Abstract
The aim of the present work is to characterize the vegetal raw material of the Calycophyllum spruceanum (Benth.) Hook. f. ex K. Schum., Rubiaceae, known as "mulateiro", and to evaluate the influence of extractive parameters for attainment of standardized aqueous extractive solutions. The physical-chemical characterization of the samples was performed using pharmacopoeic and not pharmacopoeic methodologies. A 2³ factorial design was used to evaluate the influence of extraction techniques (infusion/decoction), drug: solvent ratio (2.5 and 7.5%), and extraction time (5 and 15 min) on the total tannin content of aqueous extractive solutions from C. spruceanum. The extractive solution that showed higher total tannin and dry residue content had their physical-chemical characteristics determined. The results suggest that an aqueous extractive solution from rinds of C. spruceanum barks with higher tanning yield (9.9 g%), must be standardized using decoction as extraction methodology, with 2.5% of vegetal drug for an extraction time of 15 min. The results of the physical-chemical characterization suggest that environmental factors modify the properties of this species and, therefore, they can influence the quality and security of a product derived from this medicinal plant.
mulateiro; physicial-chemical characterization; extractive solution
Technological development of aqueous extracts from Calycophyllum spruceanum (Benth.) Hook. f. ex K. Schum., Rubiaceae, (mulateiro) using factorial design
Leidyana M. da Costa; Viviane A. dos Santos; Débora T. Ohana; Emerson S. Lima; Maria de M. Pereira; Tatiane P. de Souza* * E-mail: tpsouza@ufam.edu.br, Tel.: +55 92 3305 5000, Fax: +55 92 3633 3241.
Faculdade de Ciências Farmacêuticas, Universidade Federal do Amazonas, Rua Alexandre Amorim 330, 69010-300 Manaus-AM, Brasil
ABSTRACT
The aim of the present work is to characterize the vegetal raw material of the Calycophyllum spruceanum (Benth.) Hook. f. ex K. Schum., Rubiaceae, known as "mulateiro", and to evaluate the influence of extractive parameters for attainment of standardized aqueous extractive solutions. The physical-chemical characterization of the samples was performed using pharmacopoeic and not pharmacopoeic methodologies. A 23 factorial design was used to evaluate the influence of extraction techniques (infusion/decoction), drug: solvent ratio (2.5 and 7.5%), and extraction time (5 and 15 min) on the total tannin content of aqueous extractive solutions from C. spruceanum. The extractive solution that showed higher total tannin and dry residue content had their physical-chemical characteristics determined. The results suggest that an aqueous extractive solution from rinds of C. spruceanum barks with higher tanning yield (9.9 g%), must be standardized using decoction as extraction methodology, with 2.5% of vegetal drug for an extraction time of 15 min. The results of the physical-chemical characterization suggest that environmental factors modify the properties of this species and, therefore, they can influence the quality and security of a product derived from this medicinal plant.
Keywords: mulateiro; physicial-chemical characterization; extractive solution.
Introduction
The acceptance of the use of natural products by the ordinary population, in addition to the general dissatisfaction regarding the safety and cost of conventional medicines, are among the factors contributing to the increasing consumption of herbal medicines (Marques, 1992). In this context, the researches aimed at developing pharmaceutical forms containing products derived from medicinal plants, with efficacy, safety and constant quality have gained a growing number of researchers all over the world and especially in Brazil (Petrovick et al., 1997).
The quality control of a product involves several steps ranging from the gathering of raw material, through the entire production process, culminating with the analysis of the final product. According to Farias (2001), the quality of the raw material does not guarantee the effectiveness of products, but is an important factor.
Vegetable species belonging to the Rubiaceae family are important as a source of economic and therapeutic value (Di Stasi, 2002). Among these species we can cite the Calycophyllum spruceanum (Benth.) Hook. f. ex K. Schum., Rubiaceae, generally known as "mulateiro", a native tree from the Amazon region, which can also be found in Colombia (capirona) Bolivia (guayabochi) and Peru (capirona black) (Estrella, 1995). In the Amazon the "mulateiro" bark is used in poultice form as an anti-inflammatory, antifungal, healing and rejuvenating agent (Almeida, 2003). In Peru it is used to treat eye infections. In Paraguay it is used to treat diabetes and in Colombia it is used against parasites and skin diseases (Revilla, 2001). Studies performed with isolated secoiridoids from ethanol extract of C. spruceanum presented anti-trypomastigote in vitro activity (Portillo & Villa, 2001). Due to the no elucidation of the compounds responsible for the therapeutic activity of this species, the tannins can be used as chemical markers for quality control, considering several reports regarding possible healing and anti-inflammatory attributes related to these substances (Viana et al., 1997; Matos, 2004). The lack of studies intended to determine the physical-chemical and technological properties of this species, as well as factors that influence these properties resulting in extractive processes that interfere with the quality, effectiveness and safety of the proposed use. Therefore, it is necessary that accurate studies be performed in order to obtain a standardized extractive solution. The factorial design is a statistical tool used for process optimization in a rapid and economic way, as well as maximization of the final product quality. Besides, the mathematical model enables reliable results (Soares, 1998). Aiming to obtain a standardized extractive solution, the goal of this work was to evaluate the influence of extractive parameters concerning the preparation of an extractive solution of C. spruceanum.
