related species share similar FEVs patterns (Fig. 2). Perimarginal veins were only found in the unidentified material (represented by vouchers: Aparecida-da-Silva 3804; Pereira-Silva 5199, 16436; and Walter 4191), which displayed intramarginal veins (Fig. 3B, C), or rarely marginal ones (Fig. 3A, D).
Hyptidendron primary and secondary veins A. Hyptidendron glutinosum (Benth.) Harley, highlighting the primary pinnate venation (black arrows) and semicraspedodromous secondary vein (red arrows). B. Hyptidendron rondonicum (Harley) Harley, highlighting the primary pinnate venation (black arrows) and semicraspedodromous secondary vein (red arrows). C. Hyptidendron dictiocalyx (Benth.) Harley, highlighting the primary pinnate venation (black arrows) and secondary semicraspedodromous vein (red arrows). D. Hyptidendron leucophyllum (Pohl ex Benth.) Harley, highlighting the primary pinnate venation (black arrows) and secondary semicraspedodromous vein (red arrow). E. Hyptidendron leucophyllum (Pohl ex Benth.) Harley highlighting the secondary veins ending at a crenate margin (red arrow). F . Hyptidendron unilaterale (Epling) Harley, highlighting the secondary veins ending in a serrate margin (red arrow).
Hyptidendron tertiary, quaternary and quintenary veins and FEVs. A. Hyptidendron glutinosum (Benth.) Harley, highlighting tertiary (black arrow), quaternary (red arrow) and quintenary (blue arrow) venation. B. Hyptidendron eximium (Epling) Harley & J.F.B.Pastore highlighting tertiary (black arrow), quaternary (red arrow) and quintenary (blue arrow) venation. C. Hyptidendron amethystoides (Benth.) Harley, highlighting tertiary (black arrow), quaternary (red arrow) and quintenary (blue arrow) venation. D. Hyptidendron asperrimum (Spreng.) Harley, highlighting tertiary (black arrow), quaternary (red arrow) and quintenary (blue arrow) venation. E. Hyptidendron vauthieri (Briq.) Harley, highlighting FEVs absent (black arrow). F. Hyptidendron roseum Antar, Harley & J.F.B.Pastore, highlighting unbranched FEVs (black arrow).
Hyptidendron cerradoense Antar & Harley venation. A. Marginal veins (black arrows). B. Intramarginal venation (black arrows). C. Intramarginal veins (black arrow). D. Marginal veins, highlighting the high caliber of the perimarginal veins. E. Tertiary, (black arrow), quaternary (red arrow) and quintenary (blue arrow).
Taxonomic treatment
Hyptidendron cerradoense Antar & Harley, sp. nov. (Fig. 4). Type: BRAZIL: Goiás: Cavalcante, Vila Veneno - rio São Félix km 4, Área de Influência da futura Hidrelétrica de Cana Brava, influência indireta, 13°32'10''S 48°3'25''W, 27 June 2001, Pereira-Silva & Carvalho-Silva 5199 (Holotype: CEN (00043108)).
