Abstracts
BACKGROUND: Patients with inflammatory bowel disease may have nutritional deficiencies. AIM: To verify the adequacy of dietary intake of patients with Crohn's disease and ulcerative colitis. METHODS: To assess food intake of 55 patients, 28 with Crohn's disease and 27 with ulcerative colitis treated in the gastroenterology clinic, was used the 24-Hour Food Recall and Food Frequency Questionnaire. The inflammatory activity of the disease was evaluated by serum C-reactive protein and Harvey and Bradshaw Index. For comparison of means t test was used, and the average on non-parametric, the Mann-Whitney test, with level of significance p <0.05. RESULTS: The patients were aged between 19 and 63 years and time since diagnosis was 7.9 years (1 to 22). According to the food intake was identified deficiency in energy intake, fiber, iron, potassium, sodium, magnesium, calcium, menadione, riboflavin, niacin, folate, pantothenic acid, tocopherol and cholecalciferol in Crohn's disease and ulcerative colitis, active or in remission. The intake of vegetables, fruits, dairy products and beans were low, and intake of fats and sweets was higher than the recommendations. CONCLUSION: There was a deficiency in food intake both in Crohn's disease and in ulcerative colitis, in activity and in remission. These deficiencies can adversely affect the disease course, and justify the need for nutritional intervention with these patients.
Crohn disease; Proctocolitis; Eating
RACIONAL: Pacientes com doença inflamatória intestinal podem apresentar deficiências nutricionais. OBJETIVO: Verificar a adequação da ingestão alimentar de pacientes com doença de Crohn e retocolite ulcerativa inespecífica. MÉTODOS: Para avaliação da ingestão alimentar de 55 pacientes, 28 com doença de Crohn e 27 com retocolite ulcerativa atendidos em ambulatório de gastroenterologia, utilizou-se o Recordatório Alimentar de 24 Horas e o Questionário de Frequência Alimentar. A atividade inflamatória da doença foi avaliada pelos níveis séricos de proteína C reativa e o Índice de Harvey e Bradshaw. Para comparação de médias foi usado o teste t não pareado e, para as médias não paramétricas, o teste de Mann-Whitney, considerando nível de significância valor de p<0,05. RESULTADOS: Os pacientes tinham idade entre 19 e 63 anos e tempo de diagnóstico de 7,9 anos (1 a 22). De acordo com a ingestão alimentar identificou-se deficiência na ingestão de energia, fibras, ferro, potássio, sódio, magnésio, cálcio, menadiona, riboflavina, niacina, folato, ácido pantotênico, tocoferol e colecalciferol na doença de Crohn e na retocolite ulcerativa em atividade ou em remissão. A ingestão de legumes, frutas, laticínios e feijão foi baixa, e a de doces e gorduras foi maior que as recomendações. CONCLUSÃO: Houve deficiência na ingestão alimentar tanto na doença de Crohn como na retocolite ulcerativa, em atividade e em remissão. Essas deficiências podem afetar negativamente o curso da doença e justificam a necessidade de intervenção nutricional com esses pacientes.
Doença de Crohn; Proctolite; Ingestão de alimentos
ORIGINAL ARTICLE
Correspondence
ABSTRACT
BACKGROUND: Patients with inflammatory bowel disease may have nutritional deficiencies.
AIM: To verify the adequacy of dietary intake of patients with Crohn's disease and ulcerative colitis.
METHODS: To assess food intake of 55 patients, 28 with Crohn's disease and 27 with ulcerative colitis treated in the gastroenterology clinic, was used the 24-Hour Food Recall and Food Frequency Questionnaire. The inflammatory activity of the disease was evaluated by serum C-reactive protein and Harvey and Bradshaw Index. For comparison of means t test was used, and the average on non-parametric, the Mann-Whitney test, with level of significance p <0.05.
RESULTS: The patients were aged between 19 and 63 years and time since diagnosis was 7.9 years (1 to 22). According to the food intake was identified deficiency in energy intake, fiber, iron, potassium, sodium, magnesium, calcium, menadione, riboflavin, niacin, folate, pantothenic acid, tocopherol and cholecalciferol in Crohn's disease and ulcerative colitis, active or in remission. The intake of vegetables, fruits, dairy products and beans were low, and intake of fats and sweets was higher than the recommendations.
CONCLUSION: There was a deficiency in food intake both in Crohn's disease and in ulcerative colitis, in activity and in remission. These deficiencies can adversely affect the disease course, and justify the need for nutritional intervention with these patients.
Headings: Crohn disease. Proctocolitis. Eating.