Material and Methods
Plant material
The plant material consisted of the stem bark of Calycophyllum sprucenaum (Benth.) Hook. f. ex K. Schum., Rubiaceae, collected in the district of Purupuru-AM, in the City of Careiro-Castanho, at different periods of the year. Sample type I was collected in August 2006 (dry season) and sample type II was collected in February 2007 (rain season). The botanical identification of the material was recorded at the herbarium of the National Institute for Amazonian Research (INPA). The collected material was dried, first at room temperature for 48 h, and then taken to the oven with circulating air at temperature of 45±2 °C until weight stabilization was obtained. After the material was ground in knives mills, with a 1 mm mesh, thus providing the raw material (MPV).
Characterization of raw material
Sieve analysis
Approximately 50 g of MPV were submitted to forced vibration passing through sieves with mesh openings corresponding to 1.00, 0.800, 0.710, 0.600, 0.500, 0.400, 0.330 and 0.250 mm, using a sieve (Bertel-SP) to 60 vibrations/min for 15 min. This procedure was performed in triplicates. The determination of particle size was performed by graphical method by constructing curves of retention and pass (Voigt, 2000).
Determination of loss on drying
About 2.0 g of the MPV, exactly weighed, were placed in flat-bottomed dish, previously tared, and dried at a temperature of 105±2 °C for a period of 2 h, then flat-bottomed dish were cooled in a desiccator during 30 min and weighed. This procedure was repeated hourly until constant weight was obtained. The results were expressed as percentage of weight on the quantity of drugs from each of the samples by the average of three determinations (Farmacopéia Brasileira IV, 1988).
Determination of total ash
Porcelain crucibles were previously ignited in a muffle at 450 °C for 30 min. They were then cooled in a desiccator for 30 min and given weight. Approximately 3.0 g of MPV, exactly weighed, were placed in crucibles previously tared and submitted to ignite in muffle at 450 ºC for 2 h. The set was then cooled in a desiccator for 30 min, followed by weighing. The technique was repeated until constant weight was obtained. The results were expressed as percentage by weight of ash (% w/w) by the average of three determinations(Farmacopéia Brasileira IV, 1988).
Determination of extractable matter
About 1.0 g of the MPV was heated to boiling temperature with 100 mL of water for 10 min. After cooling, it was restored to the initial weight with the addition of water mass corresponding to that of the evaporated water. The solution was filtered, ignoring the initial 20 mL. 20 mL of the filtrate were transferred to a tared flat-bottomed dish and evaporate to dryness on a water-bath. The residue was placed in an oven at a temperature of 105±2 °C for 2 h. It was then cooled in a desiccator for 20 min, weighed and put back in the oven until constant weight was obtained. The extractable matter was calculated as weight percentage by the average of three determinations according to the equation below (Bundesvereinigung, 1986):
where TE: Extractive content (g%); g: dry residue (g); FD: constant (FD=5); m: mass of initial sample (g); pd: loss on drying (g%).
Preparation and standardization of extractive aqueous solutions using factorial design
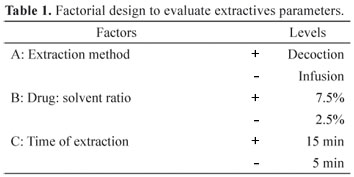
The influence of extractive parameters was evaluated using a 23 factorial design (Montgomery, 1991), where the independent variables studied were: the extraction method, the drug: solvent ratio and the extraction time. The dependent variables were the dry residue and the total tannin content (Table 1). All extraction solutions were prepared using water as solvent.
Statistical analysis
For statistical analysis of factorial design multivariate ANOVA was performed using Statistica® 6.0 program.
Characterization of the standardized extractive solution.
The extractive solution with the highest amount of total tannin was characterized by determining
Dry residue
An aliquot of 20.0 mL of extraction solution was exactly weighed in flat-bottomed dish previously tared and evaporated to dryness in water-bath, occasionally stirring. After evaporation of the solvent, the set was taken to the oven 105±2 ºC until constant weight was obtained. The result was expressed as mean and standard deviation of three determinations (Bundesvereinigung, 1986).