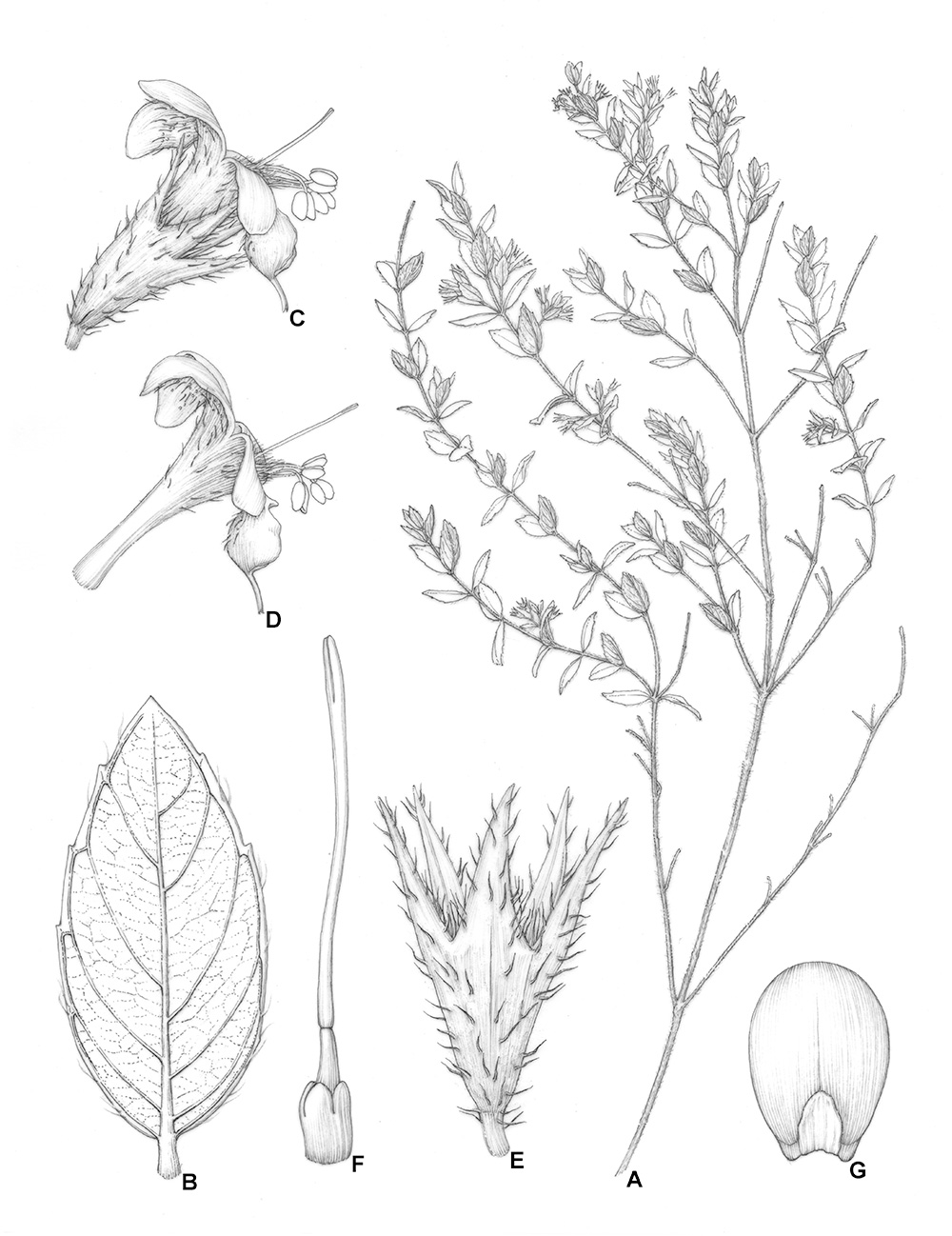
Hyptidendron cerradoense Antar & Harley A. Branch bearing leaves and inflorescences. B. Leaves, adaxial surface. C. Flower, side view. D. Corolla, side view. E. Mature calyx. F. Gynoecium and style, showing stylopodium. G. Nutlet. A-G. Illustration by Klei Sousa based on Pereira-Silva 5199 (CEN).
Hyptidendron cerradoense is morphologically related to Hyptidendron arbusculum by sharing similar leaf measurements, margins with few teeth and cymes 1-3(-4) flowered. These two species can be differentiated, as Hyptidendron cerradoense possesses perimarginal veins (vs. absent), blades elliptic, narrow elliptic or narrow ovate (vs. widely ovate, ovate, elliptic, widely elliptic, rarely very widely ovate), petioles 0.7-1.6 cm long (vs. petioles 1.5-2.8(-3.5) mm long), leaf margins entire to 4 teeth on each side of leaf (vs. 2-7 teeth on each side of leaf) and calyces externally pubescent to densely pubescent with glandular stipitate hairs of varying lengths and scattered long uniseriate hairs, which can be dense and hispid (vs. pubescent with glandular stipitate hairs of similar length).
