Abstracts
In the current study, we tested the effect of 27 plant extracts and fractions from different botanical families on the activity of Pdr5p from yeast plasma membrane, responsible for the multidrug resistance phenotype in yeast cells. Some of the extracts were able to produce a good inhibition in the fixed concentration (200 µg/mL) and were selected for a deeper investigation. Dose-response curves were obtained for the crude ethanol extracts of Bathysa australis (A. St.-Hill.) Benth. & Hook f., Mabea fistulifera Mart. and Virola oleifera (Schott) A. C. Sm. with concentrations ranging up to 400 µg/mL. The lower IC50 value was obtained for Virola oleifera, 22.8 µg/mL, followed by Bathysa australis, 35.3 µg/mL, and Mabea fistulifera, 42.5 µg/mL. After fractionation of the crude extracts by liquid-liquid partition with different organic solvents and each fraction was tested again, only some of the fractions retained the ability to inhibit the enzymatic activity. When analyzed by HPLC/DAD, the active fractions showed the presence of flavonoid derivatives, already reported for their ability to inhibit Pdr5p ATPase activity, as well as other classes of secondary metabolites such as lignans and alkaloids.
Multidrug resistance; yeast; Pdr5p; plant extracts; Atlantic Forest
No presente estudo, testamos o efeito de 27 extratos e frações de plantas de diferentes famílias botânicas sobre a atividade da proteína Pdr5p de membranas plasmáticas de leveduras, responsável pelo fenótipo de resistência a múltiplas drogas em leveduras. Alguns dos extratos foram capazes de produzir uma boa inibição na concentração fixa de 200 µg/mL e foram selecionados para uma investigação mais aprofundada. Curvas de dose-resposta foram obtidas para os extratos brutos etanólicos de Bathysa australis (A. St.-Hill.) Benth. & Hook f., Mabea fistulifera Mart. e Virola oleifera (Schott) A. C. Sm., com concentrações até 400 µg/mL. O menor valor de IC50 foi obtido para Virola oleifera, 22,8 µg/mL, seguido por Bathysa australis, 35,3 µg/mL e Mabea fistulifera, 42,5 µg/mL. Após o fracionamento dos extratos brutos por partição líquido-líquido com diferentes solventes orgânicos, cada fração foi novamente testada, sendo que apenas algumas das frações mantiveram a habilidade de inibir a atividade enzimática. Quando analisadas por HPLC/DAD, as frações ativas demonstraram a presença de derivados de flavonóides, que já demonstraram ter a habilidade de inibir a atividade ATPasica da Pdr5p, assim como outras classes de metabólitos secundários, tais como lignanas e alcalóides.
Resistência a múltiplas drogas; levedura; Pdr5p; extratos de plantas; Mata Atlântica
ARTIGO
Effect of different extracts from the Brazilian Atlantic Forest on the Pdr5p ATPase activity
Efeito de extratos de plantas da Floresta Atlântica Brasileira sobre a atividade da Pdr5p ATPase
Luciana Pereira RangelI,** ** These authors contributed equally for this work ; Lisandra Ferreira de AbreuII,** ** These authors contributed equally for this work ; Ana Rodrigues de AndradeI; Suzana Guimarães LeitãoIII; Gilda Guimarães LeitãoII; Antonio Ferreira-PereiraI,* * E-mail: apereira@micro.ufrj.br, Tel. +55-21-25626494, Fax +55-21-25608344. ,** ** These authors contributed equally for this work
ILaboratório de Bioquímica Microbiana, Departamento de Microbiologia Geral, Universidade Federal do Rio de Janeiro, 21941-902 Rio de Janeiro-RJ, Brazil
IINúcleo de Pesquisas de Produtos Naturais, Universidade Federal do Rio de Janeiro, 21941590 Rio de Janeiro-RJ, Brazil
IIIDepartamento de Produtos Naturais e Alimentos, Faculdade de Farmácia, Universidade Federal do Rio de Janeiro, 21941590 Rio de Janeiro-RJ, Brazil
ABSTRACT