Density
It was performed by using a pycnometer. The results were expressed as the mean of three determinations (Farmacopéia Brasileira IV, 1988).
pH
The pH of the extractive solution was measured at 25 °C in a pot calibrated with buffer solutions of phosphate and acetate, pH 7 and 4, respectively. The result was expressed as the mean of six determinations (Farmacopéia Brasileira IV, 1988).
Quantitative analysis of total tannin (Hartke & Mutschler, 1987; Martins, 1998; Soares et al., 2006)
Determination of total polyphenols: total polyphenols were obtained by diluting the extraction solution in 100.0 mL of distilled water (stock solution). Later, the mother solution was successively diluted. An aliquot of the sample solution (2.0 mL) was added to the Folin-Denis (2.0 mL) and sodium carbonate 20% (16 mL). The absorbance was measured at 750 nm on a spectrophotometer, just 2 min after the addition of sodium carbonate.
Determination of non-tanning fraction: using as complexing agent 150 mg of casein and complexation time of 1 h. The reading was performed as described in the determination of total polyphenols.
Determination of total tannin: the total tannin content was calculated as the difference between the total polyphenol content and the fraction of non-tanning, according to the equations below:
Where PT: Total polyphenos (g%); FNT: non-tannin fraction (g%); TT: total tannin (g%); A1: total polyphenol absorbance; A2: non-tannin fraction absorbance; FD: dilution factor; m: mass of initial sample (g); p: loss on drying (g);  : absorption coefficient of gallic acid.
: absorption coefficient of gallic acid.
Results and Discussion
Physical-chemical characterization
The results of the characterization of the MPV (Table 2) show that both materials studied had residual moisture adequate for storage, which according to the literature is in the range 8-14% humidity (Farmacopéia Brasileira, 1988). However, there are significant differences (p<0.05) in the other parameters analyzed (extractive content and ash content). This fact can be explained by the different seasons of collection of plant material, confirming that even the low extractive content for the MPV type II having been collected during the rainy season may have reduced their chemical constituents (List & Schimdt, 1989).
Regarding the size of the powders, there was no significant difference concerning the raw materials. This was expected since both samples underwent the same process of comminution
Factorial design
Considering that Type I sample presented the highest extractive content (40.52%), the extractive solution was obtained only from that sample, using water as a solvent extractor. Following the selected factorial design , the extractive solution obtained from sample type I, showed total tannin values ranging from 1.20 to 9.90 g% and dry residue ranging from 0.67 to 2.27 g% (Table 3).
In accordance with the results obtained, only factor A (extraction method) showed no significant influence on the responses studied. However, it can be observed that the method of extraction by decoction (top level) was less effective in extracting the chemical markers than infusion (lower level). This decrease can be explained by the high temperature and intense heat, a continuous process, to which it is subjected in an extraction by decoction, thus causing reduction in both the tannin content and the dry residue.
Moreover, factor B (drug:solvent ratio) caused a significant antagonistic effect on the responses studied. It caused a negative effect on the tannin content and a positive effect on the dry residue. It was observed that increasing the drug proportion, 2.5% to 7.5%, the efficiency of extraction of tannins was decreased, increasing however the extraction of other soluble solids. The negative effect can be explained by the fact that there was saturation of the solvent and therefore stagnation in extraction of tannins (Silva et al., 2009). However, with increasing vegetable drug proportion, other compounds could be extracted , which are represented by the high content of dry residue. However, Factor C (extraction time) produced a positive and significant effect on both dependent variables studied, causing an increase in the extraction yield (Figure 1 and 2).
The analysis of interactions between factors (IAB, IBC, IAC and IABC) has shown that the interaction of higher intensity was the interaction between A and C. When factors A and C change simultaneously from the lower level (A = C = infusion and 5 min) to the upper level (A = C = decoction and 15 min), the tannin content is increased (Figure 1 and 2). Therefore, while the first factor in isolation causes a negative effect on the responses, when it is coupled with C factor, this effect becomes positive, thus increasing the levels of the dependent variables studied.
Under these conditions, the extractive solution corresponding to the interaction AC had their technological characteristics determined, described in Table 4, and considerate appropriate for standardization.
Conclusions
We observed significant differences between the samples studied (Type I or Type II), collected at different times of the year, suggesting that soil and climatic conditions alter the physicochemical characteristics of this species, which are parameters that may be used to establish chemical criteria and technology for quality control of this species.
