Abstracts
Antibiotics are considered the main therapeutic option to treat bacterial infections; however, there is the disadvantage of increasing bacterial resistance. Thus, the research of antimicrobials of plant origin has been an important alternative. This work aimed at determining the in vitro antibacterial activity of the essential oil of Origanum vulgare L. (Lamiaceae) on multiresistant bacteria isolated from biological materials. 24 strains of nosocomial bacteria were used and divided into six different species that were inhibited by the essential oil in the preliminary "screening" which was accomplished by the diffusion technique in agar. MIC was determined by the microdilution method, beginning with solutions with the final concentrations: 8 up to 0.125% with the following results: The four samples (100%) of Escherichia coli, Enterococcus faecalis and MRSA were inhibited by the essential oil at the concentration of 0.125%. Three samples (75%) of Acinetobacter baumannii at 0.125% and a sample (25%) at 0.5%; Klebsiella pneumoniae (75%) at 0.125% and 25% at 0.25%; Pseudomonas aeruginosa (75%) at 0.5% and 25% at 0.25%. MIC varied from 78 to 83%. It was concluded through the obtained data that there was not difference in the minimum bactericidal concentration (0.5%) of the referred oil for Gram positive as well for Gram negative microorganisms.
Origanum vulgare; bacterial infections; multiresistant bacteria
Os antibióticos permanecem como a principal opção terapêutica para tratar infecções bacterianas, no entanto, existe a desvantagem de aumentarem a resistência bacteriana, e como alternativa, destaca-se a pesquisa de antimicrobianos de origem vegetal. Neste trabalho objetivou-se determinar in vitro a atividade antibacteriana do óleo essencial de Origanum vulgare L. (Lamiaceae) (orégano), sobre bactérias multirresistentes isoladas de materiais biológicos. Foram usadas 24 linhagens de bactérias de origem hospitalar, divididas em seis espécies distintas, que foram inibidas pelo óleo essencial no "screening" preliminar, realizado utilizando-se a técnica de difusão em ágar. A CIM foi determinada pelo método de microdiluição, partindo-se de soluções com as concentrações finais: 8 até 0,125% com os seguintes resultados: As quatro amostras (100%) de Escherichia coli, Enterococcus faecalis e MRSA foram inibidas pelo óleo essencial na concentração de 0,125%. Três amostras (75%) de Acinetobacter baumannii por 0,125% e uma amostra (25%) por 0,5%; Klebsiella pneumoniae (75%) por 0,125% e 25% por 0,25%; Pseudomonas aeruginosa (75%) por 0,5% e 25% por 0,25%. A CIM variou de 78 a 83%. Concluiu-se com base nos dados obtidos, que não houve diferença na concentração bactericida mínima (0,5%) do referido óleo tanto para os microrganismos Gram positivos quanto para os Gram negativos.
Origanum vulgare; infecções bacterianas; bactérias multirresistentes
ARTIGO
Antibacterial activity of the essential oil of Origanum vulgare L. (Lamiaceae) against bacterial multiresistant strains isolated from nosocomial patients
Atividade antibacteriana do óleo essencial de Origanum vulgare L. (Lamiaceae) contra bactérias multiressistentes isoladas de pacientes nosocomial
Adalberto Coelho da Costa* * E-mail: adalbcoelho@yahoo.com.br ; Bernadete Helena Cavalcanti dos Santos; Lauro Santos Filho; Edeltrudes de Oliveira Lima
Departamento de Ciências Farmacêuticas, Universidade Federal da Paraíba, 58051-900 João Pessoa-PB, Brazil
ABSTRACT
Antibiotics are considered the main therapeutic option to treat bacterial infections; however, there is the disadvantage of increasing bacterial resistance. Thus, the research of antimicrobials of plant origin has been an important alternative. This work aimed at determining the in vitro antibacterial activity of the essential oil of Origanum vulgare L. (Lamiaceae) on multiresistant bacteria isolated from biological materials. 24 strains of nosocomial bacteria were used and divided into six different species that were inhibited by the essential oil in the preliminary "screening" which was accomplished by the diffusion technique in agar. MIC was determined by the microdilution method, beginning with solutions with the final concentrations: 8 up to 0.125% with the following results: The four samples (100%) of Escherichia coli, Enterococcus faecalis and MRSA were inhibited by the essential oil at the concentration of 0.125%. Three samples (75%) of Acinetobacter baumannii at 0.125% and a sample (25%) at 0.5%; Klebsiella pneumoniae (75%) at 0.125% and 25% at 0.25%; Pseudomonas aeruginosa (75%) at 0.5% and 25% at 0.25%. MIC varied from 78 to 83%. It was concluded through the obtained data that there was not difference in the minimum bactericidal concentration (0.5%) of the referred oil for Gram positive as well for Gram negative microorganisms.
