Abstracts
An analysis of the constituents of coconut (Cocos nucifera L.) water from two fruit varieties (green and yellow) by hydrodistillation and solvent extraction showed the presence of alcohols, ketones, thiols, carboxylic acids, phenols, and esters. Substantial antioxidant activity was observed, using the DPPH assay, for the samples obtained by hydrodistillation and petroleum ether extraction of both coconut varieties.
Cocos nucifera; Arecaceae; coconut water; chemical composition; antioxidant activity
Uma análise dos componentes da água-de-coco (Cocos nucifera L.) de duas variedades da fruta (verde e amarelo) por hidrodestilação e extração com solvente, mostrou a presença de álcoois, cetonas, tióis, ácidos carboxílicos, fenóis, e ésteres. Significativa atividade antioxidante foi observada, usando o método DPPH, para as amostras obtidas por hidrodestilação e extração de éter de petróleo para ambas as variedades do coco.
Cocos nucifera; Arecaceae; água-de-coco; composição química; atividade antioxidante
ARTIGO
Constituents and antioxidant activity of two varieties of coconut water (Cocos nucifera L.)
Constituintes químicos e atividade antioxidante de duas variedades de água de coco (Cocos nucifera L.)
Aluísio M. da FonsecaI; Ayla M. C. BizerraI; João Sammy N. de SouzaI; Francisco José Q. MonteI; Maria da Conceição F. de OliveiraI; Marcos C. de MattosI; Geoffrey A. CordellII; Raimundo Braz-FilhoI; Telma L. G. LemosI, * * E-mail: tlemos@ufc.br, Tel. +55-83-3366-9366
IDepartamento de Química Orgânica e Inorgânica, Laboratório de Biocatálise e Produtos Naturais, Universidade Federal do Ceará, 60451-97 Fortaleza-CE, Brazil
IINatural Products Inc., Evanston, IL 60203 USA
ABSTRACT
An analysis of the constituents of coconut (Cocos nucifera L.) water from two fruit varieties (green and yellow) by hydrodistillation and solvent extraction showed the presence of alcohols, ketones, thiols, carboxylic acids, phenols, and esters. Substantial antioxidant activity was observed, using the DPPH assay, for the samples obtained by hydrodistillation and petroleum ether extraction of both coconut varieties.
Keywords: Cocos nucifera, Arecaceae, coconut water, chemical composition, antioxidant activity.
RESUMO
Uma análise dos componentes da água-de-coco (Cocos nucifera L.) de duas variedades da fruta (verde e amarelo) por hidrodestilação e extração com solvente, mostrou a presença de álcoois, cetonas, tióis, ácidos carboxílicos, fenóis, e ésteres. Significativa atividade antioxidante foi observada, usando o método DPPH, para as amostras obtidas por hidrodestilação e extração de éter de petróleo para ambas as variedades do coco.
Unitermos: Cocos nucifera, Arecaceae, água-de-coco, composição química, atividade antioxidante.
INTRODUCTION
Cocos nucifera L. (Arecaceae) is a 20-30 m high tropical palm tree cultivated, preferentially, on beach coastal areas. Its fruits are popularly known as "coco", forming a bunch or a cluster of fruits in different stages of development (Reddy et al., 2005).
In the northeast of Brazil, State of Ceará, the coconut tree is available as two main varieties, giant and dwarf, being subdivided in green, yellow, and red varieties. The water from the green variety of coconut is widely consumed as a soft drink, and is known to possess beneficial health properties (Farr, 1994; Campbell-Falck et al., 2000; Pummer et al., 2001; Mepba and Achinewhu, 2003; Agra et al., 2007 and 2008), whereas the liquid produced from the yellow and red coconut varieties type is little used as a drink, because it is not so sweet as the green variety, and thus it is less cultivated.
Coconut water has a sui generis flavor, being sweet and slightly acid (Borse et al., 2007). It contains proteins, fats and minerals such as sodium, potassium, magnesium and calcium electrolytes (Chumbimuni-Torres and Kubota, 2006; Jirovetz et al., 2003). The nutritional composition of coconut water has been well documented (Santoso et al., 1996), but to the best of our knowledge there is no report comparing the chemical compositions of the water produced by the green and yellow varieties of coconuts.