Subshrubs or shrubs 30-50 cm tall, slightly aromatic or aromatic, xylopodium present; stems woody, branched, 2-4 mm diam., younger stems quadrangular, canaliculate, pubescent with long uniseriate eglandular hairs, which can be curved and soft or erect and sharp and then the surface hispid, also rarely small sessile glands and gland-tipped hairs, older stems ± squared and slightly canaliculate or not canaliculate, less hairy, with longitudinal grooves, internodes 0.3-1.5(-2.7) cm long. Cauline leaves mostly congested near the apex or somewhat spreading along the branches, densely imbricate near the apex, sometimes almost all leaves imbricate, longer than internodes, less commonly smaller or with similar size, mostly diminishing in size towards stem apex, lamina 0.8-1.5 × 0.3-0.7 cm, chartaceous to coriaceous, concolorous or slightly discolorous, with abaxial surface paler, elliptic, narrow elliptic or narrow ovate, base rounded or cuneate, sometimes unequal, apex acute, sometimes slightly apiculate, apiculus ca. 0.5 mm long, adaxial surface glabrous or glabrescent with few gland-tipped hairs and small sessile glands, venation mostly inconspicuous, midvein plane, secondary veins prominulous, perimarginal vein present, intramarginal or marginal, abaxial surface glabrous or glabrescent with few gland-tipped hairs and tiny sessile glands, midvein occasionally with sparse long uniseriate eglandular hairs, venation reticulate, primary and secondary veins prominent, tertiary veins not so conspicuous, margins ciliate, mostly hispid with long uniseriate eglandular hairs, sometimes with gland-tipped hairs, serrulate, entire to 1/2 of leaf margin, rarely completely entire, not revolute, (0-)1-4 teeth on each side of leaf, with tooth apex swollen, acute or obtuse; petiole 0.7-1.6 cm long, canaliculate, expanded in the base, sparsely pubescent or glabrescent with gland-tipped hairs, sessile glands and rare uniseriate curved eglandular hairs. Inflorescence not forming a well-define terminal thyrsoid structure, but with dichasial axillary cymes, concentrated near apex, subtended by bracts similar to leaves with same shape, with similar size or smaller, 0.35-1.0 × 0.1-0.35 cm, longer or smaller than cymes, mature cymes 0.7-1.7 cm long, 1-3(-4)-flowered, not obscured by bracts, rarely slightly obscured by bracts, peduncles 0.4-3.5(-7.5) mm long, pubescent to densely pubescent with small gland-tipped hairs. Flowers with pedicels 1-3 mm long, pubescent to densely pubescent with gland-tipped hairs, rarely few long uniseriate eglandular hairs close to calyx attachment, and subtended by linear bracteoles, 0.8-2.7 × 0.1 mm, pubescent to densely pubescent with gland-tipped hairs and rarely few long uniseriate eglandular hairs, mostly in apex; calyx at anthesis (3.8-)5.5-6.4 mm long, green, tube (2.4-)3.0-4.0 mm long, ± infundibuliform, straight, ribbed, externally pubescent to densely pubescent with different height gland-tipped hairs and scattered long uniseriate hairs, which can be dense and hispid, mostly in the base and ribs, tube internally glabrescent with few hairs and with a faint ring of long uniseriate hairs in the throat, calyx lobes subequal, 1.5-3.4 mm long, with base deltate and apex long acuminate, straight, externally with indumentum as on tube but with a concentration of long uniseriate eglandular hairs, internally pubescent with small gland-tipped hairs and margin with long uniseriate eglandular hairs, calyx in fruit 8.4-9.5 mm long, indumentum less dense, tube 5.0-6.0 mm long, ± cylindrical, ribbed, calyx lobes 2.7-4.0 mm long, subequal, straight; corolla lilac, (5.5-)8.1-8.3 mm long, tube (3.1-)4.9-5.1 mm long, ± cylindrical, becoming slightly enlarged near throat, 0.6-0.9 mm wide, externally with base glabrous becoming sparsely villous with curved uniseriate hairs and small sessile glands, internally with curved entangled non-glandular hairs, close to insertion of posterior pair of stamens, lobes spreading, externally with the same indumentum as tube but with a concentration of sessile glands, lobes internally glabrous, anterior lobe large, boat-shaped with long, almost caudate apex; posterior pair of stamens with filaments densely villous with long curved, entangled, uniseriate, eglandular hairs, anterior pair with filaments glabrous except by few long, uniseriate hairs near the anther; gynoecium with style jointed, with a well-developed stylopodium protruding above the ovary, and apically with two slender stigmatic lobes. Nutlets 1 per flower, 3.0-3.6 × 1.9-2.1 mm, ellipsoid or obovoid, not flattened, not winged, castaneous, not shiny, glabrous, rugulose, with deep abscission scars, slightly mucilaginous when wetted.