Keywords: Origanum vulgare, bacterial infections, multiresistant bacteria.
RESUMO
Os antibióticos permanecem como a principal opção terapêutica para tratar infecções bacterianas, no entanto, existe a desvantagem de aumentarem a resistência bacteriana, e como alternativa, destaca-se a pesquisa de antimicrobianos de origem vegetal. Neste trabalho objetivou-se determinar in vitro a atividade antibacteriana do óleo essencial de Origanum vulgare L. (Lamiaceae) (orégano), sobre bactérias multirresistentes isoladas de materiais biológicos. Foram usadas 24 linhagens de bactérias de origem hospitalar, divididas em seis espécies distintas, que foram inibidas pelo óleo essencial no "screening" preliminar, realizado utilizando-se a técnica de difusão em ágar. A CIM foi determinada pelo método de microdiluição, partindo-se de soluções com as concentrações finais: 8 até 0,125% com os seguintes resultados: As quatro amostras (100%) de Escherichia coli, Enterococcus faecalis e MRSA foram inibidas pelo óleo essencial na concentração de 0,125%. Três amostras (75%) de Acinetobacter baumannii por 0,125% e uma amostra (25%) por 0,5%; Klebsiella pneumoniae (75%) por 0,125% e 25% por 0,25%; Pseudomonas aeruginosa (75%) por 0,5% e 25% por 0,25%. A CIM variou de 78 a 83%. Concluiu-se com base nos dados obtidos, que não houve diferença na concentração bactericida mínima (0,5%) do referido óleo tanto para os microrganismos Gram positivos quanto para os Gram negativos.
Unitermos: Origanum vulgare, infecções bacterianas, bactérias multirresistentes.
INTRODUCTION
The spread of drug resistant pathogens is one of the most serious threats to successful treatment of microbial diseases. The bacterial resistance acquired against antibiotics, becoming it difficult to fight them, mainly, when they are isolated using biological material collected from hospitalized patients. In these cases the colonization of the skin is, initially, constituted with normal microbiota, and when it is injured contributes for the bacterial multiplication. Therefore, it is verified that, after the five first days of internment of the patient this biota is substituted by Gram negative bacteria and Staphylococcus strains present in the nosocomial environment, with the possibility of the presence of infections caused by some species of fungi and viruses (Martins, 2001). The most important pathogens involved in nosocomial infections, including of great burnt patients are: Staphylococcus aureus methicillin resistant, Enterococcus and multiresistant Pseudomonas aeruginosa, that constitute a great problem in units for burnt treatment, increasing the etiology of cutaneous infections and eventually septicemia in patients with severe infections (Khardori & Kanchanapoom, 2002).
In spite of the advances introduced in the attendance to the burnings victims, factors responsible now by the significant surviving and total rehabilitation, occurred in the majority of the patients, the infections remain as the main complication, with as consequence the prolongation of the nosocomial stay. However, the occurrence of multiresistant bacterial strains constitutes an additional problem by the difficulty on choosing a correct antimicrobial therapy, with effective support by the microbiology laboratory (Fernandes & Ribeiro Filho, 2000).
The microorganisms come acquiring multiresistance to antibiotics, and this fact conduct the clinical treatment using each time more the therapeutic properties of determined plants used for fighting the infections (White, 1970). In this way, and considering their pharmacological properties, different plants are studied and used for treatment of diverse types of illnesses (Thylstrup & Fejerskov, 1995). Thus, the fact of the microorganisms acquire quickly resistance to antibiotics, justify the investment, each bigger time in natural products, and the scientists are always alert in the search of new drugs, from the vegetal species, with the objective of to fight the multiresistant microorganisms (Oliveira et al., 2007; Silva et al., 2007; Coutinho et al., 2008).
Considering this premise, the study of the antibacterial action of the essential oil of Origanum vulgare against strains of multiresistant bacteria, from nosocomial origin and isolated from clinical materials from diverse anatomical sites in the body was motivated.
Since ancient ages essential oils (EOs) and other extracts of plants have evoked interest as sources of natural products, they are also called volatile are aromatic oily liquids obtained from plant material (flowers, buds, seeds, leaves, herbs, wood, fruits and roots). They can be obtained by expression, fermentation, or extraction but the method of steam distillation is most commonly used for commercial production of EOs (Van de Braak & Leijten, 1999).
It has long been recognised that some EOs have antimicrobial properties (Boyle, 1955; Nascimento et al., 2007; Costa et al., 2008) and these have been reviewed in the past like the antimicrobial properties of spices. However, the relatively recent enhancement of interest in 'green' consumerism has lead to a renewal of scientific interest in these substances (Nychas, 1995; Tuley de Silva, 1996).
