Abstracts
Two plants were investigated for their properties against the local effects of Bothrops alternatus venom in rabbits, namely Curcuma longa L. (Zingiberaceae) and Aristolochia cymbifera L. (Aristolochiaceae). The experiments showed that, Curcuma longa extract topic application was the most effective treatment against local symptoms (edema, hemorrhages and necrosis) caused by Bothrops venom, whereas the results with Aristolochia cymbifera L. were doubtful, because they led to a larger damaged area during the early phase of the inflammation process, besides skin mummification.
Curcuma longa; Aristolochia cymbifera; Bothrops alternatus; snake venom
Extratos de duas plantas, Curcuma longa L. (Zingiberaceae) e Aristolochia cymbifera L. (Aristolochiaceae), foram utilizados no tratamento local do envenenamento por Bothrops alternatus em coelhos. Este estudo demonstrou que o extrato tópico de Curcuma longa foi o mais efetivo tratamento contra os efeitos locais (edema, hemorragia e necrose) causados pelo veneno botrópico. O tratamento com o extrato de Aristolochia cymbifera apresentou resultados duvidosos, pois causou uma grande área de lesão no início do processo inflamatório, além de mumificação da pele.
Curcuma longa; Aristolochia cymbifera; Bothrops alternatus; veneno de cobra
ARTIGO
Plant extracts for topic therapy of Bothrops alternatus envenomation
Tratamento tópico com extratos de plantas no envenenamento por Bothrops alternatus
Marilia Martins MeloI,* * E-mail: marilia@vet.ufmg.br, Tel. +55-31-34992229 ; Maria LúciaII; Gerhard G. HabermehlIII
IDepartamento de Clínica e Cirurgia Veterinárias, Escola de Veterinária, Universidade Federal de Minas Gerais, Avenida Antônio Carlos, 6.627, 30123-970, Belo Horizonte, MG, Brasil
IIFaculdade de Farmácia, Universidade de Alfenas, Rod. MG 179, km 0, 37130-000, Alfenas, MG, Brasil
IIIChemisches Institut, Tierärztliche Hochschule, Bischofsholer Damm 15, D-30173, Hannover, Germany
ABSTRACT
Two plants were investigated for their properties against the local effects of Bothrops alternatus venom in rabbits, namely Curcuma longa L. (Zingiberaceae) and Aristolochia cymbifera L. (Aristolochiaceae). The experiments showed that, Curcuma longa extract topic application was the most effective treatment against local symptoms (edema, hemorrhages and necrosis) caused by Bothrops venom, whereas the results with Aristolochia cymbifera L. were doubtful, because they led to a larger damaged area during the early phase of the inflammation process, besides skin mummification.
Keywords:Curcuma longa, Aristolochia cymbifera, Bothrops alternatus, snake venom.
RESUMO
Extratos de duas plantas, Curcuma longa L. (Zingiberaceae) e Aristolochia cymbifera L. (Aristolochiaceae), foram utilizados no tratamento local do envenenamento por Bothrops alternatus em coelhos. Este estudo demonstrou que o extrato tópico de Curcuma longa foi o mais efetivo tratamento contra os efeitos locais (edema, hemorragia e necrose) causados pelo veneno botrópico. O tratamento com o extrato de Aristolochia cymbifera apresentou resultados duvidosos, pois causou uma grande área de lesão no início do processo inflamatório, além de mumificação da pele.
Unitermos:Curcuma longa, Aristolochia cymbifera, Bothrops alternatus, veneno de cobra.
INTRODUCTION
The majority of snake bites accidents in South America are inflicted by species classified in the genus Bothrops (Barraviera; Pereira, 1994). The bothropic venom induces a qualitatively similar pathophysiological picture, characterized by: immediate and prominent local tissue damage (edema, hemorrhage and mionecrosis); vascular alterations (hemorrhage and hypovolemic shock), coagulation disorders (defibrination) that might evolve into acute renal failure (Barraviera; Pereira, 1994; Kamiguti et al., 1996).