The ANOVA showed that the factor extraction method and the factor drug proportion caused a negative effect on tannin content in the extractive solution and the transition from low to upper level provides a decrease in the response. But the factor time of extraction was the most significant factor on both dependent variables studied.
Therefore, the conditions for obtaining an extraction solution from bark of Calycophyllum spruceanum with increased yield of tannin (9.9 g%) should be standardized in decoction, using 2.5% of raw material for an extraction timeof 15 min.
Acknowledgement
The authors thank CNPq for its financial support.
Received 21 Sep 2009
Accepted 18 Aug 2010
- Almeida MC 2003. Aspectos ecofisiológicos da germinação de sementes de mulateiro (Calycophyllum spruceanum Benth) - Rubiaceae - Instituto de Biociências da Universidade Estadual Paulista. Available at http://www.biblioteca.unesp.br Acess on 24 October 2006.
- Bundesvereinigung 1986. Deutscher Apothekerverbände (Hrgsb.). Deutscher Arzneimittel-Codex. Frankfurt: Govi, Stuttgart: Deutscher Apotheker. v. 1. Codex-Probe 4.
- Di Stasi LC, Hiruma CA 2002. Plantas medicinais da Amazônia e na Mata Atlântica 2 ed. São Paulo: Editora Unesp.
- Estrella E 1995. Plantas Medicinalis Amazonicas: realydad e perspectivas Manaus: Editora TCA.
- Farias MR 2001. Avaliação da qualidade de matérias-primas vegetais. In: Simões CMO, Schenkel EP, Gosmann G, Mello JCP, Mentz, LA, Petrovick, PR, Farmacognosia: da planta ao medicamento 3.ed. Porto Alegre: UFSC/UFRGS. p.199-222.
- Farmacopéia Brasileira 1988. 4 ed. São Paulo: Atheneu Editora.
- Hartke K, Mutschler E 1987. Deutsches Arzneibuch-9 Kommentar. Ausgabe 1986. Stuttgart: Wissenschaftliche, p. 305-307.
- List PH, Shimidt PC 1989. Phytopharmaceutical Technology. Boca Raton: New York.
- Matos FJA 2004. Constituintes químicos ativos e propriedades biológicas de plantas medicinais brasileiras. Fortaleza: Editora UFC.
- Martins AG 1998. Influência de fatores tecnológicos na avaliação analítica e farmacológica de extratos secos nebulizados de Maytenus ilicifolia Martius ex Reiss Programa de Pós-graduação em Ciências Farmacêuticas da Universidade Federal do Rio Grande do Sul, Porto Alegre.
- Marques LC 1992. Produção e comercialização de fitoterápicos no Paraná: uma abordagem de vigilância sanitária Curitiba, 232p. Tese de Mestrado, Universidade Federal do Paraná
- Montgomery DC 1991. Diseño y análisis de experimentos México: Iberoamérica.
- Petrovick PR, González Ortega G, Bassani VL 1997. From a medicinal plant to a pharmaceutical dosage form. A (still) long way for the Brazilian medicinal plants. Rev Cien Cult 49: 364-369.
- Portillo A, Villa R 2001. Antifungal activity of Paraguayan plants used in traditional medicine. J Ethnopharmacol 88: 93-98.
- Revilla J 2001. Plantas da Amazônia: oportunidades econômicas e sustentáveis Manaus: co-edição Sebrae/INPA.
- Silva IV, Ferreira MS, Wanderley AG, Fernandes MG, Soares LA, De Souza TP 2009. Influence of extractive parameters on the preparation of a solution from Psidium guajava L. Lat Am J Pharm 28: 116-120.
- Soares LAL, González Ortega G, Bassani VL, Petrovick PR 1998. Desenvolvimento tecnológico de solução extrativa aquosa de Phyllanthus niruri L. (quebra-pedra) empregando planejamento fatorial. Cad Farm 14: 21-26.
- Soares LAL, Maia A, Oliveira AL, Petrovick PR, González Ortega G 2006. Avaliação de complexos formados por catequina e macromoléculas. Acta Farm Bonaer 25: 10-16.
- Viana GS, Bandeira MAM, Moura LC, Souza MVP, Matos FJ, Ribeiro RA 1997. Analgesic and antiinflamtory effects of the tannin fraction from Myracrodruon urundeuva Fr. All. Phytother Res 11: 118-122.
- Voigt R 2000. Pharmazeutische Technologie 9.ed. Uberarb.
Publication Dates
-
Publication in this collection
04 Mar 2011 -
Date of issue
Feb 2011
History
-
Received
21 Sept 2009 -
Accepted
18 Aug 2010