In the current study, we tested the effect of 27 plant extracts and fractions from different botanical families on the activity of Pdr5p from yeast plasma membrane, responsible for the multidrug resistance phenotype in yeast cells. Some of the extracts were able to produce a good inhibition in the fixed concentration (200 µg/mL) and were selected for a deeper investigation. Dose-response curves were obtained for the crude ethanol extracts of Bathysa australis (A. St.-Hill.) Benth. & Hook f., Mabea fistulifera Mart. and Virola oleifera (Schott) A. C. Sm. with concentrations ranging up to 400 µg/mL. The lower IC50 value was obtained for Virola oleifera, 22.8 µg/mL, followed by Bathysa australis, 35.3 µg/mL, and Mabea fistulifera, 42.5 µg/mL. After fractionation of the crude extracts by liquid-liquid partition with different organic solvents and each fraction was tested again, only some of the fractions retained the ability to inhibit the enzymatic activity. When analyzed by HPLC/DAD, the active fractions showed the presence of flavonoid derivatives, already reported for their ability to inhibit Pdr5p ATPase activity, as well as other classes of secondary metabolites such as lignans and alkaloids.
Keywords: Multidrug resistance, yeast, Pdr5p, plant extracts, Atlantic Forest.
RESUMO
No presente estudo, testamos o efeito de 27 extratos e frações de plantas de diferentes famílias botânicas sobre a atividade da proteína Pdr5p de membranas plasmáticas de leveduras, responsável pelo fenótipo de resistência a múltiplas drogas em leveduras. Alguns dos extratos foram capazes de produzir uma boa inibição na concentração fixa de 200 µg/mL e foram selecionados para uma investigação mais aprofundada. Curvas de dose-resposta foram obtidas para os extratos brutos etanólicos de Bathysa australis (A. St.-Hill.) Benth. & Hook f., Mabea fistulifera Mart. e Virola oleifera (Schott) A. C. Sm., com concentrações até 400 µg/mL. O menor valor de IC50 foi obtido para Virola oleifera, 22,8 µg/mL, seguido por Bathysa australis, 35,3 µg/mL e Mabea fistulifera, 42,5 µg/mL. Após o fracionamento dos extratos brutos por partição líquido-líquido com diferentes solventes orgânicos, cada fração foi novamente testada, sendo que apenas algumas das frações mantiveram a habilidade de inibir a atividade enzimática. Quando analisadas por HPLC/DAD, as frações ativas demonstraram a presença de derivados de flavonóides, que já demonstraram ter a habilidade de inibir a atividade ATPasica da Pdr5p, assim como outras classes de metabólitos secundários, tais como lignanas e alcalóides.
Unitermos: Resistência a múltiplas drogas, levedura, Pdr5p, extratos de plantas, Mata Atlântica.
INTRODUCTION
The search for new pharmacologically active agents obtained by screening natural sources such as microbial and plant extracts has led to the discovery of many clinical useful drugs that play a major role in the treatment of human diseases (Shu, 1998). A recent review pointed out that approximately 60% of the antitumor and antiinfective agents that are commercially available or in late stages of clinical trials today are of natural product origin (Cragg et al., 1997). Historically, the majority of the natural product-based drugs including cyclosporine, paclitaxel, and camptothecin derivatives were first discovered by traditional cell-based in vitro assays (antibacterial, antifungal, antiviral, antiparasitic, or cytotoxic assays) before their real molecular biological targets were identified (Shu, 1998).
Antiinfective and antitumor targets have been, historically, the effective research area for natural products screening programs. Today, with the advent of genomics research and newer molecular biology tools for developing bioassays, more sophisticated biological assays in addition to cell-based assays are being employed routinely in the drug discovery paradigm.