If antivenom administration is initiated rapidly after envenomation, neutralization of systemic effects usually is achieved successfully, but neutralization of local tissue damage is a more difficult task. In a number of snake bite cases, lack of neutralization of local effects results in permanent sequelae, with tissue loss (Jorge et al., 1999; Wen, 2000). Due to relevance of local effects in envenomations induced by snakes of the genus Bothrops, several research groups have studied this problem from different perspectives (Santos et al., 2003; Ferreira et al., 2003; Fonseca et al., 2004; Melo et al., 2005).
The use of phytotherapy against the snake venom has long been recognized, even in modern times but only for the last 20 years has it gained closer scientific attention (Mors et al., 2000).
Curcumin is an important constituent of rhizomes of Curcuma longa L. (Zingiberaceae) and it is responsible for the yellow color of the rhizome (Amman; Wahl, 1990). The compound possesses a significant anti-inflammatory activity in acute as well as in chronic models of inflammation (Srimal; Dhawan, 1972). Also, it has been shown that curcumin is a potent inhibitor of leukotriene B4 formation in rat peritoneal polymorphonuclear neutrophils (Amman et al., 1992).
One fraction, namely ar-turmerone, was isolated from C. longa L. This fraction neutralized both the hemorrhagic activity present in Bothrops jararaca venom and the lethal effect of Crotalus durissus terrificus venom in mice. Immunological studies demonstrated that this fraction also inhibited the proliferation and natural killer activity of human lymphocytes (Ferreira et al., 1992).
The roots of Aristolochia species are traditional remedies against snake venom. Most of the Aristolochia species chemically studied produced in their roots peculiar organic nitro-compounds, having a phenanthreme nucleus: the aristolochic acids and aristolactams (Mors et al., 2000). França et al. (2003 and 2005) reported the isolation of terpenoids, lignoids, antraquinone and vanillin from Aristolochia birostris.
As shown above, despite the therapeutic properties attributed to C. longa L. and Aristolochia species, these plants have not been well investigated for its biological activities against the venom of Bothrops alternatus. The main purpose of this study was to evaluate the therapeutic efficacy of the isopropanol-aqueous extract from rhizomes of Curcuma longa L. and leaves of Aristolochia cymbifera L. using bothropic envenomation as experimental model in rabbits.
MATERIAL AND METHODS
Plant material
Rhizomes of Curcuma longa L. (Zingiberaceae) and green leaves of Aristolochia cymbifera L. (Aristolochiaceae) were collected from cultivated specimens in the Jardim Botânico, Universidade Federal de Minas Gerais, Brazil (approximately 800 m height) in February 2005. A voucher specimen of Aristolochia cymbifera is deposited at UFMG herbarium (BHCH 931).
For the first extract, 327 g of fresh rhizomes of Curcuma longa L. were cut and macerated with 100 mL of distillated water plus 400 mL of isopropanol for 24 hours at room temperature (25 °C). And for the second extract, 126.5 g of green leaves of Aristolochia cymbifera were subjected to the same procedure. Both aqueous-isopropanol extracts obtained were concentrated to circa 50% under vacuum until isopropanol elimination. The final concentration of Curcuma longa and Aristolochia cymbifera in 1.0 ml was 1.62 g and 0.38 g respectively.
Animals
Desiccated venom of Bothrops alternatus (12.5 µg) in 100 µL of saline was injected into the shaved dorsal back skin (intradermally) of 25 white New Zealand rabbits (mean weight 900 g).
The rabbits were divided into five groups, with five animals in each: group I - topic application of Curcuma longa L. extract (1.0 mL); group II - subcutaneous treatment of C. longa L. extract (1.0 mL); group III - topic application of Aristolochia cymbifera L. extract (1.0 mL); group IV - subcutaneous treatment of A. cymbifera L. extract (1.0 mL); group V (control group): topic application of distilled H20 (1.0 mL). These treatments were performed for 30 min, 2 h and 4 h after venom inoculation (a.v.i.).
Clinical evaluation
Edema activity was estimated by pachymeter measurement after 30 min, 2 h, 4 h, 24 h and 72 h a.v.i. The development of the hemorrhage was estimated by diameter determination with a special ruler in millimeters at the same time intervals as described above (Melo et al., 2005).
Blood was collected from the heart, with and without Na2EDTA, and the hematological and serum biochemical parameters (total protein, blood urea nitrogen, creatinine, alkaline phosphatase, triglicerides, aspartate amino-transferase and cholesterol) were evaluated (Kaneko et al., 1997).