Phenology: Hyptidendron cerradoense was found with flowering specimens in May and June and with fruiting specimens in September and November.
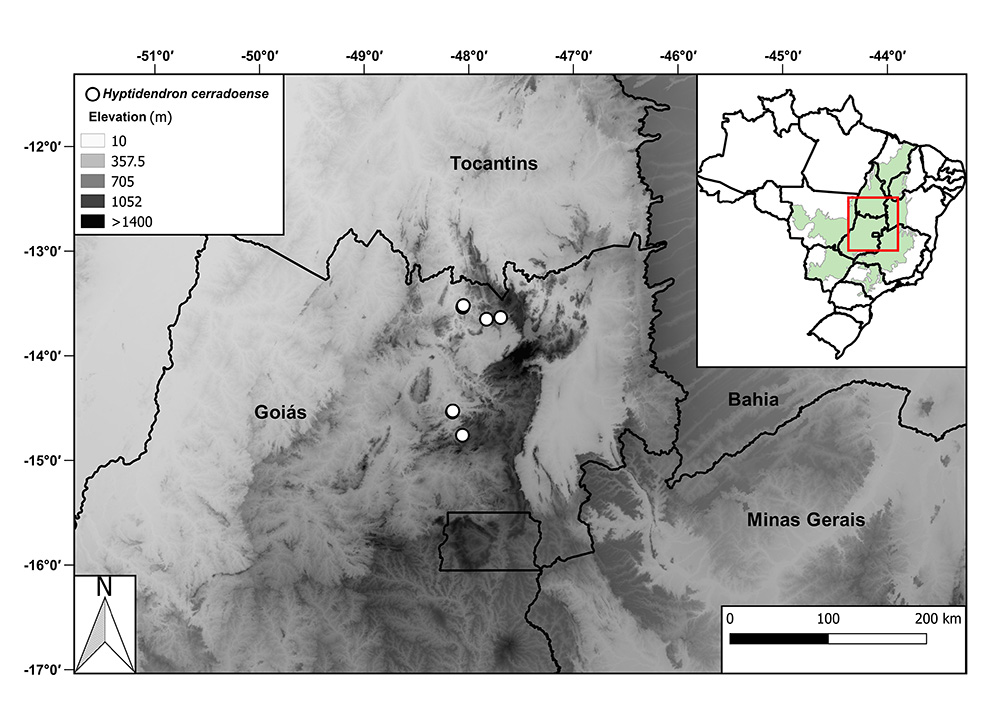
Distribution and Habitat: Hyptidendron cerradoense is endemic to Cavalcante and Niquelândia municipalities, known from seven collections (Fig. 5). It can be found from 350 to 1000 m elevation in campo sujo, cerrado sensu stricto, cerrado rupestre and campo cerrado habitats, all of these included in the Cerrado domain.
Distribution of Hyptidendron cerradoense Antar & Harley (white circles). In the small map, the green shape shows the extension of the Cerrado domain.
Preliminary Conservation Status: The Area of Occupancy (AOO) is 28 km² and the Extent of Occurrence (EOO) is 3,408 km². Hyptidendron cerradoense is known from just seven collections in five localities. In Cavalcante municipality, one of the localities is inside the protected area Reserva Natural da Serra do Tombador. In Niquelândia municipality populations are threatened by the intense mining activity in the region (Pastore 2016Pastore JFB. 2016. A new endangered species of Polygala (Polygalaceae) from Niquêlandia, Goiás, Brazil. Phytotaxa 288: 96-100.) and the population represented by the collection Walter 4191 (CEN) was probably destroyed due to flooding for the construction of a hydroelectric power plant. In view of that, the conservation status of this species is assessed as Endangered according to criteria B1ab(ii, iii)+2ab(ii, iii) (IUCN 2012IUCN. 2012. IUCN Red List categories and criteria: Version 3.1. 2nd. edn. Cambridge, IUCN. ).