Consequently, in recent years a notable number of natural product-derived agents have been discovered by employing mechanism-based screening approaches involving cellular or biochemical targets in their assay design. In addition, a large number of natural products, especially plant-derived drugs, continue to be discovered on the basis of traditional or empirical local medicinal practices (Shu, 1998; Amaral et al., 2006; Barbosa-Filho et al., 2006; Saúde-Guimarães and Faria, 2007; Rocha et al., 2007).
Multidrug resistance is a serious problem for chemotherapy nowadays. This phenomenon consists in cellular resistance to several structurally and functionally unrelated drugs (Gottesman and Pastan, 1993), generally extruded from the cytoplasm by transporters that belong to the ABC (ATP Binding Cassette) Family (Higgins, 1992). The most studied member of this family is P-glycoprotein (P-gp), codified by the gene ABCB1 (previously called MDR1) (Roninson et al., 1986), which is responsible for resistance of cancer cells to drugs.
Homologous ABC transporters can be found in other organisms, such as Candida glabrata (Cdr1p and Pdh1p (Sanglard et al., 1999; Lupetti et al., 2002)). These fungal ABC transporters share homology (around 70%) with Saccharomyces cerevisiae Pdr5p (Decottignies et al., 1994), an ABC transporter involved in yeast multidrug resistance, which is also homologous to mammalian P-gp. Besides sequence homology, these transporters are also functional homologues because they share many drug substrates and are inhibited by the same compounds (Wolfger et al., 2001). For these reasons, Pdr5p is a good model for the search of novel multidrug resistance inhibitors.
This work shows that plant extracts can be a good source of new modulators for this class of transporters.
MATERIAL AND METHODS
Plant material
Plants were collected from two Atlantic Forest fragments (Bela Fama Forest - Santana do Deserto city, MG and Boa Vista Forest - Levy Gasparian city, RJ), Brazil. Taxonomic identifications were done by Sebastião J. da Silva Neto from Instituto Jardim Botânico do Rio de Janeiro, Brazil. Voucher specimens are deposited at the Herbarium of the Federal University of Rio de Janeiro (see Table 1 for voucher numbers).
Preparation of extracts
The air-dried and powdered leaves (20 g of each) were exhaustively extracted with ethanol 96° GL. The obtained extracts were filtered and evaporated under reduced pressure on a rotary evaporator. Crude ethanol extracts of Bathysa australis, Mabea fistulifera and Virola oleifera were fractionated by liquid-liquid partition between MeOH : H2O 9 : 1 and Hexane, CH2Cl2, EtOAc and BuOH, in this order.
Preparation of plasma membranes
Yeast cells from the mutant strain AD124567 overexpressing Pdr5p and multideleted in genes encoding the Pdr3p regulator and five ABC transporters (Yor1p, Snq2p, Pdr10p, Pdr11p, and Ycf1p) (Dyor1::hisG, Dsnq2::hisG, Dpdr10::hisG, Dpdr11::hisG, Dycf1::hisG, Dpdr3::hisG) (Decottignies et al., 1998) were grown in 2% glucose, 1%yeast extract, 2% peptone and harvested in the exponential phase of growth. After washing with 10 mM NaN3, the cell wall was digested with zymolase 20T (Seigakagu®) at 37 ºC for 60 min, in a proportion of 6.5 mg zymolase for each 1600 initial DO and 58 µL b-mercaptoethanol for each 15 mL of zymolase buffer (2.8 M sorbitol, 0.1 M KH2PO4, 10 mM NaN3). Unbroken cells and cell debris were removed by low-speed centrifugation (4,500 × g for 10 min). The supernatant was centrifuged for 40 min at 12,000 × g, as described before (Goffeau and Dufour, 1988) in order to remove possible contaminants as mitochondria and finally, the resulting supernatant was centrifuged at 20,000 × g. The final pellet, highly enriched in plasma membranes, was stored at -70 °C. The strain AD124567 was kindly provided by Joseph Nader (Unité de Biochimie Physiologique, Université Catholique de Louvain, - Belgium).