Necrosis and other lesions were evaluated in skin, muscle, heart, lungs, liver, spleen and kidneys, by necropsy and histopathology. After 72 h, the animals received anesthesia (thiopental 50 mg/kg) intramuscularly and were sacrificed and submitted to necropsy, except for one animal in each group. Tissue fragments (1.0 cm) were fixed with 10% formalin buffered solution. After 24 hours in the formalin solution, the fragments were transferred to flasks containing ethyl alcohol (70%, 80%, 90% and 100%) and allowed to standard for two hours in each concentration, then embedded in paraffin, sectioned at 5 µm, and stained by Hematoxilin/Eosin (Prophet et al., 1992).
Statistical analysis
The completely randomized design was employed. Mean and standard deviation were calculated for hemorrhage diameter and edema within each group and statistical analysis was performed by Duncan test, and P-value of less than 0.05 level of significance was considered to indicate significance.
RESULTS AND DISCUSSION
Clinical evaluation
After 15 minutes past venom inoculation, all the animals presented apathy, hyporexia, increased corporal temperature (hyperthermia) and exhaustion, although the normal nervous reflexes were present. There was also a decrease of urination and defecation.
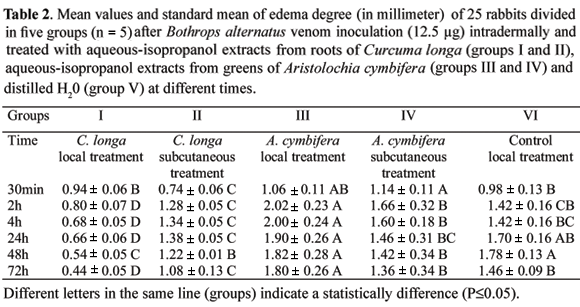
The topic application of the extract of Curcuma longa L. (group I) avoided the increase of the hemorrhagic halo on the inoculation site decreasing the diameter of 21.8 ± 2.1 mm within 30 min to 5.2 ± 0.45 mm within 72 h (Table 1). Also, the edema decreased from to 0.94 ± 0.06 mm (30 min) to 0.44 ± 0.05 mm (72 h) (Table 2). Many researches are trying to find the best and most efficient treatment capable of neutralizing the local inflammation induced by Bothrops venom. Three diarylheptanoids - curcumin 15 (diferuyl-methane), demethoxycurcumin and bis demethoxycurcumin, make up the yellow dye of rhizomes of turmeric, Curcuma longa. These curcuminoids are the only natural pigments of this class. Well known as a spice and coloring matter, turmeric is also widely used as medicinal in oriental tradition, the treatment of snake bite poisoning being one of its uses. In the structure of curcumin, two ferulic acid moieties are linked via a methylene bridge, resulting in a conjugated diketone, which, in solution, through keto-enol tautomerism, produces a strong chelating centre. Many elements have been shown to form chelates with the curcuminoids. There are indications that curcumin also interacts strongly with biological macromolecules, like serum proteins, albumin and hyaluronic acid (Mors et al., 2000). Among the many pharmacological properties shown by curcuminoids, anti-inflammatory, hepatoprotective, inhibition of lipoxygenase, inhibitors of the enzyme acetylcholinesterase and prostaglandin-endoperoxide synthetase stand out (Amman & Wahl, 1990; Barbosa-Filho et al., 2006). Another unsaturated ketone of turmeric, ar-turmerone, neutralizes both the hemorrhagic activities present in Bothrops jararaca venom, and lethal effect of Crotalus durissus terrificus venom in mice (Ferreira et al., 1992).
The subcutaneous treatment with the extract of Curcuma longa L. (group II) did not avoid the increase of hemorrhagic halo at the inoculation site. The halo diameter passed from an averaged 23.8 1.64 mm (30 min a.v.i.) to 29.4 2.96 mm within 72 h (Table 1). For the animal left alive for observation, after the 4th day a.v.i., the hemorrhagic halo gradually reduced and, at day 15, the skin peeled off with total healing at day 20. Edema, besides the smaller (P < 0.05) in this group (0.74 0.06 mm) when compared with other groups (Table 2), 2 and 4h later, increased and showed similar to control group and in 24 h, one animal had total skin loss at the wound. All the animals were bleeding at the inoculation site after 48 h. These observations suggest that parenteral treatments should be avoided as well as repeated blood collections, due to the metalloproteinases and substances like thrombin that are part of the composition of the bothropic venom (Kamiguti et al., 1996).