In recent years, there has been considerable interest in finding sources of natural antioxidants, especially from fruits and vegetables which have provided a measure of protection against the process of oxidative damage in the human species (Jacob and Burri, 1996). Antioxidant compounds from plants, particularly flavonoids and other polyphenols, have been reported to inhibit the propagation of free radical reactions, and to protect the human body from disease (Kinsella et al., 1993). Moreover, antioxidant activities have been associated with acids (Loki and Rajamohan, 2003), aromatic compounds (Agnaniet et al., 2005; Letizia et al., 2000), aldehydes (Yi and Kim, 1982), and esters (Narain et al., 2007). Coconut water also possesses antioxidant properties (Leong and Shui, 2002; Skrede et al., 2004). This property was verified mainly in the coconut water in natura, and it decreases drastically with the use of thermal treatments, or the actions of acids or alkaline on coconut water, and with the degree of maturation of the fruits (Mantena et al., 2003).
The present work reports a study of the low molecular weight, hydrodistillable or petroleum ether extractable constituents of the coconut water from the green and yellow varieties of coconut (Figure 1) collected in Fortaleza, Ceará, Brazil, and their antioxidant activities using the DPPH assay.
MATERIAL AND METHODS
General procedures
GC-FID: The quantitative analysis was carried out on a Shimadzu GC-17A gas chromatograph using a dimethylpolysiloxane DB-5 fused silica capillary column (30 m x 0.25 mm, film thickness 0.25 µm). H2 was used as the carrier gas at a flow rate of 1 mL/ min and 30 psi inlet pressure; split, 1:30; temperature program: 35-180 ºC at 4 ºC/ min, then heated at a rate of 17 ºC/ min to 280 ºC and held isothermal for 10 min; injector temperature, 250 ºC; detector used FID, detector temperature, 250 ºC.
GC/MS: analysis of the oils was performed on a Hewlett-Packard 5971 GC/MS instrument employing the following conditions: dimethylpolysiloxane DB-1 fused silica capillary column (30 m x 0.25 mm, 0.1 µm film thickness); carrier gas: He (1 mL/ min); injector temperature: 250 ºC; detector temperature: 200 ºC; column temperature: 35-180 ºC at 4 ºC/ min, then 180-250 ºC at 10 ºC/ min; mass spectra: electronic impact 70 eV. Individual components were identified by two computer library MS searches using retention indices as a pre-selection routine (Stenhagen et al., 1974) and visual inspection of the mass spectra from the literature for confirmation (Adams, 2001).
Plant material
The samples of Cocos nucifera fruits were harvested, from Icarai (Caucaia) County, State of Ceará, Brazil, in February, 2007. The two varieties were authenticated by Professor Edson P. Nunes, and the voucher specimens (Nº35458 and Nº 35459) were deposited at the Herbarium Prisco Bezerra (EAC) of Departamento de Biologia, Universidade Federal do Ceará.
Extraction of the constituents from samples of coconut water by hydrodistillation
The coconut water (2 L) from the two varieties was separated from the coconut fruit in a glass container (several fruits from the same tree). The essential oil of the coconut water was extracted by steam distillation in a Clevenger-type apparatus for two hours. Hydrodistillation was carried out in triplicate. The hydrolyte (20 mL) was extracted with petroleum ether (3 x 10 mL), and the solvents were evaporated under vacuum, yielding 32 mg and 45 mg for the green (sample 1) and yellow (sample 2) coconut water varieties, respectively. The extracts obtained from the two varieties were analyzed by GC/MS and GC-FID and the results are presented in Table 1. In addition, the extracts were submitted to the DPPH assay, and the results are presented in Table 2.
Extraction of the constituents from samples of coconut water using petroleum ether
Coconut waters from the two varieties (green and yellow) (2 L) were extracted with petroleum ether (3 x 300 mL). The solvents were dried with anhydrous Na2SO4 and concentrated under vacuum yielding residues of 0.26 g (sample 1') from the green variety and 0.30 g (sample 2') from the yellow variety. The extracts obtained from the two varieties were submitted to silica gel column chromatography using petroleum ether as eluent. Fractions were pooled according to their TLC profile, resulting in 30 mg of sample 1' and 56 mg of sample 2'. Samples were analyzed by GC/MS and GC-FID and the results are presented in Table 1. The extracts were also submitted to the DPPH assay, and the results are presented in Table 2.