INTRODUCTION
Inflammatory bowel disease (IBD) is the general name given to a group of inflammatory disorders of unknown cause involving the gastrointestinal tract, which can be divided into two main groups: non-specific chronic ulcerative colitis (UC) and Crohn's disease (CD) . The cause of IBD is still unknown and there is interaction between genetic, environmental and immune influences33.
Nutritional status in IBD may be affected by reduction in food intake caused by gastrointestinal symptoms, malabsorption and for drug treatment, with larger changes in the acute phase of illness. Besides the reduction in food intake due to gastrointestinal symptoms such as anorexia, nausea and vomiting, patients may have increased nutritional needs as a result of inflammatory activity and complications of the disease25.
It is unclear whether dietary manipulation combined with drug therapy might improve symptoms or induce remission. Food intolerances observed vary and should be treated individually26. Several nutritional deficiencies have been described in IBD, such as iron, cyanocobalamin, folate, calcium and zinc18. Folate deficiency can cause anemia and is also implicated in increased risk for cancer of the colon and rectum24. Vitamin D regulates the functioning of the immune system and tissue proliferation and differentiation20, and its important role in bone metabolism, together with calcium28. Another important nutrient is vitamin K, present in bone formation and reabsorption7.
Considering the changes that cause nutrient deficiency disease and the influence of food intake, this study aims to evaluate the adequacy of dietary intake of patients with IBD at different stages of inflammatory activity.
METHODS
Cross-sectional study whose inclusion criteria were presence of diagnosis of inflammatory bowel disease, people physically able to anthropometric evaluation, age over 18 years. From 17 to April 14, 2008, 55 patients treated at the Clinic of Gastroenterology, Federal University of Paraná in Curitiba, Brazil who participated in the study were informed about the research objectives and signed a consent form in accordance with the approval by the Ethics Committee of Hospital de Clínicas number 1121.160/2005-10.
Information concerning the time of diagnosis of disease, previous operations, associated with other diagnoses and medications were obtained from medical records. Patients were asked about the presence of gastrointestinal symptoms, preferences and/or food intolerances, changes in food intake considering for how long and why, fluid intake per day, and the practice of physical activity or not.
To determine the inflammatory activity of CD and UC were considered values of C-reactive protein (CRP) by nephelometry performed considering normal values less than 0.50 mg/dl. Was considered the PCR tests in the assessment or request made in the last 15 days. For patients with CD, was used disease activity index proposed by Harvey and Bradshaw17 during the medical consultation. Patients with scores less than five points were considered in remission, between five and seven mildly active disease, eight and 16 moderate activity, and greater than 16 severe activity3.
Total energy expenditure was calculated considering the value of resting energy expenditure x 1.39. Nutritional needs were calculated according to the Dietary Reference Intakes considering the recommendations in accordance with the Recommended Dietary Allowance or Adequate Intake according to sex and age. To assess the adequacy of protein intake were considered the values established by the Recommended Dietary Allowance of 0.8 to 1.2 g/kg/day and fiber intake were considered AI values established as for men of 38 g/day and for women 25 g/day, aged from 19 to 50 years, and 30 g/day for men and 21 g/day women over 51 years15. To assess the adequacy of macronutrient intake, were considered an appropriate percentage from 45 to 65% of total energy expenditure of carbohydrates, 10-35% protein and 20-30% lipids15. To analyze the intake of retinol, iron, zinc, thiamin, riboflavin, niacin, pyridoxine, folate, cyanocobalamin, ascorbic acid, tocopherol, selenium, magnesium and phosphorus was considered the Recommended Dietary Allowance. In the adjustment of consumption of menadione, manganese, pantothenic acid, biotin, potassium, sodium, calcium, and cholecalciferol, was considered the values of AI, because these nutrients have no Estimated Average Requirement and therefore have no points of reference for Recommended Dietary Allowance10,11,12,13,14,15.
For analysis of food consumption were asked to patients throughout the history food, who reported their usual dietary intake 24 hours. At that time, patients also answered a food frequency questionnaire, which was analyzed according to the Food Guide for the Brazilian population4. For the calculation of dietary intake was used the software Dietwin Nutritional Assessment6.
Results were presented considering mean and standard deviation. To compare means was used the unpaired t test, and the average non-parametric the Mann-Whitney test, level of significance p <0.05
RESULTS
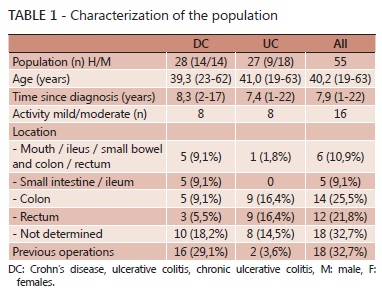
Were evaluated 55 patients with inflammatory bowel disease, aged between 19 and 63 years and time since diagnosis of 7.9 years (1 to 22). All were literate and 80% (44) lived in Curitiba and metropolitan area; 58% (32) were female and aged 40 years (Table 1).