The animals of group III (subcutaneous treatment of Aristolochia cymbifera L.) presented within 30 min a.v.i., the mean hemorrhagic halo diameter of 25.8 4.3 mm, that gradually increased up to 35.4 1.9 mm within 24 h, the biggest value(P < 0.05) when compared with other groups (Table 1). In the animal left alive for observation, the hemorrhagic halo on days 4 and 5, had largest diameter (38.0 mm) that later reduced up to the 12th day when the skin peeled off the wound. On the 13th day, a cicatrization area was evident which reduced from the 17th day and totally disappeared on the 20th day. The edema degree was 1.06 0.12 mm at 30 min, increasing to 2.02 0.22 m at 2 h, and decreasing at subsequent times (Table 2). All animals were bleeding over the wound, and within 24 h, showed a great damage area.
The animals of group IV (treatment subcutaneous of extract of Aristolochia cymbifera L.) presented the largest hemorrhagic halo average (P < 0.05) within 30 min (29.2 1.8 mm) that increased up to 32.4 2.9 mm within 2 h a.v.i. (Table 1). Also, within 30 min a.v.i., the local edema was the biggest (P < 0.05) (1.14 0.11 mm) that increased up to 1.66 mm within 2 h a.v.i. (Table 2). Forty-eight hours after inoculation of the venom, all animals had dermal ulceration at the inoculation site. In the animal left alive for observation, the skin was dry, with a mummified aspect, peeling off at the 12th day and started the scar reduction by the 13th day.
For groups treated with extract of Aristolochia cymbifera L. (groups III and IV), mummification of skin wound was observed, besides the rabbits showed excessive discomfort when the extracts were applied.
In the group V (control), the hemorrhagic halo diameter was of 21.8 1.3 mm within 30 min a.v.i. that gradually increased up to 28.2 2.7 mm within 4 h. In one animal that was maintained alive, the extensive lesion that occurred at the inoculation site culminated with death at the 13th day.
Although, animals of the groups III and IV (Aristolochia cymbifera L.) have survived, a better evaluation of this extract is necessary. These extracts led to a larger damaged area than control group during the early phase of the inflammation process, besides discomfort in the animals and skin mummification.
Blood profile and serum biochemistry
All rabbits showed anemia (microcitic hypochromic), polychromatic erythrocytes, anysocitosis and rubrocytes due to the bleeding (Santos et al., 2003). In all treated rabbits (groups treated with C. longa and A. cymbifera extract), a leukopenia was observed which was different from group V (control) that showed leukocitosis. Curcuma longa L. has a compound (Ferreira et al., 1992), and possibly also Aristolochia cymbifera, which possesses significant anti-inflammatory activity and probably this leukopenia can be explained by this.
Despite of platelets were within the normal parameters, smaller values were observed in group V (control.). Smaller value of total protein was also observed in Group V (4.71 g/dL), due to the lost skin, plasmatic transudation and bleeding (Oliveira, 2005).
A mild increase of blood urea nitrogen (BUN) was observed in all animals, however group IV (treated subcutaneously with the extract of Aristolochia cymbifera L.) showed the highest values, as described by Oliveira (2005). Renal failure was not observed. All rabbits urinated normally, not showing anuria and the creatinine levels were normal. Despite Barraviera and Pereira (1994) claimed this as a rare complication of snake accident, this symptom could not be observed, due to the quantity of venom inoculated.
It was observed a decrease of alkaline phosphatase and increase of triglicerides in all rabbits, especially in group IV (treated subcutaneously with the extract of Aristolochia cymbifera L.). This picture is described in hypothyroidism, lowered metabolism (Kaneko et al., 1997). Also, highest values of aspartate amino-transferase (AST) were observed in group IV, probably due to the muscular and cardiac lesion (Zenckers degeneration) described in histopathological findings.
Cholesterol was normal in all the groups except in group V which showed a decrease value.