Antioxidant assay
The hydrogen or electron donation abilities of the compounds were measured from the bleaching of the purple-colored ethanol solution of 1,1-diphenyl-2-picrylhydrazyl (DPPH). This spectrophotometer assay uses the stable radical DPPH as a reagent (Bandeira et al., 2006; Burits and Bucar, 2000; Hegazi and El Hady, 2002). The sample solution of material (500 µL) at four concentrations (1.0, 0.5, 0.25, and 0.125 µg/µL) was mixed with the same volume of 60 µM DPPH solution and allowed to stand for 30 min at room temperature. The absorbance was then measured at 520 nm using a spectrophotometer and the inhibition of free radical DPPH in percent (I%) was calculated using the formula below; where Ablank is the absorbance of the control reaction (containing all of the reagents except for the test compound), and Asample is the absorbance of the test compound. The compound concentration demonstrating 50% inhibition (IC50) was calculated from the plot of inhibition percentage against sample concentration. Tests were carried out in triplicate (Tepe et al., 2005). Samples 1, 1', 2, 2', and DPPH were dissolved in ethanol. Trolox and BHT were used as positive control samples and the results are presented in Table 2.
Statistical analysis
The results were expressed as mean ± S.E.M. One-way analysis of variance (ANOVA) was used. In the antioxidant activity assay, one-way ANOVA test was used follow by Tukey test (P < 0.001).
RESULTS AND DISCUSSION
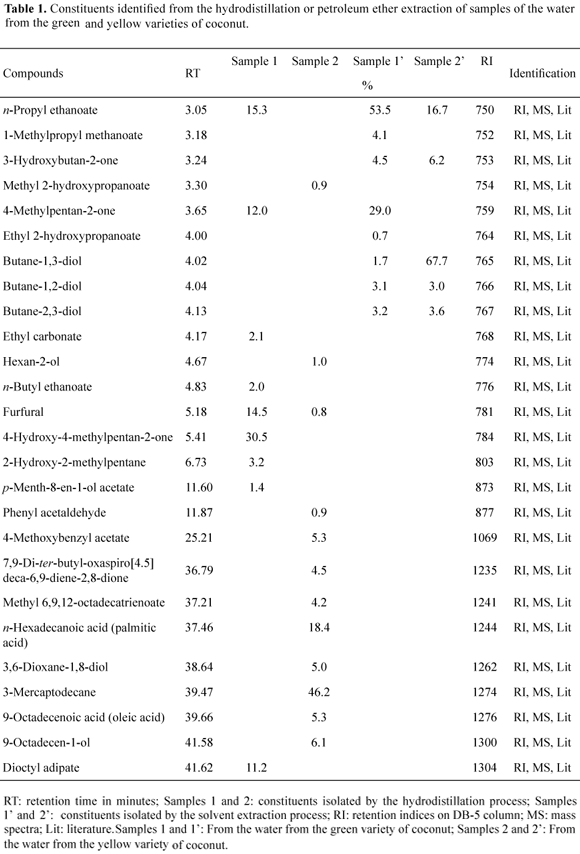
The essential oils obtained by the hydrodistillation process from the two varieties of coconut water, green (sample 1), and yellow (sample 2), showed different chemical compositions. The essential oil from the water of the green coconut variety was characterized by a high level of ketones (42.5%) and esters (29.9%), followed by aldehydes (14.5%) and alcohols (3.2%), and representing 90.1% of the total sample. The major constituents were identified as, 4-hydroxy-4-methylpentan-2-one (30.5%) and n-propyl ethanoate (15.3%). The essential oil analysis from the water of the yellow coconut variety was characterized by the presence of thiols (46.2%), carboxylic acids (23.7%), alcohols (12.1%), esters (10.4%), lactones (4.5%), and aldehydes (1.7%), and represented 98.6% of the total sample. The major components of the essential oil of this sample were 3-mercapto-decane (46.2%) and n-hexadecanoic acid (18.4%).