Origanum vulgare is a very versatile plant and although it has been known for a long time, as a popular medicine, only now is it starting to be recognised for its potential therapeutic role such as diaphoretic, carminative, antispasmodic, antiseptic and tonic properties. Although it has a wide spectrum of antimicrobial activity, which has been the subject of several in vitro and in vivo investigations, there is a lack of knowledge on its effectiveness against multiresistant bacteria isolated from nosocomial patients.
The aim of the present study was to evaluate the susceptibility of multiresistant bacteria isolates, obtained from patients attended at a burnt unit in an emergency hospital, to Origanum essential oil and its major components carvacrol and thymol.
In this way our objective was: a) to determine the bacterial etiology of infections in patients taken care of in a emergency and trauma hospital; b) to identify the incidence of multiresistant pathogens as etiologic agents of the infectious processes; and c) to observe "in vitro" the antibacterial activity of the EOs, on isolated bacteria from clinical samples, determining the minimum inhibitory concentration (MIC) and the minimum bactericidal concentration (MBC) of this composition.
MATERIAL AND METHODS
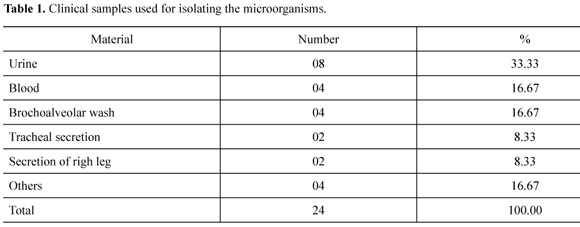
Clinical samples (170) obtained from patients attended at the Hospital de Emergência e Trauma Senador Humberto Lucena (HETSHL) were used. Reference hospital for its specialty and located in the urban area of João Pessoa- State of Paraíba, Brazil and classified by the Brazilian System of Health (SUS) for attendance of urgency procedures. The biological materials was sampled from diverse anatomical small sites and processed for bacterial isolation in the Clinical Microbiology Laboratory in the Health Center Sciences at the Universidade Federal da Paraíba. The clinical samples are described on Table 1.
Processing and identification
The samples had been inoculated in the plates of Agar Blood, Manitol Salt Agar and MacConkey Agar (Difco), in order for the development of different microrganisms. It was prioritized, the isolation of Staphylococcus aureus, Enterococcus faecalis, Klebsiella pneumoniae, Escherichia coli, Pseudomonas aeruginosa and Acinetobacter baumannii, considered as the main multiresistant pathogens. The bacterial identification was carried through standardized routine methods, with the characterization of Gram positive cocci and species of Enterobacteriaceae e Gram Negative Not Fermenters Rods (GNNFR), as established in routine techniques (Koneman et al., 2001; Santos Filho, 2003).
Test of susceptibility to antimicrobials
The susceptibility tests were performed by the disc diffusion method (Bauer et al., 1966) using the drugs recommended by the NCCLS (2004): amicacyn (AMI), cefepime (CPM), ceftazidime (CAZ), ciprofloxacin (CIP), levofloxacin (LEV), penicillin (PEN), ampicillin (AMP), ampicillin-sulbactam (SAM), amoxicillina-clavulanic acid (AMC), gentamicyn (GEN), cefotaxime (CFO), ceftriaxon (CFT), aztreonam (ATZ), imipenem (IMI), meropenem (MER) piperacillin (PIP), piperacillin-tazobactam (PTZ), ticarcilin-clavulanic acid (TIM), oxacillin (OXA), vancomicyn (VAN)
Characterization of the samples
The bacteria that shown profile of resistance, with the following characteristics was used for the study of the antimicrobial activity of the essential oil: methicillin resistant S. aureus (MRSA), E. faecalis, K. pneumoniae and E. coli extended spectrum beta-lactamases producers (ESBL), strains Pseudomonas aeruginosa and Acinetobacter baumannii resistant to ceftazidime and Carbapenems.
Samples of S. aureus oxacillin-resistant (MRSA) had been confirmed using E-test® for determination of MIC and the confirmation of the resistance (Araújo et al., 2000). Samples of K. pneumoniae and E. coli producers of extended spectrum beta-lactamases (ESBL) had been confirmed using the criteria recommended by the NCCLS (2004), in which the values for the sizes of inhibition zones are established, with the test of diffusion with discs, for the presumptive selection of samples potentially producers of ESBL. Strains presenting this behavior were submitted to the confirmatory tests (Honorio et al., 2001).
Bacterial stains
From the 170 bacterial isolates, 24 bacterial strains divided into six groups of different species were selected for this study, in the following sequence: S. aureus (04), E. faecalis (04), K. pneumoniae (04), E. coli (04), P. aeruginosa (04) and A. baumannii (04). After the preliminary isolation in the identification routine scheme the strains were identified and kept in Nutrient Agar (Biobras) until its use in posterior tests.