ATPase assays
ATP hydrolysis was measured by incubating the sample at 37 ºC in a final volume of 500 µL containing 3 mM ATP-Na, 4 mM MgCl2, 1 mM EGTA, 0.5 mM ouabain (inhibitor of plasma membrane Na+,K+-ATPase), 10 nM thapsigargin (inhibitor of sarcoplasmic reticulum Ca2+-ATPase), 1 mM NaN3 (inhibitor of mitochondrial F1F0-ATPase), 50 mM KNO3 (inhibitor of phosphatases) and 100 mM Tris (adjusted for pH 7.5 with HCl). Assays were carried out as described before (Decottignies et al., 1994). Crude ethanol extracts or their fractions were added from stock solutions in dimethylsulfoxide up to 8% (v/v) final concentration. The DMSO concentrations used were tested previously and no interference was observed up to a 10% concentration. The data obtained with dose-response curves were used to calculate the IC50 values for each of the extracts.
HPLC Analyses
The HPLC profiles of the extracts of the plants were performed in a gradient elution mode with methanol : water (pH 3.0 with acetic acid) as eluent, 1 mL/min., using a linear gradient from a start mixture of 20:80 to an intermediate of 80:20 over 30 min, and then to 100% methanol over 1 min, which then held isocratic for 5 min. An ultrasonic bath, Thornton model T28220 was used to degas the HPLC solvents (5 min.) and to dissolve the samples. The HPLC equipment was a Lachrom HPLC System (Merck, Darmstadt, Germany) equipped with an interface D7000, pump L-7100, diode array detector (DAD) L-7450A and solvent degasser L-7612. The injections were done manually with an injector valve (Rheodyne) equipped with a 20 µL sample loop. A Lichrosorb RP-18 column (Merck, Darmstadt, Germany, 5 µm particle size, 250 x 4.6 mm i.d.) N. 738342, was coupled to a guard column Lichrocart 250-4 HPLC cartridge (Merck, Darmstadt, Germany). The UV detection was performed with a DAD in an integration range from 240 nm to 260 nm.
Statistical analysis
All experiments were carried out at least three times and the results are expressed as mean ± S.E. Statistical analysis and IC50 values were calculated using the computer program Sigma Plot version 8.0 (SPSS Science Marketing).
RESULTS
Saccharomyces cerevisiae Pdr5p has a substrate and an inhibitor profile which is similar to mammalian P-glycoprotein and other fungal ABC transporters, responsible for chemotherapeutic treatments failure. For this reason, added to the fact that yeast can be used as a tool for obtaining large amounts of protein for biochemical studies (Decottignies et al., 2001; Ferreira-Pereira et al., 2003), Pdr5p was chosen as a model for studying plant compounds as multidrug resistance reversal agents.
In order to select the most promising plant extracts, we performed a screening experiment of inhibition of Pdr5p ATPase activity, using a fixed crude ethanol extract concentration of 200 mg/mL. Plant species used in this work are listed in Table 1 as well as their effect on Pdr5p ATPase activity. The results are shown in percentages of the control activity, which was considered as 100%.
Most of the tested extracts presented low or no effect on Pdr5p ATPase activity. Some of them, however, were able to produce a good inhibition in the fixed concentration. From these, Bathysa australis, Mabea fistulifera and Virola oleifera were available in a suitable amount, and for this reason, were the ones selected for a deeper investigation. Dose-response curves were obtained for the crude ethanol extracts with concentrations ranging from 0 to 400 µg/mL (Figure 1). The three extracts presented similar curve profiles. The lower IC50 value was obtained for Virola oleifera, 22.8 µg/mL, followed by Bathysa australis, 35.3 µg/mL, and Mabea fistulifera, 42.5 µg/mL. Based on these results, these crude ethanol extracts were subsequently fractionated by liquid-liquid partition. The resulting hexane, dichloromethane, ethyl acetate and butanol fractions, after solvent evaporation and solubilization in 20% dimethylsulfoxide, were tested again on Pdr5p ATPase activity (200 µg/mL) (Figure 2).