The classification of disease activity according to the PCR was compared with disease activity by Harvey Bradshaw index in patients with CD; both PCR as this index classified 30.8% of patients in activities and 69.2% in remission. Of the patients with UC were active 39% and 61% in remission. Of these patients, 17 received corticosteroid therapy.
Less than 20% of patients reported physical activity as walk, football in weekends, gymnastics and weight training performed with an average frequency of one to three times a week.
CD patients, in activity or in remission, had lower energy intake that apparently needs, but was not significant. Protein intake was higher than necessary, with a significant difference for patients in remission (p <0.05). All CD patients showed low intake of potassium, magnesium, calcium, cholecalciferol and tocopherol (p <0.005). It was found that activity in CD patients also had low intake of sodium, riboflavin, retinol, folate, pantothenic acid (p <0.05) and menadione (p <0.005), while patients in remission had low intake of niacin (p <0.05), sodium and riboflavin (p <0.005). CD patients in remission had retinol intake and manganese higher than needed (P <0.005) (Table 2).
Patients with active UC had a lower intake of energy and kcal/kg (p <0.05), with lower energy intake of patients with UC in remission (p <0.01). Both in activity or in remission had low intake of potassium, magnesium, calcium, cholecalciferol and tocopherol (p <0.005). Patients with active UC had low intakes of thiamin, folate, pantothenic acid (p <0.005), riboflavin, pyridoxine and ascorbic acid (p <0.05). Patients with UC in remission had low intake of retinol (p <0.005), phosphorus, folate (p <0.05) and pantothenic acid (p <0.01). The intake of manganese was greater than the needs (p <0.05) for all patients with UC (Table 3).
According to food frequency, there was low intake of vegetables, fruits, dairy products and beans, adequate intake of starchy and excessive intake of sweets and fats (Table 4). The most common food intolerances reported by patients were related to lactose (50%), fruits and vegetables (33%), meat (20%), beans/soy and sweet (12%), condiments (9%) and fat/fried foods and oilseeds (9%). Intolerance to bread/pasta, coffee and soft drinks were reported less frequently (5%) patients.
DISCUSSION
Studies have found that indirect calorimetry in relation to resting energy expenditure in patients with IBD in remission showed no difference compared with healthy controls9. The same was observed in active CD patients without steroids30, but activity in patients in remission and who received corticosteroids had higher resting energy expenditure than controls2.
Although there is no change in energy needs, it is observed that there is inadequate food intake in patients with IBD, both active and in remission. In the study by Geerling BJ et al.16 patients with newly diagnosed IBD, in remission, had higher intake of carbohydrates and less protein compared with healthy controls, according to the dietary history. CD patients showed no difference in micronutrient intake, whereas patients with UC ate significantly less calcium, riboflavin and vitamin C. Evaluating the three-day food records of 54 patients with CD in remission Filippi et al.9 was found inadequate diet, with significantly lower intake of vitamin C and pyridoxine intake than controls and deficient in pyridoxine, folic acid, ascorbic acid, cholecalciferol, tocopherol, calcium and zinc. When evaluated the dietary intake of outpatients in seven days of food records, Aghdassi et al.1 found no significant difference between patients in remission or activity, however poor intake was observed for retinol, tocopherol, ascorbic acid, thiamine, riboflavin, niacin, folate, pyridoxine, calcium, zinc and iron in both groups of patients. In the study based on food intake in 1543 subjects, there was greater variability in the food groups in nutrient intake, possibly because nutrients can come from different food sources27.
Although studies in the literature have used different methodologies for assessment of food intake deficiencies, in this study were similar.
The reported food intolerance, especially lactose, fruits, vegetables and beans may have influenced the low consumption of these groups. The portions were lower than the daily intake recommendations of the Ministry of Health of Brazil4.
The decreased intake of fruits and vegetables in this study was similar to that observed in in 32 patients with IBD32. Reducing the intake of fruits and vegetables results lower intake of fiber, which may be associated during active disease, to reduce intestinal motility and bacterial fermentation and to prevent abdominal symptoms1. However, some patients continue with their restrictions after the disease activity is controlled, which contributes to lower intake of micronutrients and antioxidants9. The exclusion of fruits and vegetables also contributed to the low intake of folate in patients with UC19.