Essential oil
The Origanum vulgare essential oil was supplied by the company FERQUIMA - Industry and Commerce Ltda (Vargem Grande, São Paulo, Brazil). This provider produces and commercializes essential oils and its quality parameters (appearance, color, purity, odor, density -20 ºC, refraction index -20 ºC) were described in an accompanying technical report. The procedure of its extraction was carried through the method of hydro distillation in industrial scale.
Screening of the evaluation of the antibacterial activity of essential oils
Initially, saline solution was prepared with bacterial strains suspensions in assay tubes with 5 mL of sterile saline solution (NaCl 0.85% p/v). After that, such suspensions were agitated during two minutes with in a Vortex. After agitation, each suspension had its comparative turbidity adjusted to that presented by the tube 0.5 of the McFarland scale, which corresponds approximately to an inoculum of 108 UFC/mL for bacteria (Chin Lu, 1971; Tavares, 1984; Drutz, 1987; Cleeland & Squires, 1991).
Following with aid of a sterile swab absorbed with the prepared bacterial suspension, all surface of a Mueller Hinton (Difco) agar plate was inoculated and paper filter discs absorbed with 0.02 mL of the products submitted to analysis were used. Finally, the plate was incubated 35-37 ºC during 18 the 24 hours (Bauer et al., 1966; Chin Lu, 1971; McGinnis, 1980; Vincent & Vincent, 1994). It was considered as positive for antibacterial activity the cases where the product applied on the plates inoculated with the bacterial suspension made formation of inhibition area equal or superior to 10 mm of diameter around the disc (Naqui et al., 1991; Lima et al., 1993; Cole, 1994; Alves et al., 2000; Souza et al., 2005).
Determination of the minimum inhibitory concentration (MIC) using the microdilution technique
The MIC of the essential oil was determined against 24 bacterial strains isolated from clinical samples. It was established varied concentrations of the essential oil in the following scheme: in a sterile assay tube 3.2 mL of the essential oil were placed, added with 0.04 mL of TWEEN 80 and q.s.p. 5 mL with distilled water, and agitated during two minutes in a Vortex. Following a serial dilution, each sterile tube with 2.5 mL of sterile distilled water was added of 2.5 mL of previous dilution and agitated again during two minutes (Allegrini & Simeon, 1972). With this sequence of procedures, the final obtained concentrations correspond to 8, 4,2, 1, 0.5, 0.25 and 0.125 dilutions.
The determination of the MIC of the O. vulgare EOs was carried through the microdilution technique using sterilized microplates with 96 (12 x 08) wells with deep flat and proper cover, where was placed 100 µL of the essential oil solution. After that, 100 µL of nutrient broth doubly concentrated were added and inoculated with the assayed microorganism. In the eighth line of the microdilution plate, 200 µL of the broth inoculated with the microbial suspension (positive control of the assayed strain viability) were placed, in this way that the first sequence of wells presented the highest concentration of the essential oil, and the seventh line, presents it lesser concentration of the studied oil. The system was incubated at 35-37 ºC during 24 hours (Viljoen et al., 2003; Sahin et al., 2004).
For the reading of the microdilution assay the colorimetric method was used, which consists of the addition of 20 µL of the water solution of the resazurin die (Sigma-Aldrich) in the 0.01% concentration, which is recognized as a colorimetric marker of oxide-reduction reaction (Mann & Markan, 1998; Salvat et al., 2001; Burt & Reinders, 2003). The change of coloration of the die (blue for rose) indicates microbial growth. The lesser concentration of the oil essential capable to inhibit the growth of strain assayed, verified for the blue coloration of the die is considered as a measure of the MIC (Espinel-Ingrof et al., 1992).
Determination of minimum bactericidal concentration (MBC)
For the determination of the MBC, 50 µL of the four last concentrations of the oil were removed, in the microdilution plate with growth absence, and were inoculated in a plate contend Nutrient Agar (Biobras). The same ones were incubated at 35 ± 1 ºC during 24-48 hours and, after this, the reading was made. The minimum bactericidal concentration (MBC) was considered starting at the point that did not allow to the reactivation of the microorganism in the medium without antimicrobials (Benoudia et al., 1988; Smith Palmer et al. 1998; Salvat et al., 2001; Sahin et al., 2004).
RESULTS AND DISCUSSION
Of the carried through microbiological analyses in 170 clinical samples of some biological materials, 24 bacterial strains (sixteen Gram negative and eight Gram positive) were selected considering that they presented the profile for the considered study.