In Figure 2A, it is shown that the ethyl acetate and the butanol fractions of Virola oleifera were able to produce a higher effect than that obtained with the original crude ethanol extract, while the dichloromethane and hexane fractions were less or as efficient as the crude ethanol extract of this plant.
Virola oleifera, a plant from the South Eastern region of Brazil, has been used in traditional medicine for the treatment of diseases of the respiratory tract, rheumatism and asthma, as well as gastric or duodenal ulcers (Fernandes et al., 1994). The genus Virola is known to be an abundant source of lignans and neolignans (Fernandes et al., 1997). One lignan, oleiferin-C and two flavonoid glycosides, astilbin and quercitrin, previously isolated from the leaves of Virola oleifera, exhibited a good analgesic activity (Kuroshima et al., 2001).
Four lignans (lignan 7-ol, galbacin, eupamatenoid-8 and aristolignin), were also isolated from the leaves of this species, as components of the chlorophyll-free dichloromethane fraction of the ethanol extract (Fernandes et al., 1993). It is interesting to note that the hexane and dichloromethane fractions, where the lignans are expected to be found, are the less active ones, for this plant species. On the other hand, flavonoid glycosides are expected to be found in the ethyl acetate/butanol fractions. In fact, the HPLC profile of the ethyl acetate fraction showed peaks corresponding to flavonol compounds (Table 2), at Rt 2.88 min. lmax.255.3 and 353.9 nm) and 3.25 min. lmax.256.2 and 349.7 nm (Mabry et al., 1970). We also observed a peak at Rt 4.25 min. (Table 2), which showed a benzenoid band (lmax.272.8 nm) that could be due to a lignan derivative.
Figure 2b shows that the ethyl acetate extract from Mabea fistulifera was the most efficient one, while the butanol, dichloromethane and hexane fractions were less efficient or as efficient as the original M. fistulifera crude ethanol extract. Previous work showed that the ethanol extract from the fruits of this plant presented significant lethality to brine shrimp larvae. The isolation of a bioactive naringenin coumaroyl glycoside and two other inactive naringenin derivatives, along with gallic acid ethyl ester was reported (Garcez et al., 1997). The HPLC chromatogram of the ethyl acetate fraction of the ethanol extract of M. fistulifera showed peaks at Rt 2.45 and 3.31 min. (Table 2) which UV spectra are compatible with a benzenoid compound (Silverstein et al., 1991). In fact, gallic acid was isolated from the ethyl acetate fraction by our group, along with minor gallotannins (structure under elucidation).