The report of lactose intolerance is an important finding of this paper, and can influence calcium intake. Experimental oral tolerance to lactose in 67 patients with IBD and 25 controls, showed no significant difference between groups22. Insufficient calcium intake in patients with IBD (n = 152) was higher than in healthy controls (n = 73). The major deficiencies were found in 47% (n = 71) of patients who were lactose-restricted diet, although the tolerance tests were abnormal in only 25% (n = 18) of patients31. These findings indicate the importance of an observed individual tolerance to milk and dairy products, identifying whether the acceptance of these foods is related to lactose intolerance or allergy to milk proteins8.
Among the studied patients, was observed that there was deficiency of iron intake only in patients with UC in remission, similar to what Heatley18 found in his paper that iron deficiency was more common in patients with UC.
Intolerance of bread or pasta in patients with IBD reported by some patients may be explained by the production of Saccharomyces cerevisiae antibodies (ASCA positive), especially in individuals with localized disease in the small intestines5.
The lower intake of fruits, vegetables and milk and higher intakes of animal protein, cereals, fats and simple carbohydrates in patients with UC was associated with a higher risk of developing colon cancer29. The intake of processed foods and highly refined, characteristic of the modern diet is detrimental mainly by adding the risk factor related to consumption of sulfite present in processed meats23. The beliefs of these patients may also influence food intake of important nutrients19.
It must be put in mind, that the present study evaluated only nutrient intake according to dietary recall, not considering the use of supplements such as folate, calcium and vitamin D used by some patients. It is known that drug treatment of IBD with sulfasalazine decreases the absorption of folic acid and corticosteroids reduce calcium absorption33. It is well established that patients treated with corticosteroids should receive supplementation of cholecalciferol 800-1000 IE / 1000 mg / d calcium ion21. The diet should be balanced and without restrictions, and patients, especially those in remission, should be encouraged to improve their eating habits32.
CONCLUSION
There was deficiency in food intake in both CD and UC in active and in remission. These deficiencies are mainly related to the intake of macronutrients, energy and fiber and micronutrients, including calcium, folic acid, cholecalciferol, tocopherol, menadione, retinol, riboflavin, pyridoxine, thiamine and niacin. The shortcomings can adversely affect the course of the disease, and justify the need for nutritional intervention with these patients.
REFERENCES
6. Dietwin® (Software de Avaliação Nutricional). Versão 2.2,24. Brasil, 1995-2007.
- 1. Agdassi E, Wendland BE, Stapleton M, Raman M, Allard JP. Adequacy of Nutritional Intake in a Canadian Population of Patients With Crohn's Disease. J Am Diet Assoc. 2007; 107(9):1575-1580.
- 2. Al-Jaouni R, Hébuterne X, Pouget I, Rampal P. Energy metabolism and substrate oxidation in patients with Crohn´s disease. Nutrition. 2000, 16:173-178.
- 3. Best WR. Predicting the Crohn's Disease Activity Infex From the Harvey-Bradshaw Index. Inflamm Bowel Dis. 2006; 12(4):304-310.
- 4. Brasil. Ministério da Saúde. Guia alimentar para a população brasileira. Brasília, DF; 2005.
- 5. Brunner B, Scheurer U, Seibold F. Differences in Yeast Intolerance Between Patients With Crohn's Disease and Ulcerative Colitis. Dis Colon Rectum. 2007; 50(1):83-88.
- 7. Duggan P, O´Brien M, Kiely M, McCarthy J, Shanahan F, Cashman KD. Vitamin K status in patients with Crohn's disease and relationship to bone turnover. Am J Gastroenterol 2004; 99(11): 2178-2185.
- 8. Ferguson LR, Shelling AN, Browning BL, Huebner C, Petermann I. Genes, diet and inflammatory bowel disease. Mutat Res. 2007; 622:70-83.
- 9. Filippi J, Al Jouani R, Wiroth JB, Hébuterne X, Schneider SM. Nutritional Deficiencies in Patients With Crohn's Disease in Remission. Inflamm Bowel Dis. 2006; 12(3):185-191.
- 10. Food and Nutrition Board. Institute of Medicine. Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D. And Fluoride. Washington DC: National Academy Press; 1997.
- 11. Food and Nutrition Board. Institute of Medicine. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin and Choline. Washington DC: National Academy Press; 1998.
- 12. Food and Nutrition Board. Institute of Medicine. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. Washington DC: National Academy Press; 2000.
- 13. Food and Nutrition Board. Institute of Medicine. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. Washington DC: National Academy Press; 2000.
- 14. Food and Nutrition Board. Institute of Medicine. Dietary Reference Intakes for Water, Potassium, Sodium, Chloride, and Sulfate. Washington DC: National Academy Press; 2004.