Etymology: The specific epithet refers to the Cerrado domain in which the species is endemic. The Cerrado is the richest savanna biome with over 12300 species of angiosperms recognized for Brazil, of which ca. 30 % are endemic (Flora e Funga do Brasil 2022Flora e Funga do Brasil. 2022. Instituto de Pesquisas Jardim Botânico do Rio de Janeiro. http://floradobrasil.jbrj.gov.br. 22 Jul. 2022.
http://floradobrasil.jbrj.gov.br...
). Despite this high number of richness and endemism, the domain has been continuously suffering from area lost, with more than 50 % of its original area already replaced (Beuchle et al. 2015Beuchle R, Grecchi RC, Shimabukuro YE, et al. 2015. Land cover changes in the Brazilian Cerrado and Caatinga biomes from 1990 to 2010 based on a systematic remote sensing sampling approach. Applied Geography 58: 116-127. ), putting it as one of the hotspots of conservation (Mittermeier et al. 2011Mittermeier RA, Turner WR, Larsen FW, Brooks TM, Gascon C. 2011. Global biodiversity conservation: the critical role of hotspots. In: Zachos E, Habel JC. (eds.) Biodiversity hotspots. London, Springer Publishers. p. 3-22.).
Specimens examined: BRAZIL. Goiás: Cavalcante, UHE Cana Brava. Arraial São Félix. Margem direita do Rio Tocantins. Margem direita do Rio São Félix, 13°31'10''S 48°3'4''W, 9 Sep 2000, Bucci 1382 (UFG); Cavalcante, E Cavalcante-Minaçu, km 75, entrada à direita da rodovia com destino ao rio São Félix, Serra do Tombador, 06 Nov 2012, G. Pereira-Silva et al. 16436 (CEN); Cavalcante, Reserva Natural da Serra do Tombador, área atrás da sede, área queimada out/17 após 12 anos, 13º39'05''S, 47º49'51''W, 26 Jun 2018, C.A.S. Rodrigues 26 (CEN); Niquelândia, 14°45'36,01''S 48°3'36,01''W, 17 Sep 2018, Boldrim et al. 4038 (CEN); Niquelândia, 4 km do povoado de Muquém em direção a Niquelândia, 14°31'41''S 48°9'8''W, 8 May 1998, Aparecida da Silva et al. 3804 (IBGE, K, US); Niquelândia, área de influência do AHE Serra da Mesa, estrada de terra Niquelândia - Muquém, cerca de 3 km antes de Muquém, 14°32'17''S 48°9'21''W, 3 Jun 1998, Walter et al. 4191 (CEN, HUEFS).
Affinities and morphological notes: Hyptidendron cerradoense is unique in the genus by the presence of a perimarginal vein. The new species is also similar to H. vepretorum by sharing similar leaf measurements and cymes 1-3 flowered, differing from it by the perimarginal vein present (vs. absent), lamina indumentum glabrous or glabrescent with few gland-tipped hairs and small sessile glands (vs. abaxial surface pubescent, densely pubescent or rarely villous with gland-tipped hairs and long eglandular uniseriate hairs), leaf margin entire or with 1-4 teeth on each side of leaf (vs. (1-)3-14 teeth on each side of leaf) and cymes not obscured by bracts, rarely slightly obscured by bracts (vs. mostly obscured by bracts, at least partially).
At first we thought of recognizing two separate taxa from Niquelândia and Cavalcante municipalities, respectively, as these two populations share interesting differences in peduncle size, with populations from Cavalcante with reduced peduncles up to 1.7 mm long and populations from Niquelândia with peduncles from 3-7.5 mm long. However, after careful morphological analyses, although populations from Cavalcante and Niquelândia are ca. 200 km distant, we considered them as part of the same species. Although the peduncle size is somewhat relevant for Hyptidendron taxonomy (e.g. Antar et al. 2021Antar GM, Harley RM, Pastore JFB, Gonella PM, Sano PT. 2021. Hyptidendron pulcherrimum (Hyptidinae - Lamiaceae) a new narrowly endemic species from Minas Gerais, Brazil. Adansonia 43: 1-8.), this feature isolated and measured in just a few specimens, could not be used solely to recognize two different taxa. Furthermore, specimens from Serra do Tombador (Pereira-Silva 16436; Rodrigues 26) also have some other unique features as conspicuously imbricate leaves, reduced pedicels and leaves mostly entire with perimarginal marginal venation. However, as it shares most of its morphological features with the other populations from Cavalcante and Niquelândia, we prefer to maintain it within the H. cerradoense concept. Further collections and observation in vivo may be useful to better understand this variation.