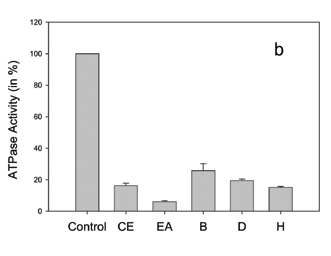
Concerning Bathysa australis extracts (Figure 2c), we observed that the ethyl acetate, dichloromethane and hexane fractions presented a very similar inhibition effect on Pdr5p ATPase activity in comparison to its crude ethanol extract, while the butanol fraction produced a very small inhibition. In Brazil there are seven species from the genus Bathysa, known as false Quina, all of them occurring in the South and Southeast regions of the Atlantic Rain Forest. The use in Brazilian Folk Medicine of Bathysa australis mucilage for wound healing is reported (Filho, 1999). As far as we know, there is no report on the phytochemistry of B. australis. Also, the chemistry of the genus is not well known, with only one paper reporting the isolation of paeonol, an acetophenone derivative, from roots of B. meridionalis (Weeks et al., 1977). The HPLC chromatogram of the ethyl acetate fraction from this plant (Table 2) showed a peak at Rt 15.17 min., which UV bands are in the range of an indole alkaloid (three main lmax. bands: 220-245 nm, 270-310 nm and 290-320 nm) (Thylor and Farnsworth, 1973). Other peaks, at Rt 16.24 and 17.47 min. are characteristic of flavonoid derivatives. The UV spectra for those peaks present absorption maxima at lmax.267.2 and 344.3 nm, and lmax.256.2 and 341.7 nm, respectively, compatible with luteolin derivatives lmax.242sh, 253, 267, 291sh and 349 nm (Mabry et al., 1970). It is interesting to note that, among five Rubiaceae species tested - Bathysa australis, Guettarda virbunoides, Psychotria vellosiana, Simira glaziovii and S. sampaioana, and two Apocynaceae species, Malouetia arborea and Peschiera affinis, Bathysa stands out as the most active. These two botanical families are known to be a rich source of indole alkaloids (Dewick, 1997). These differences in activity could be due to different alkaloid structures and/or the presence of synergistic compounds such as flavonoids, previously described as possessing Pdr5p inhibitory activity (Conseil et al., 2000).
It can be seen, for Mabea fistulifera and Virola oleifera extracts that in both cases, at least one fraction was able to produce a larger effect than the observed for the original crude ethanol extract. This is not true for Bathysa australis, since the three fractions that presented a relevant effect were only as efficient as the crude ethanol extract. This can be due to many factors such as concentration of active constituents in one specific fraction (Mabea and Virola) or the separation of synergistic compounds into different fractions (Bathysa). As a matter of fact, all active fractions indicated the presence of flavonoids, known for their ability to inhibit Pdr5p activity. Previous studies indicate that flavonoids constitute a new class of modulators with bifunctional interactions at vicinal ATP-binding site and steroid-interacting region within a cytosolic domain of P-glycoprotein (Conseil et al., 1998). The authors comment that flavones/flavonols (like quercetin or apigenin) bind more strongly than flavanones (naringenin), isoflavones (genistein) or glycosylated derivatives (rutin). Also, Di Pietro and co-workers (Di Pietro et al., 2002) state that prenylated flavonoids bind with high affinity, strongly inhibiting drug interaction and nucleotide hydrolysis, that corroborate with idea that these class of compounds could be a tool to study and understand the mechanism of multidrug resistance.
DISCUSSION
Resistance to chemotherapy is a common clinical problem in patients with infectious diseases as well as in patients with cancer. This includes a several opportunistic infections caused by different microorganisms in patients with acquired immunodeficiency syndrome, which is very disturbing since we have an enormous increase in bacteria and opportunistic fungi infections that usually are very resistant to normal treatment. Therefore, it is necessary to find some strategies to reverse this drug resistance and the study of plant extracts could be one of them. In this work, we investigated three different plants (selected from 27 previously tested - Table 1): Bathysa australis, Mabea fistulifera and Virola oleifera, in order to verify the effect of extracts on the enzymatic activity of Pdr5p from yeast plasma membrane, the enzyme responsible for the multidrug resistance phenotype. The crude ethanol extracts from these plants were able to inhibit the enzymatic activity with high affinity, which can be observed by the IC50 values ranging from 22.8 to 42.5 µg/mL. When partitions obtained from the ethanol extracts after extraction with different solvents were tested, we observed that in both plants the fraction ethyl acetate were more effective showing a step of separation and concentration of some substance(s) that may be a possible inhibitors of enzymatic activity. Analysis of these fractions using HPLC demonstrated the presence of flavonoids that have been considered good inhibitors and/or modulators of multidrug resistance activity (Boumendjel et al., 2002). Further investigations of the phytochemistry of the active plants are being conducted and we are working on these partitions with the aim of isolating the active compounds to study their effects on the purified enzyme and/or mammal cells that overexpress P-glycoprotein. We fully expect that these results will aid understanding not only Pdr5p but also many other ABC transporters and help in the discovery of new medicine for the treatment of the human diseases in which these transporters play a role.