The compounds of the coconut water from the green and yellow varieties were analyzed in samples obtained by extraction using petroleum ether as solvent. Eight compounds were identified in sample 1' (green variety), and were characterized as esters (58.3%), ketones (33.5%), and diols (8.0%), representing 99.8% of the total extract. For the specimen of the yellow variety, five components were identified and characterized as diols (74.3%), esters (16.7%), and ketones (6.2%), representing 97.2% of total extract. All of the five constituents identified in the sample 2' (yellow variety) are present in the composition of the green variety, sample 1', but at different concentrations. For the green variety, the major constituents were n-propyl ethanoate (53.5%) and 4-methylpentan-2-one (29.0%), whereas for the yellow variety, the major constituents were butane-1,3-diol (67.7%) and n-propyl ethanoate (16.7%). The butane-1,3-diol identified in both samples (sample 1' and sample 2') is an important chiral synthon for various optically active compounds, such as azetidinone derivatives leading to penem and carbapenem antibiotics (Matsuyama et al., 2001).
Although the chemical composition of the extracts was different between the hydrodistillation and solvent extraction methods, some similarities were observed. The ester n-propyl ethanoate appears in sample 1 (15.3%) (hydrodistillation process) and in sample 1' (53.5%, petroleum ether extract), and the ketone 4-methylpentan-2-one was also present in sample 1 (12%) and the sample 1' (29%). In addition, by the hydrodistillation process, the aldehyde furfural was shown to be present in both varieties (green and yellow) in the percentage of 14.5% and 0.8%, respectively.
The results of the free radical scavenging effects of the samples 1, 1', 2, and 2' from the hydrodistillation and solvent extraction of both varieties showed concentration-dependant activity. The free radical scavenging effects of samples 1 and 2 were 79.4% and 83.5% at a concentration of 1.0 µg/µL, while it was 76.6%, and 79.3% for 0.50 µg/µL, 64.8% and 67.2% at a concentration 0.25 µg/µL, and 41.2% and 32.5% at a concentration of 0.125 µg/µL, respectively, showing significant antioxidant activity compared with Trolox and BHT (Table 2). The samples obtained from the petroleum ether extractions, sample 1' and sample 2', showed only moderate free radical scavenging activity, with inhibition levels of 31.5 and 34.9% at a concentration of 1.0 µg/µL respectively, and 16.4 and 27.7% inhibition at a concentration of 0.25 µg/µL, respectively.
Alcohols, ketones, lactones, aldehydes, and esters having short carbon chains were detected in the essential oil of the coconut water from the two varieties analyzed, and are probably responsible, in part, for the aroma of coconut water. In addition, the ester n-propyl ethanoate was present in both extraction processes, and probably is one of the compounds responsible for the flavor of coconut water.
The free radical scavenging effects of the samples from hydrodistillation and solvent extraction of the water from the two coconut varieties were compared to the positive controls Trolox and BHT in the DPPH free radical system. High scavenger activity was found in the samples obtained from the hydrodistillation process, and moderate activity was observed in the samples from the solvent extraction. The antioxidant activity of these fractions from coconut water is of interest as a potential natural food antioxidant additive, nutraceutical, and requires further evaluation.
ACKNOWLEDGMENTS
This work was supported by funds and fellowships from the following Brazilian government agencies CAPES, CNPq, and FUNCAP/Pronex.
Received 21 October 2008; Accepted 8 January 2009
- Adams RP 2001. Identification of essential oils components by gas chromatography/trap mass spectrometry. Illinois: Allured Publishing Corporation.
- Agnaniet H, Bikanga R, Bessiere JM, Menut C 2005. Aromatic plants of tropical central Africa. Part XLVI. Essential oil constituents of Cassia alata (L.) from Gabon. J Essent Oil Res 17: 410-412.
- Agra MF, França PF, Barbosa-Filho JM 2007. Synopsis of the plants known as medicinal and poisonous in Northeast of Brazil. Rev Bras Farmacogn 17: 114-140.
- Agra MF, Silva KN, Basílio IJLD, França PF, Barbosa-Filho JM 2008. Survey of medicinal plants used in the region Northeast of Brazil. Rev Bras Farmacogn 18: 472-508.
- Bandeira PN, Fonseca AM, Costa SMO, Lins MUDS, Pessoa ODL, Monte FJQ, Nogueira NAP, Lemos TLG 2006. Antimicrobial and antioxidant activities of the essential oil of resin of Protium heptaphyllum. Nat Prod Commun 1: 117-120.
- Borse BB, Rao LJM, Ramalakshmi K, Raghavan B 2007. Chemical composition of volatiles from coconut sap (neera) and effect of processing. Food Chem 101: 877-880.