The results presented on Table 2 demonstrate that of the four strains of A. baumannii, three (01, 02 and 03) corresponding to 75% were inhibited by the essential oil of O. vulgare at the 0.125% concentration. The strain 04 (25%) was inhibited by the 0.5% concentration. The four strains (100%) of E. coli, S. faecalis and S. aureus were inhibited with the 0.125% concentration. From the four strains of K. pneumoniae, three (75%), strains 01, 02 and 04 were inhibited at the 0.125% concentration, on the other hand, the strain 03 was inhibited in the 0.25% concentration. Considering the strains of P. aeruginosa, three of them (01, 02 and 03) corresponding to 75% were inhibited at the 0.5% concentration, however, the strain 04 (25%) was inhibited in a lesser concentration (0.25%). Emphasize that, good results with these normally multiresistant bacteria were obtained with the referred oil, while Hammer et al. (1997) had determined the MIC of different oils using extracts at < 2% concentration. Moreover, Souza at al. (2006), although testing the action of the O. vulgare oil against other bacterial species, had also obtained good results. The MBC corresponded to 100% when was used the 0.5% concentration in the twenty four tested strains showing the effectiveness of the oil. Santurio et al. (2007) testing the action of O. vulgare against serum variant of Salmonella enterica also had determined a very good MBC.
CONCLUSION
From the obtained data we can concluded that: the essential oil of O. vulgare in low concentration was capable to prevent the growth of the strains: A. baumannii, E. coli, K. pneumoniae, P. aeruginosa, E. faecalis and S. aureus methicillin resistant (MRSA) and the majority of these bacteria had the minimum inhibitory concentration (MIC) equal to the minimum bactericidal concentration (MBC). And a significant number of these microorganisms were also inhibited by a concentration slightly lower.
It was observed that there was no difference in the minimum bactericidal concentration (MBC) of the Origanum vulgare essential oil as much to the Gram positive as well as for the Gram negative microorganisms.
REFERENCES
Allegrini J, Simeon M 1972 Study of essential oil antibacterial power. Prod Prob Pharm 27: 891-899.
Alves AJ 2000. Atividade biológica de óleos essenciais e fitoconstituintes sobre espécies de Mycobacterium. Tese de Doutorado. UFPB. 143p.
Araújo BAC, Oliveira AL, Santos Filho L 2000. Isolamento de amostras multirresistentes de Staphylococcus aureus em estetoscópios usados no ambiente hospitalar. RBAC 32: 285-288.
Bauer AW, Kirby WM, Sherris JC. Turck M 1966. Antibiotic susceptibility testing by a standardized single disc method. Am J Clin Pathol 45: 393-496.
Benoudia H, Hassar M, Benjilalli B 1988. Lês propriétés antiséptiques dês huiles essentielles in vitro, testées contre dês germes pathogénes hospitaliers. Fitoterapia 59: 115-119.
Boyle W 1955. Spices and essential oils as preservatives. The American Perfumer and Essential Oil Review 66: 25- 28.
Burt SA, Reinders RD 2003. Antibacterial activity of selected plant essential oils against Escherichia coli O157:H7. Lett Appl Microbiol 26: 162-167.
Chin Lu Y 1971. In vitru study of pyrrolnitrin. J Am Med Assoc 70: 19-22.
Cleeland R, Squires E 1991. Evaluation of new antimicrobials in vitro and in experimental animal infections In: Lorian, V. Antibiotics in Laboratory Medicine. 3 ed. Baltimore: Williams; Wilkins, 739-788 p.
Cole MD 1994. Key antifungal, antibacterial and anti-insect assays - Critical review. Biochem Syst Ecol 22: 837- 856.
Costa VCO, Tavares JF, Agra MF, Falcão Silva VS, Facanali R, Vieira MAR, Marques MOM, Siqueira-Júnior JP, Silva MS 2008. Composição química e modulação da resistência bacteriana a drogas do óleo essencial das folhas de Rollinia leptopetala R. E. Fries. Rev Bras Farmacogn 18: 245-248.
Coutinho HDM, Costa JGM, Siqueira-Júnior JP, Lima EO 2008. In vitro anti-staphylococcal activity of Hyptis martiusii Benth against methicillin-resistant Staphylococcus aureus-MRSA strains. Rev Bras Farmacogn 18 (Supl.): 670-675.
Drutz DJ 1987. In vitro antifugical susceptibility testing and measurement of levels of antifungical agents in body fluids. Rev Infect Dis 9: 411-415.
Espinel-Ingroff A, Kish Jr CW, Kerkering TM, Fromtling RA, Bartizal K, Galgiani JN, Villareal K, Pfaller MA, Gerarden T, Rinaldi MG 1992. Collaborative comparison of broth macrodilution and microdilution antifungal susceptibility tests. J Clin Microbiol 30: 3138-3145.
Fernandes AT, Ribeiro Filho N 2000. Infecção em Queimados. In: Fernandes, A. T., Fernandes, M. O. V., Ribeiro Filho, N. Infecção Hospitalar e suas Interfaces na Área da Saúde. São Paulo: Atheneu, 1: 657- 669.
Hammer KA, Carson CF, Rilley TV 1999. Antimicrobial activity of essential oils and other plant extracts. J Appl Microbiol 86: 985-990.