ACKNOWLEDGMENTS
We thank Dr. Angela Hampshire for a critical review of this manuscript and Joseph Nader (Physiological Biochemistry Unit (FYSA) - Université Louvain-la-Neuve/Belgium) for yeast strains. The present work was supported by Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação Universitária José Bonifácio (FUJB).
Received 30 September 2007; Accepted 5 December 2007
- Amaral FMM, Ribeiro MNS, Barbosa-Filho JM, Reis AS, Nascimento FRF, Macedo RO 2006. Plants and chemical constituents with giardicidal activity. Rev Bras Farmacogn 16(Supl.): 696-720.
- Barbosa-Filho JM, Medeiros KCP, Diniz MFFM, Batista LM, Athayde-Filho PF, Silva MS, Cunha EVL, Almeida JRGS, Quintans-Júnior LJ 2006. Natural products inhibitors of the enzyme acetylcholinesterase. Rev Bras Farmacogn 16: 258-285.
- Boumendjel A, Di Pietro A, Dumontet C, Barron D 2002. Recent advances in the discovery of flavonoids and analogs with high-affinity binding to p-glycoprotein responsible for cancer cell multidrug resistance. Med Res Rev 22: 512-529.
- Conseil G, Cortay HB, Dayan G, Jault JM, Barron D, Di Pietro, A 1998. Flavonoids: a class of modulators with bifunctional interactions at vicinal ATP- and steroid-binding sites on mouse P-glycoprotein. Proc Natl Acad Sci USA 95: 9831-9836.
- Conseil G, Decottignies A, Jault JM, Comte G, Barron D, Goffeau A, Di Pietro A 2000. Prenyl-flavonoids as potent inhibitors of the Pdr5p multidrug ABC transporter from Saccharomyces cerevisiae. Biochemistry 39: 6910-6917.
- Cragg GM, Newman DJ, Snader KM 1997. Natural products in drug discovery and development. J Nat Prod 60: 52-60.
- Decottignies A, Kolaczkowski M, Balzi E, Goffeau A 1994. Solubilization and characterization of the overexpressed PDR5 multidrug resistance nucleotide triphosphatase of yeast. J Biol Chem 269: 12797-12803.
- Decottignies A, Grant AM, Nichols JW, de Wet H, McIntosh D, Goffeau A 1998. ATPase and multidrug transport activities of the overexpressed yeast ABC protein Yor1p. J Biol Chem 273: 12612-12622.
- Decottignies A, Rogers B, Kolaczkowski M, Carvajal E, Balzi E, Conseil G, Niimi K, Di Pietro A, Monk BC, Goffeau A 2001. The pleitropic drug ABC transporters from Saccharomyces cerevisiae In: Paulsen I.T., Lewis K. (Eds) Microbial multidrug efflux Wymondham, Horizon Scientific Press, p.157-176.
- Dewick PM 1997. Medicinal Natural Products, New York, John Wiley & Sons.
- Di Pietro A, Conseil G, Pérez-Victoria JM, Dayan G, Baubichon-Cortay H, Trompier D, Steinfels E, Jault JM, de Wet H, Maitrejean M, Comte G, Boumendjel A, Mariotte AM, Dumontet C, McIntosh DB, Goffeau A, Castanys S, Gamarro F, Barron D 2002. Modulation by flavonoids of cell multidrug resistance mediated by P-glycoprotein and related ABC transporters. Cell Mol Life Sci 59: 307-322.
- Fernandes AMAP, Barata LES, Ferri PH 1993. Lignans and a neolignan from Virola oleifera leaves. Phytochemistry 32: 1567-1572.
- Fernandes AMAP, Barata LES, Ferri PH 1994. Absolute configuration of the lignan oleiferins from Virola oleifera. Phytochemistry 36: 533-534.