Post-mortem findings
The following post-mortem findings were observed in most rabbits: a great area of cutaneous ulceration (compromising of the dermis and subcutaneous tissue) outside of the inoculation area with intense hemorrhage (in whole back-lumbar area, deepening for the muscular tissue with petechial hemorrhages) and edema. The heart was found with flaccid ventricular wall, cyanotic and with prominent vases. There was presence of coagula in both heart chambers. The lungs were rosy with presence of subpleural petechial hemorrhage and edema. Kidneys were pale and petechial hemorrhages were observed in thymus and spleen. The spleen was flaccid in consistence as well intestinal loops. In group V, all the animals showed hemorrhages in the paws and at the anus and the kidneys had petechial hemorrhages and several retraction areas (infarct).
The accentuated edema resulted possibly, of the capillary alterations and the effect of the proteolytic fractions of the venom (Gutierrez; Lomonte, 1989). The petechial and ecchymotic hemorrhage in many tissues can be attributed to the toxic action of the hemorragins (bothroalternin) and of other fractions (Castro et al., 1998), particularly frequent in group V. In spite of normal number platelets for almost all the groups, the animals presented hemorrhage in several tissues. It is important to point out that the platelets need to be as numerous as functionally normal (Troy, 1988).
Microscopic findings
Comparing skin microscopic results among the different groups indicated that the least effective treatments were employed for groups III and VI (Aristolochia cymbifera L. extract). The groups treated with Aristolochia cymbifera behaved as the control group showing Zenckers degeneration in the heart, dilation of the hepatic capillary sinusoids with deposition of acidophilus material (amyloidosis), glomeruli with deposition of acidophilus material and strong necrosis of the epidermis with complete destruction of the subcutaneous and muscular tissue (Queiroz; Petta, 1984) with acute inflammation (Table 3).
Roots from Aristolochia serpentaria L. (Aristolochiaceae) are described as snake venom antidote (Mors et al., 2000). Although the animals of groups III and IV have survived, the results with topic application of the extracts of Aristolochia cymbifera L. for Bothrops envenomation were not satisfactory. A better evaluation of these extracts is necessary, considering the capability to cause pain and discomfort in the animals, resulting in the mummification of the wound, as well as the least benefit observed both in biochemical and histopathological exams.
These results led to the conclusion that Curcuma longa L. (Zingiberaceae) extract topic application was the most effective treatment against local symptoms (edema, hemorrhages and necrosis) caused by snake envenomation with Bothrops alternatus.
Clinical evidence of efficacy of plant extracts should be provided and whenever possible some indication of the mechanism of action and what components of the original plant contribute to this action is desirable. Further studies are being done with aiming to identifying other plant extracts effective against Bothrops venom.
ACKNOWLEDGEMENTS
We would like to thank Internationales Büro of the Federal Ministry of Science and Technology in Bonn. On the Brazilian side the project is supported by Conselho Nacional de Pesquisa e Tecnologia (CNPq).
Received 06/18/06. Accepted 11/21/06
- Amman HPT, Anazodo MI, Safayhi H, Dhawan BN, Srimal RC 1992. Curcumin: A potent inhibitor of leukotriene B4 formation in rat peritoneal polymorphonuclear neutrophils (PMNL). Planta Med 58: 226.
- Amman HPT, Wahl MA 1990. Pharmacology of Curcuma longa. Planta Med 57: 1-7.
- Barbosa-Filho JM, Medeiros KCP, Diniz MFFM, Batista LM, Athayde-Filho PF, Silva MS, Cunha EVL, Almeida JRGS, Quintans-Júnior LJ 2006. Natural products inhibitors of the enzyme acetylcholinesterase. Rev Bras Farmacogn 16: 258-285.
- Barraviera B, Pereira PCM 1994. Acidentes por serpentes do gênero Bothrops In: Barraviera, B. (Coord.) Venenos animais: uma visão integrada, Rio de Janeiro: Publicações Científicas.
- Castro HC, Dutra DLS, Oliveira-Carvalho AL, Zingali RB 1998. Bothroalternin, a thrombin inhibitor from the venom of Bothrops alternatus. Toxicon 36: 1903-1912.