- Burits M, Bucar F 2000. Antioxidant activity of Nigella sativa essential oil. Phytother Res 14: 323-328.
- Campbell-Falck D, Thomas T, Falck TM, Tutuo N, Clem K 2000. The intravenous use of coconut water. Am J Emerg Med 18: 108-111.
- Chumbimuni-Torres KY, Kubota LT 2006. Simultaneous determination of calcium and potassium in coconut water by a flow-injection method with tubular potentiometric sensors. J Food Comp Anal 19: 225-230.
- Farr S 1994. 2001 A soft drink odyssey. Food Manuf 69: 29-50.
- Hegazi AG, El Hady FKA 2002. Egyptian propolis: 3. Antioxidant, antimicrobial activities and chemical composition of propolis from reclaimed lands. Z Naturforsh 57c: 395-402.
- Jacob RA, Burri BJ 1996. Oxidative damage and defense. Am J Clin Nutr 63: 9855-9905.
- Jirovetz L, Buchbauer G, Ngassoum MB 2003. Solid-phase-microextraction-headspace aroma compounds of coconut (Cocos nucifera) milk and meat from cameroon. Ernährung Nutr 27: 300-303.
- Kinsella JE, Frankel E, German B, Kanner J 1993. Possible mechanism for the protective role of antioxidants in wine and plant foods. Food Technol 47: 85-89.
- Leong LP, Shui G 2002. An investigation of antioxidant capacity of fruits in Singapore markets. Food Chem 76: 69-75.
- Letizia CS, Cocchiara J, Wellington GA, Funk C, Api AM 2000. p-Methoxybenzyl acetate. Food Chem Toxicol 38: S7-S9.
- Loki AL, Rajamohan T 2003. Hepatoprotective and antioxidant effect of tender coconut water on carbon tetrachloride induced liver injury in rats. Ind J Biochem Bioph 40: 354-357.
- Mantena SK, Jagadish, Badduri SR, Siripurapu KB, Unnikrishnan MK 2003. In vitro evaluation of antioxidant properties of Cocos nucifera Linn. water. Nahrung 47: 126-131.
- Matsuyama A, Yamamoto H, Kawada N, Kobayashi Y 2001. Industrial production of (R)-1,3-butanediol by new biocatalysts. J Mol Catal B: Enzymat 11: 513-521.
- Mepba HD, Achinewhu SC 2003. Effects of processing on protein nutritive quality of coconut Cocos nucifera products. Plant Food Hum Nutr 58: 15-25.
- Narain N, Galvão MS, Madruga MS 2007. Volatile compounds captured through purge and trap technique in caja-umbu (Spondias sp.) fruits during maturation. Food Chem 102: 726-731.
- Pummer S, Heil P, Maleck W, Petroianu G 2001. Influence of coconut water on hemostasis. Am J Emerg Med 19: 287-289.
- Reddy KV, Das M, Das SK 2005. Filtration resistances in non-thermal sterilization of green coconut water. J Food Eng 69: 381-385.
- Santoso U, Kubo K, Ota T, Tadokoro T, Maekawa A 1996. Nutrient composition of kopyor coconuts (Cocos nucifera L.). Food Chem 51: 299-304.
- Skrede G, Larsen VB, Aaby K, Jorgensen AS, Birkeland SE 2004. Antioxidative properties of commercial fruit preparations and stability of bilberry and black currant extracts in milk products. J Food Sci 69: 351-356.
- Stenhagen E, Abrahamson S, McLafferty FW 1974. Registry of Mass Spectra Data. New York: John Wiley & Sons.
- Tepe B, Daferera D, Sokmen A, Sokmen M, Polissou M 2005. Antimicrobial and antioxidant activities of the essential oil and various extracts of Salvia tomentosa Miller (Lamiaceae). Food Chem 90: 333-340.
- Yi BH, Kim DH1982. Antioxidant activity of maltol, kojic acid, levulinic acid, furfural, 5-hydroxymethylfurfural, and pyrazine. Han 'guk Sikp 'um Kwahakhoechi, 14: 265-270.
Publication Dates
-
Publication in this collection
18 Aug 2009 -
Date of issue
Mar 2009
History
-
Accepted
08 Jan 2009 -
Received
21 Oct 2008