- Fernandes AMAP, Prado AL, Barata LES, Paulo MQ, Azevedo NR, Ferri PH 1997. A Method to separate lignoids from Virola Leaves. Phytochem Analysis 8: 18-21.
- Ferreira-Pereira A, Marco S, Decottignies A, Nader J, Goffeau A, Rigaud JL 2003. Three-dimensional reconstruction of the Saccharomyces cerevisiae multidrug resistance protein Pdr5p. J Biol Chem 278: 11995-11999.
- Filho PG 1999. Estudos taxonômicos do gênero Bathysa Presl (Rubiceae, Rondeletiae) no Brasil. Rodriguésia 50: 49-75.
- Garcez WS, Garcez FR, Pellicciari I, Hara SM, Ferreira FC, Nakasse LY, Siqueira JM 1997. A bioactive narigenin coumaroyl glucoside from Mabea fistulifera subsp. Robusta. Planta Med 63: 386-386.
- Goffeau A, Dufour JP 1988. Plasma membrane ATPase from the yeast Saccharomyces cerevisiae. Methods Enzymol 157: 528-533.
- Gottesman MM, Pastan I 1993. Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu Rev Biochem 62: 385-427.
- Higgins CF 1992. ABC transporters: from microorganisms to man. Annu Rev Cell Biol 8: 67-113.
- Kuroshima KN, de Campos F, de Souza MM, Yunes RA, Delle Monache F, Filho VC 2001. Phytochemical and pharmacological investigations of Virola oleifera leaves. Z Naturforsch C 56: 703-706.
- Lupetti A, Danesi R, Campa M, Del Tacca M, Kelly S 2002. Molecular basis of resistance to azole antifungals. Trends Mol Med 8: 76-81.
- Mabry TM, Markham KR, Thomas MB 1970. The Systematic Identification of Flavonoids New York, Springer-Verlag.
- Rocha FD, Pereira RC, Kaplan MAC, Teixeira VL 2007. Produtos naturais de algas marinhas e seu potencial antioxidante. Rev Bras Farmacogn 17: 631-639.
- Roninson IB, Chin JE, Choi KG, Gros P, Housman DE, Fojo A, Shen DW, Gottesman MM, Pastan I 1986. Isolation of human mdr DNA sequences amplified in multidrug-resistant KB carcinoma cells. Proc Natl Acad Sci USA 83: 4538-4542.
- Saúde-Guimarães DA, Faria AR 2007. Substâncias da natureza com atividade anti-Trypanosoma cruzi. Rev Bras Farmacogn 17: 455-465.
- Sanglard D, Ischer F, Calabrese D, Majcherczyk PA, Bille J 1999. The ATP binding cassette transporter gene CgCDR1 from Candida glabrata is involved in the resistance of clinical isolates to azole antifungal agents. Antimicrob Agents Chemother 43: 2753-2765.
- Shu YZ 1998. Recent natural products based drug development: a pharmaceutical industry perspective. J Nat Prod 61: 1053-1071.
- Silverstein RM, Bassler GC, Morril TC 1991. Spectrometric Identification of Organic Compounds NewYork : John Wiley & Sons.
- Thylor W, Farnsworth NR 1973. The Vinca Alkaloids: Botany, Chemistry and Pharmacology New York, Marcel Dekker.
- Weeks RA, Dobberstein RH, Farnsworth NR 1977. Isolation of paenol from Bathysa meridionalis. J Nat Prod 40: 515-523.
- Wolfger H, Mamnun YM, Kuchler K 2001. Fungal ABC proteins: pleiotropic drug resistance, stress response and cellular detoxification. Res Microbiol 152: 375-389.
Publication Dates
-
Publication in this collection
14 Apr 2008 -
Date of issue
Mar 2008
History
-
Accepted
05 Dec 2007 -
Received
30 Sept 2007