Honório LC, Santos IB, Assis, AML, Santos Filho L 2001. Análise do perfil de resistência, de enterobactérias produtoras de beta-lactamases de espectro ampliado (ESBL) isoladas em João Pessoa, PB. RBAC 33: 179-182.
Khardori N, Kanchanapoom T 2002. Managent of infections1 patients with severe buns impact of multiresistant pathogens. J Burns Surg Wound Care 1: 1-7.
Koneman EW, Allen SD, Janda WM, Schreckenberger PC, Júnior Winn WC 2001. Diagnóstico Microbiológico Texto e Atlas Colorido. 5. ed. Medsi, 1465 p.
Lima EO, Gompertez OF, Giesbrecht AM, Paulo MQ 1993. "In vitro" antifungal activity of essential oils obtained from officinal plants against dermatophytes. Mycoses 36: 333-336.
Mann CM, Markham JL 1998. A new method for determining the minimum inhibitory concentration of essential oils. J Appl Microbiol 84: 538-544.
Martins MA 2001. Manual de Infecção Hospitalar, Epidemiologia Prevenção, Controle. 2ª ed. Editora Medsi. Belo Horizonte MG.
McGinnis MR 1980. Laboratory handbook of medical mycology. New York: Academic Press, 411 p.
Naqui SH, Kilian MSY, Vohora SB 1991. Anti-bacterial, antifungal and antihelmintic investigations on Indian medicinal plants. Fitoterapia 62: 221-228.
Nascimento PFC, Nascimento AC, Rodrigues CS, Antoniolli AR, Santos PO, Barbosa Júnior, Trindade RC 2007. Atividade antimicrobiana dos óleos essenciais: uma abordagem multifatorial dos métodos. Rev Bras Farmacogn 17: 108-113.
NCCLS 2004. National Committee For Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7-A4. National Committee for Clinical Laboratory Standards, Wayne, PA. 2004.
Nychas GJE 1995. Natural antimicrobials from plants. In: New Methods of Food Preservation, ed. Gould, G.W. pp. 58-89. London: Blackie Academic Professional.
Oliveira RAG, Lima EO, Souza EL, Vieira WL, Freire KRL, Trajano VN, Lima IO, Silva-Filho RN 2007. Interference of Plectranthus amboinicus (Lour.) Spreng essential oil on the anti-Candida activity of some clinically used antifungals. Rev Bras Farmacogn 17: 186-190.
Sahin F, Gulluce M, Daferera D, Sokmen A, Polissiou M, Agar G, Ozer H 2004. Biological activities of the essential oils and methanol extract of Origanum vulgare ssp. vulgare in the Eastern Anatolia region of Turkey. Food Control 15: 549-557.
Salvat A, Antonnacci L, Fortunato RH, Suarez EY, Godoy HM 2001. Screening of some plants from Northern Argentina for their antimicrobial activity. Lett Appl Microbiol 32: 293-297.
Santos Filho L 2003. Manual de Microbiologia Clínica, 4a Ed. Editora Universitária, João Pessoa, UFPB, 314 p.
Santurio JM, Santurio DF, Pozzatti P, Moraes C, Franchin PR, Alves SH 2007. Atividade antimicrobiana dos óleos essenciais de orégano, tomilho e canela frente a sorovares de Salmonella enterica de origem avícola. Cienc Rural 37: 803-808.
Silva JG, Souza IA, Higino JS, Siqueira-Junior JP, Pereira JV, Pereira MSV 2007. Atividade antimicrobiana do extrato de Anacardium occidentale Linn. em amostras multiresistentes de Staphylococcus aureus. Rev Bras Farmacogn 17: 572-577.
Smith-Palmer A, Stewart J, Fyfe L 1998. Antimicrobial properties of plant essential oils and essences against five important food-borne pathogens. Lett Food Microbiol 26: 118-122.
Souza EL, Lima EO, Freire KRL, Sousa CP 2005. Inhibition action of some essential oils and phytochemicals on the growth of moulds isolated from foods. Braz Arch Biol Techn 2: 245-259.
Tavares W 1984. Manual de Antibióticos. 3ª Ed. São Paulo: Atheneu, 374p.
Thylstrup A, Fejerskov O 1995. Características clínicas e patológicas da cárie dentária. Cariologia Clínica, 2a Ed., São Paulo, Santos, cap. 6, p. 45-69.
Tuley de Silva 1996 K. (Ed.), A Manual on the Essential Oil Industry. United Nations Industrial Development Organization, Vienna.
Van de Braak SAAJ, Leijten GCJJ 1999. Essential Oils and Oleoresins: A Survey in the Netherlands and other Major Markets in the European Union. CBI, Centre for the Promotion of Imports from Developing Countries, Rotterdam, p. 116.