Discussion
Hyptidendron possesses semicraspedodromous secondary venation which is the most common type in Hyptidinae (Rudall 1980Rudall PJ. 1980. Leaf anatomy of the subtribe Hyptidinae (Labiatae). Botanical Journal of the Linnean Society 80: 319-340.). Other taxa within the subtribe can present craspedodromous type as some species in Cyanocephalus and Hyptis (Silva-Luz et al. 2012Silva-Luz CL, Gomes CG, Pirani JR, Harley RM. 2012. Flora da Serra do Cipó, Minas Gerais: Lamiaceae. Boletim de Botânica da Universidade de São Paulo 30: 109-155.); brochidodromous type as in Hyptis sect. Pachyphyllae (Rudall 1980Rudall PJ. 1980. Leaf anatomy of the subtribe Hyptidinae (Labiatae). Botanical Journal of the Linnean Society 80: 319-340.) and some other species of Hyptis (Silva-Luz et al. 2012Silva-Luz CL, Gomes CG, Pirani JR, Harley RM. 2012. Flora da Serra do Cipó, Minas Gerais: Lamiaceae. Boletim de Botânica da Universidade de São Paulo 30: 109-155.); and eucamptodromous as in some species of Hyptis (Silva-Luz et al. 2012Silva-Luz CL, Gomes CG, Pirani JR, Harley RM. 2012. Flora da Serra do Cipó, Minas Gerais: Lamiaceae. Boletim de Botânica da Universidade de São Paulo 30: 109-155.). Although taxonomic and evolutionary significance of these variations within the subtribe remains obscure, future studies, supported by phylogenies, are much desired and can sustain venation as an important feature for the subtribe’s taxonomy.
Although Hyptidendron is a relatively small genus, currently with 21 species recognized, it is noteworthy that variation in leaf venation, a genetically determined character (Roth-Nebelsick et al. 2001Roth-Nebelsick A, Uhl D, Mosbrugger V, Kerp H. 2001. Evolution and Function of Leaf Venation Architecture: A Review. Annals of Botany 87: 553-566.), is significant for its taxonomy. Our studies support the recognition of a new species that was already apparent due to other morphological and geographical differences from the known species of the genus. In view of this, the integration from classic morphological studies and anatomical studies with leaf venation, in the context of integrative taxonomy, allowed us to circumscribe and describe the new species.
Acknowledgments
We thank Gisele Gomes Nogueira Alves for helping with laboratory work and by commenting on early versions of the manuscript. We also thank the curators and staff of the visited herbaria. This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001; GMA thanks the Smithsonian for the Cuatrecasas Fellowship Award, American Society of Plant Taxonomists and Idea Wild for financial support; RMH, Honorary Research Fellow at R.B.G. Kew wishes to thank staff at the Herbarium at RBG Kew and at HUEFS, Universidade Estadual de Feira de Santana, Bahia Brazil for supporting this research; Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) provided financial support to JFBP (grant# 302452/2017-6), and PTS (grant# 310331/2019-6).
References
- Antar GM, Harley RM, Pastore JFB, Sano PT. 2019. Novelties in Hyptidendron (Hyptidinae - Lamiaceae): a new species and a rediscovery. Brittonia 71: 64-72.
- Antar GM, Harley RM, Pastore JFB, Gonella PM, Sano PT. 2021. Hyptidendron pulcherrimum (Hyptidinae - Lamiaceae) a new narrowly endemic species from Minas Gerais, Brazil. Adansonia 43: 1-8.
- Bachman S, Moat J, Hill AW, de la Torre J, Scott B. 2011. Supporting Red List threat assessments with GeoCAT: Geospatial conservation assessment tool. ZooKeys 150: 117-126.
- Beuchle R, Grecchi RC, Shimabukuro YE, et al 2015. Land cover changes in the Brazilian Cerrado and Caatinga biomes from 1990 to 2010 based on a systematic remote sensing sampling approach. Applied Geography 58: 116-127.