- 15. Food and Nutrition Board. Institute of Medicine. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients). Washington DC: National Academy Press; 2005.
- 16. Geerling BJ, Badart-Smook A, Stockbrügger RW, Brummer RJM. Comprehensive nutritional status in recently diagnosed patients with inflammatory bowel disease compared with population controls. Eur J Clin Nutr. 2000; 54:514-521.
- 17. Harvey RF, Bradshaw JM. A Simple Index of Crohn Disease Activity. Lancet 1980; 1:514.
- 18. Heatley RV. Assessing nutritional state in inflammatory bowel disease. Gut 1986; 27:61-66.
- 19. Jowett SL, Seal CJ, Phillips E, Gregory W, Barton JR, Welfare MR. Dietary beliefs of people with ulcerative colitis and their effect on relapse and nutrient intake. Clin Nutr. 2004; 23(2):161-170.
- 20. Leslie WD, Miller N, Rogala L, Bernstein CN. Vitamin D status and bone density in recently diagnosed inflammatory bowel disease: The Manitoba IBD Cohort Study. Am J Gastroenterol 2007; 102: 1-9.
- 21. Lochs H, Dejong C, Hammarqvist F, Hebuterne X, Leon-Sanz M, Schütz T, van Gemert W, van Gossun A, Valentini L, Lübke H, Bischoff S, Engelmann N, Thul P.. ESPEN Guidelines on Enteral Nutrition: Gastroenterology. Clin Nutr 2006; 25:260-274.
- 22. Madrid RB, Benarroch HS, Sánchez SM,Sánchez FG, Meroño AA, Martínez JM. Malabsorción de lactosa em pacientes con enfermedad inflamatoria intestinal inactiva: ¿está justificado excluir los productos lácteos a todos los pacientes? An Med Interna. 2004; 21(5):212-214.
- 23. Magee EA, Edmond LM, Tasker SM, Kong SC, Curno R, Cummings JH. Associations between diet and disease activity in ulcerative colitis patients using a novel method of data analysis. Nutrition Journal. 2005; 4:7.
- 24. Mathers JC. Reversal of DNA hypomethylation by folic acid supplements: possible role in colorectal cancer prevention. Gut 2005; 54: 579-581.
- 25. O'Sullivan M, O'Morain C. Nutrition in inflammatory bowel disease. Best Pract Res Clin Gastroenterol. 2006; 20(3):561-573.
- 26. Rajendran N, Kumar D. Role of diet in the management of inflammatory bowel disease. World J Gastroenterol. 2010; 16(12):1442-1448.
- 27. Palaniappan U, Cue RI, Payette H, Gray-Donald K. Implications of Day-to-Day Variability on Measurements of Usual Food and Nutrient Intakes J Nutr. 2003; 133:232.235.
- 28. Rodríguez-Borez L, Barahona-Garrido J, Yamamoto-Furusho JK. Basic and clinical aspects of osteoporosis in inflammatory bowel disease. World J Gastroenterol. 2007; 13(46): 6156-6165.
- 29. Rosman-Urbach M, Niv Y, Birk Y, Morgestern S, Schwartz B. Relationship between nutritional habits adopted by ulcerative colitis relevant do cancer development patients at clinical remission stages and molecular-genetic parameters. Br J Nutr. 2006; 95:188-195.
- 30. Schneeweiss B, Lochs H, Zauner C, Fischer M, Wyatt J, Maier-Dobersberger T, Schneider B. Energy and substrate metabolism in patients with active Crohn´s disease. J Nutr. 1999, 129:844-848.
- 31. Silvennoinen J, Lamberg-Allardt C, Kärkkäinen M, Niemelä S, Lehtola J. Dietary calcium intake and its relation to bone mineral density in patients with inflammatory bowel disease. J Intern Med 1996; 240:285-292.
- 32. Valentini L, Schaper L, Buning C, Hengstermann S, Koernicke T, Tillinger W, Greglielmi FW, Norman K, Buhner S, Ockenga J, Pirlich M, Lochs H. Malnutrition and impaired muscle strength in patients with Crohn´s disease and ulcerative colitis in remission. Nutrition 2008; 24:694-702.
- 33. Wild GE, Drozdowski L, Tartaglia C, Clandinin MT, Thomson ABR. Nutritional modulation of the inflammatory response in inflammatory bowel disease - From de molecular to the integrative to the clinical. World J Gastroenterol. 2007; 13(1):1-7.
Food intake in patients with inflammatory bowel disease
Publication Dates
-
Publication in this collection
15 Dec 2011 -
Date of issue
Sept 2011
History
-
Received
06 Dec 2010 -
Accepted
19 Apr 2011