Viljoen A, Van Vuuren S, Ernst E, Klepser M, Demirci B, Baser H, Van Wyk BE 2003. Osmitopsis astericoides (Asteraceae). The antimicrobial activity and essential oil composition of a Cape-Dutch remedy. J Ethopharmacol 88: 137-143.
Vincent JG, Vincent HW 1994. Filter paper disc modification of the Oxford cup Penicillium determination. Proc Soc Exp Biol Med New York 25: 162-164.
White A 1970. Relation between quantitative nasal cultures and dissemination of Staphylococci. J Lab Clin Med 58: 273-277.
Received 12 November 2008; Accepted 20 February 2009
- Allegrini J, Simeon M 1972 Study of essential oil antibacterial power. Prod Prob Pharm 27: 891-899.
- Alves AJ 2000. Atividade biológica de óleos essenciais e fitoconstituintes sobre espécies de Mycobacterium. Tese de Doutorado. UFPB. 143p.
- Araújo BAC, Oliveira AL, Santos Filho L 2000. Isolamento de amostras multirresistentes de Staphylococcus aureus em estetoscópios usados no ambiente hospitalar. RBAC 32: 285-288.
- Bauer AW, Kirby WM, Sherris JC. Turck M 1966. Antibiotic susceptibility testing by a standardized single disc method. Am J Clin Pathol 45: 393-496.
- Benoudia H, Hassar M, Benjilalli B 1988. Lês propriétés antiséptiques dês huiles essentielles in vitro, testées contre dês germes pathogénes hospitaliers. Fitoterapia 59: 115-119.
- Boyle W 1955. Spices and essential oils as preservatives. The American Perfumer and Essential Oil Review 66: 25- 28.
- Burt SA, Reinders RD 2003. Antibacterial activity of selected plant essential oils against Escherichia coli O157:H7. Lett Appl Microbiol 26: 162-167.
- Chin Lu Y 1971. In vitru study of pyrrolnitrin. J Am Med Assoc 70: 19-22.
- Cleeland R, Squires E 1991. Evaluation of new antimicrobials in vitro and in experimental animal infections In: Lorian, V. Antibiotics in Laboratory Medicine. 3 ed. Baltimore: Williams; Wilkins, 739-788 p.
- Cole MD 1994. Key antifungal, antibacterial and anti-insect assays - Critical review. Biochem Syst Ecol 22: 837- 856.
- Costa VCO, Tavares JF, Agra MF, Falcão Silva VS, Facanali R, Vieira MAR, Marques MOM, Siqueira-Júnior JP, Silva MS 2008. Composição química e modulação da resistência bacteriana a drogas do óleo essencial das folhas de Rollinia leptopetala R. E. Fries. Rev Bras Farmacogn 18: 245-248.
- Coutinho HDM, Costa JGM, Siqueira-Júnior JP, Lima EO 2008. In vitro anti-staphylococcal activity of Hyptis martiusii Benth against methicillin-resistant Staphylococcus aureus-MRSA strains. Rev Bras Farmacogn 18 (Supl.): 670-675.
- Drutz DJ 1987. In vitro antifugical susceptibility testing and measurement of levels of antifungical agents in body fluids. Rev Infect Dis 9: 411-415.
- Espinel-Ingroff A, Kish Jr CW, Kerkering TM, Fromtling RA, Bartizal K, Galgiani JN, Villareal K, Pfaller MA, Gerarden T, Rinaldi MG 1992. Collaborative comparison of broth macrodilution and microdilution antifungal susceptibility tests. J Clin Microbiol 30: 3138-3145.
- Fernandes AT, Ribeiro Filho N 2000. Infecção em Queimados. In: Fernandes, A. T., Fernandes, M. O. V., Ribeiro Filho, N. Infecção Hospitalar e suas Interfaces na Área da Saúde. São Paulo: Atheneu, 1: 657- 669.
- Hammer KA, Carson CF, Rilley TV 1999. Antimicrobial activity of essential oils and other plant extracts. J Appl Microbiol 86: 985-990.
- Honório LC, Santos IB, Assis, AML, Santos Filho L 2001. Análise do perfil de resistência, de enterobactérias produtoras de beta-lactamases de espectro ampliado (ESBL) isoladas em João Pessoa, PB. RBAC 33: 179-182.
- Khardori N, Kanchanapoom T 2002. Managent of infections1 patients with severe buns impact of multiresistant pathogens. J Burns Surg Wound Care 1: 1-7.
- Koneman EW, Allen SD, Janda WM, Schreckenberger PC, Júnior Winn WC 2001. Diagnóstico Microbiológico Texto e Atlas Colorido. 5. ed. Medsi, 1465 p.
- Lima EO, Gompertez OF, Giesbrecht AM, Paulo MQ 1993. "In vitro" antifungal activity of essential oils obtained from officinal plants against dermatophytes. Mycoses 36: 333-336.