- Buot IEJ. 2020. Leaf Architecture as a Promising Tool in Confirming Identity of Confusing Plant Taxa. Journal of Nature Studies 19: 134-143.
- Ellis B, Daly DC, Hickey LJ, et al 2009. Manual of leaf architecture. 1st edn. New York, The New York Botanical Garden.
- Epling C. 1949. Revisión del género Hyptis (Labiatae). Revista del Museo de La Plata, Sección Botánica 7: 153-497.
- Flora e Funga do Brasil. 2022. Instituto de Pesquisas Jardim Botânico do Rio de Janeiro. http://floradobrasil.jbrj.gov.br 22 Jul. 2022.
» http://floradobrasil.jbrj.gov.br - Harley RM. 1988. Revision of generic limits in Hyptis Jacq. (Labiatae) and its allies. Botanical Journal of the Linnean Society 98: 87-95.
- Harley RM, Pastore JFB. 2012. A generic revision and new combinations in the Hyptidinae (Lamiaceae), based on molecular and morphological evidence. Phytotaxa 58: 1-55.
- Harley RM, Antar GM. 2017. Hyptidendron albidum (Lamiaceae, Hyptidinae), a remarkable new species from northern Minas Gerais state, Brazil. Phytotaxa 308: 97-103.
- Harris JG, Harris MW. 2001. Plant identification terminology: an illustrated glossary. 2nd. edn. Spring Lake, Spring Lake Publishing.
- Hickey LJ. 1973. Classification of the architecture of dicotyledonous leaves. American Journal of Botany 60: 17-33.
- IUCN. 2012. IUCN Red List categories and criteria: Version 3.1. 2nd. edn. Cambridge, IUCN.
- IUCN Standards and Petitions Committee. 2019. Guidelines for using the IUCN Red List categories and criteria. Version 13. IUCN Standards and Petitions Subcommittee. http://www. iucnredlist.org/documents/RedListGuidelines.pdf
» http://www. iucnredlist.org/documents/RedListGuidelines.pdf - Marinho LC, Fiaschi P, Gahagen B, Santos FAR, Amorim AM. 2016. Tovomita (Clusiaceae) from the Brazilian Atlantic Forest: Taxonomy and Utility of Leaf Venation Characters at the Species Level. Systematic Botany 41: 758-774.
- Mittermeier RA, Turner WR, Larsen FW, Brooks TM, Gascon C. 2011. Global biodiversity conservation: the critical role of hotspots. In: Zachos E, Habel JC. (eds.) Biodiversity hotspots. London, Springer Publishers. p. 3-22.
- Pastore JFB. 2016. A new endangered species of Polygala (Polygalaceae) from Niquêlandia, Goiás, Brazil. Phytotaxa 288: 96-100.
- QGIS Development Team. 2018. QGIS Geographic information system. [s.l.], Open Source Geospatial Foundation Project.
- Roth-Nebelsick A, Uhl D, Mosbrugger V, Kerp H. 2001. Evolution and Function of Leaf Venation Architecture: A Review. Annals of Botany 87: 553-566.
- Rudall PJ. 1980. Leaf anatomy of the subtribe Hyptidinae (Labiatae). Botanical Journal of the Linnean Society 80: 319-340.
- Silva-Luz CL, Gomes CG, Pirani JR, Harley RM. 2012. Flora da Serra do Cipó, Minas Gerais: Lamiaceae. Boletim de Botânica da Universidade de São Paulo 30: 109-155.
- Strittmatter CGD. 1973. Nueva técnica de diafanización. Boletín de la Sociedad Argentina de Botánica 15:126-129.
- Sun X, Xue J, Lei Z, et al 2018. Taxonomic and phylogenetic significance of leaf venation characteristics in Dioscorea plants. Archives of Biological Sciences 70: 397-407.
- Thiers B. 2022, continuously updated. Index Herbariorum: a global directory of public herbaria and associated staff. New York Garden’s Virtual Herbarium. http://sweetgum.nybg.org/ih/ herbarium. php?irn=174420 15 Jan. 2022.
» http://sweetgum.nybg.org/ih/ herbarium. php?irn=174420
Publication Dates
-
Publication in this collection
12 Sept 2022 -
Date of issue
2022
History
-
Received
01 June 2021 -
Accepted
23 Mar 2022