- Ferreira LA, Henriques OB, Andreoni AAS, Vital GRF, Campos MMC, Habermehl GG, Moraes VLG 1992. Antivenom and biological effects of ar-turmerone isolated from Curcuma longa (Zingiberaceae). Toxicon 30: 1211-1218.
- Ferreira KM, Melo MM, Dantas-Barros AM 2003. Tratamento tópico de coelhos com Agaricus blazei Murril após envenenamento botrópico experimental. Rev Bras Farmacogn 13(Supl. 1): 77-80.
- Fonseca FV, Melo MM, Silva J, Pereira GP, Dantas-Barros AM 2004. Extratos de Curcuma longa L. e Kalanchoe brasiliensis Camb. no tratamento local do envenenamento por Bothrops alternatus. Rev Bras Farmacogn 14(Supl. 1): 26-29.
- França VC, Agra MF, Barbosa-Filho JM, Da-Cunha EVL, Silva MS 2003. Physcion and dihydrocarinatin from Aristolochia birostris. Biochem Syst Ecol 31: 1341-1343.
- França VC, Vieira KVM, Lima EOL, Barbosa-Filho JM, Da-Cunha EVL, Silva MS 2005. Estudo fitoquímico das partes aéreas de Aristolochia birostris Ducht. (Aristolochiaceae). Rev Bras Farmacogn 15: 326-330.
- Gutiérrez JM, Lomonte B 1989. Local tissue damage induced by Bothrops snake venoms. A Review. Mem Inst Butantan 51: 211-223.
- Jorge MT, Ribeiro LA, O'Connell JL 1999. Prognostic factors for amputation in the case of envenoming by snakes of Bothrops genus (Viperidae). Ann Trop Med Parasitol 93: 401-408.
- Kamiguti AS, Hay CRM, Theakston RDG, Zuzel M 1996. Review Article. Insights into the mechanism of haemorrhage caused by snake venom metalloproteinases. Toxicon 34: 327-642.
- Kaneko JJ, Harvey JW, Bruss ML 1997. Clinical biochemistry of domestic animals. 5ed. San Diego: Academic press.
- Melo MM, Habermehl GG, Oliveira NJF, Nascimento EF 2005. Treatment of Bothrops alternatus envenomation by Curcuma longa and Calendula officinalis extracts and ar-turmerone. Arq Bras Med Vet Zoot 57: 7-17.
- Mors WB, Nascimento MC, Pereira BMR, Pereira NA 2000. Plant natural products active against snake bite - the molecular approach. Phytochesmitry 55: 627-642.
- Oliveira NJF 2005. Avaliação clínica e laboratorial de bovinos experimentalmente inoculados com veneno de Bothrops alternatus bruto e iodado. Belo Horizonte, 181p. Tese de Doutorado - Programa de Pós-graduação em Ciência Animal, Universidade Federal de Minas Gerais.
- Prophet EB, Mills B, Arrigton JB 1992. Afip Laboratory Methods in Histotechonology Washington: Am. Registry of Pathology, 278p.
- Queiroz LS, Petta CA 1984. Histopatological changes caused by urutu snake (Bothrops alternatus) in mouse skeletal muscle. Rev Inst Med Trop São Paulo 26: 247-253.
- Santos MMB, Melo MM, Jacome DO, Habermehl GG 2003. Avaliação das lesões locais de cães envenenados experimentalmente com Bothrops alternatus após diferentes tratamentos. Arq Bras Med Vet Zoot 55: 639-644.
- Srimal RC, Dhawan BN 1972. Pharmacology of diferyloyl methane (curcumin) a non-steroidal anti-inflammatory agent. J Pharm Pharmacol 25: 447-452.
- Troy GG 1988. An overview of hemostasis. Vet Clin North Am Small Anim Pract 18: 5-20.
- Wen FH 2000. Ineficácia do antiveneno na reversão do edema e necrose em pacientes picados por serpentes do gênero Bothrops, Simpósio da Sociedade Brasileira de Toxinologia 6, São Pedro, São Paulo, 15 a 18 de março de 2000, Anais, p.78-81.
Publication Dates
-
Publication in this collection
22 May 2007 -
Date of issue
Mar 2007
History
-
Accepted
21 Nov 2006 -
Received
18 June 2006