- Mann CM, Markham JL 1998. A new method for determining the minimum inhibitory concentration of essential oils. J Appl Microbiol 84: 538-544.
- Martins MA 2001. Manual de Infecção Hospitalar, Epidemiologia Prevenção, Controle. 2Ş ed. Editora Medsi. Belo Horizonte MG.
- McGinnis MR 1980. Laboratory handbook of medical mycology. New York: Academic Press, 411 p.
- Naqui SH, Kilian MSY, Vohora SB 1991. Anti-bacterial, antifungal and antihelmintic investigations on Indian medicinal plants. Fitoterapia 62: 221-228.
- Nascimento PFC, Nascimento AC, Rodrigues CS, Antoniolli AR, Santos PO, Barbosa Júnior, Trindade RC 2007. Atividade antimicrobiana dos óleos essenciais: uma abordagem multifatorial dos métodos. Rev Bras Farmacogn 17: 108-113.
- NCCLS 2004. National Committee For Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7-A4. National Committee for Clinical Laboratory Standards, Wayne, PA. 2004.
- Nychas GJE 1995. Natural antimicrobials from plants. In: New Methods of Food Preservation, ed. Gould, G.W. pp. 58-89. London: Blackie Academic Professional.
- Oliveira RAG, Lima EO, Souza EL, Vieira WL, Freire KRL, Trajano VN, Lima IO, Silva-Filho RN 2007. Interference of Plectranthus amboinicus (Lour.) Spreng essential oil on the anti-Candida activity of some clinically used antifungals. Rev Bras Farmacogn 17: 186-190.
- Sahin F, Gulluce M, Daferera D, Sokmen A, Polissiou M, Agar G, Ozer H 2004. Biological activities of the essential oils and methanol extract of Origanum vulgare ssp. vulgare in the Eastern Anatolia region of Turkey. Food Control 15: 549-557.
- Salvat A, Antonnacci L, Fortunato RH, Suarez EY, Godoy HM 2001. Screening of some plants from Northern Argentina for their antimicrobial activity. Lett Appl Microbiol 32: 293-297.
- Santos Filho L 2003. Manual de Microbiologia Clínica, 4a Ed. Editora Universitária, João Pessoa, UFPB, 314 p.
- Santurio JM, Santurio DF, Pozzatti P, Moraes C, Franchin PR, Alves SH 2007. Atividade antimicrobiana dos óleos essenciais de orégano, tomilho e canela frente a sorovares de Salmonella enterica de origem avícola. Cienc Rural 37: 803-808.
- Silva JG, Souza IA, Higino JS, Siqueira-Junior JP, Pereira JV, Pereira MSV 2007. Atividade antimicrobiana do extrato de Anacardium occidentale Linn. em amostras multiresistentes de Staphylococcus aureus. Rev Bras Farmacogn 17: 572-577.
- Smith-Palmer A, Stewart J, Fyfe L 1998. Antimicrobial properties of plant essential oils and essences against five important food-borne pathogens. Lett Food Microbiol 26: 118-122.
- Souza EL, Lima EO, Freire KRL, Sousa CP 2005. Inhibition action of some essential oils and phytochemicals on the growth of moulds isolated from foods. Braz Arch Biol Techn 2: 245-259.
- Tavares W 1984. Manual de Antibióticos. 3Ş Ed. São Paulo: Atheneu, 374p.
- Thylstrup A, Fejerskov O 1995. Características clínicas e patológicas da cárie dentária. Cariologia Clínica, 2a Ed., São Paulo, Santos, cap. 6, p. 45-69.
- Tuley de Silva 1996 K. (Ed.), A Manual on the Essential Oil Industry. United Nations Industrial Development Organization, Vienna.
- Van de Braak SAAJ, Leijten GCJJ 1999. Essential Oils and Oleoresins: A Survey in the Netherlands and other Major Markets in the European Union. CBI, Centre for the Promotion of Imports from Developing Countries, Rotterdam, p. 116.
- Viljoen A, Van Vuuren S, Ernst E, Klepser M, Demirci B, Baser H, Van Wyk BE 2003. Osmitopsis astericoides (Asteraceae). The antimicrobial activity and essential oil composition of a Cape-Dutch remedy. J Ethopharmacol 88: 137-143.
- Vincent JG, Vincent HW 1994. Filter paper disc modification of the Oxford cup Penicillium determination. Proc Soc Exp Biol Med New York 25: 162-164.
- White A 1970. Relation between quantitative nasal cultures and dissemination of Staphylococci. J Lab Clin Med 58: 273-277.
Publication Dates
-
Publication in this collection
18 Aug 2009 -
Date of issue
Mar 2009
History
-
Accepted
20 Feb 2009 -
Received
12 Nov 2008