Abstract
This study describes the isolation of a flavonoid fraction from leaves of Ocotea notata (Nees & Mart.) Mez, Lauraceae, the identification of six major compounds (an A-type proanthocyanidin trimer [3], isoquercitrin [4], reynoutrin [5], miquelianin [6], quercitrin [7], afzelin [8]) and four minor compounds (catechin [1], epicatechin [2], quercetin [9], kaempferol [10]) present in the fraction and its activity against the Herpes simplex virus type 1 (HSV-1) and type 2 (HSV-2). The 50% effective concentrations values (EC50) calculated from the dose-response curve and the selectivity indices (SI) against the virus were: EC50 35.8 µg/mL and SI 5.5 to HSV-1 and EC50 23.5 µg/mL and SI 8.5 to HSV-2. The flavonoid fraction was more active against HSV-2 than HSV-1. The mechanisms of antiviral action of the flavonoid fraction against the virus were also evaluated. The percentage inhibition (PI) obtained for HSV-2 was higher than 90% in the following assays: virucidal, pre-treatment of cells, treatment of cells after viral adsorption and treatment of cells after viral penetration. For HSV-1, the flavonoid fraction had no effect in pre-treatment of cells and showed 60% of inhibition in virucidal assay.
antiherpes; flavonoids; Ocotea notata; HSV-1; HSV-2
Antiherpetic activity of a flavonoid fraction from Ocotea notata leaves
Rafael GarrettI, * * Correspondence Rafael Garrett Núcleo de Pesquisas de Produtos Naturais, Centro de Ciências da Saúde, Universidade Federal do Rio de Janeiro, Bloco H, 21941-590, Rio de Janeiro-RJ, Brazil rafael_garrett@iq.ufrj.br Tel. +55 21 2562 7121 ; Maria Teresa V. RomanosII; Ricardo M. BorgesI; Marcelo G. SantosIII; Leandro RochaIV; Antonio Jorge R. da SilvaI
INúcleo de Pesquisas de Produtos Naturais, Centro de Ciências da Saúde, Universidade Federal do Rio de Janeiro, Brazil
IIDepartamento de Virologia, Centro de Ciências da Saúde, Universidade Federal do Rio de Janeiro, Brazil
IIIDepartamento de Ciências, Universidade do Estado do Rio de Janeiro, Brazil
IVLaboratório de Tecnologia de Produtos Naturais, Faculdade de Farmácia, Universidade Federal Fluminense, Brazil
ABSTRACT
This study describes the isolation of a flavonoid fraction from leaves of Ocotea notata (Nees & Mart.) Mez, Lauraceae, the identification of six major compounds (an A-type proanthocyanidin trimer [3], isoquercitrin [4], reynoutrin [5], miquelianin [6], quercitrin [7], afzelin [8]) and four minor compounds (catechin [1], epicatechin [2], quercetin [9], kaempferol [10]) present in the fraction and its activity against the Herpes simplex virus type 1 (HSV-1) and type 2 (HSV-2). The 50% effective concentrations values (EC50) calculated from the dose-response curve and the selectivity indices (SI) against the virus were: EC50 35.8 µg/mL and SI 5.5 to HSV-1 and EC50 23.5 µg/mL and SI 8.5 to HSV-2. The flavonoid fraction was more active against HSV-2 than HSV-1. The mechanisms of antiviral action of the flavonoid fraction against the virus were also evaluated. The percentage inhibition (PI) obtained for HSV-2 was higher than 90% in the following assays: virucidal, pre-treatment of cells, treatment of cells after viral adsorption and treatment of cells after viral penetration. For HSV-1, the flavonoid fraction had no effect in pre-treatment of cells and showed 60% of inhibition in virucidal assay.
Keywords: antiherpes, flavonoids, Ocotea notata, HSV-1, HSV-2
Introduction
Herpesvirus is one of the most common human pathogen and the infections caused by its different virus types are widely distributed around the world (Brady & Bernstein, 2004; Hill et al., 2008). Among the Herpes simplex virus, the types 1 (HSV-1) and 2 (HSV-2) are the main human pathogens. The HSV-1 infection, also known as labial herpes, usually occurs by the contact with infected saliva during infancy. It causes diseases like gingivostomatitis, keratitis and encephalitis. On the other hand, HSV-2 infection, also known as genital herpes, is commonly associated with the beginning of the sexual activity in adolescence and it is responsible for genital diseases (Sacks et al., 2004; Mehnert & Candeias, 2005). Additionally, HSV infections may result from primary contact with the virus or reactivation of a latent infection (Hook et al., 1992). Several drugs are currently available for the treatment of HSV infections. Acyclovir is a potent drug and shows low cytotoxicity hence it is considered the first drug of choice (Kleymann, 2003; De Clercq, 2004). However, the emerging of resistant viral strains has been encouraging the search for new antiherpetic alternatives (Bacon et al., 2003; De Clercq, 2005; Fritz et al., 2007, Mundinger & Efferth, 2008). Different steps of the herpesvirus replication cycle can be chosen as targets for antiherpetic compounds. They could inactivate the virus particle or block its penetration at an early step of the infection, prevent the virus uncoating, inhibit the viral genome replication and others (Gomes et al., 2008).
Different compounds isolated from vegetal sources, including terpenes, phenolics, polyphenols and glycosides display antiherpes activities (Bourne et al., 1999; Benencia & Courreges, 2000; Chiang et al., 2002; Nohara, 2004; Kutluay et al., 2008). Over 4000 flavonoids have been identified to date in the plant kingdom. These compounds play an important role in plant protection against pathogens, UV-B radiation and in plant dispersion by providing attractive flower colors to pollinators. They are found in vegetables, fruits, seeds, flowers as well as in wine, tea and propolis (Harborne & Williams, 2000; Grotewold, 2006). The number of published papers about antiviral activity of flavonoids had increased in the last two decades. The most cited flavonoid classes displaying antiherpes activity are flavonols and flavones (Hayashi et al., 1997; Amaral et al., 1999; Ma et al., 2001; Chiang et al., 2003; Lyu et al., 2005; Fritz et al., 2007; Gomes et al., 2008; Schnitzler et al., 2009; Martins et al., 2011).
Ocotea notata (Nees & Mart.) Mez, Lauraceae, is a medium-sized tree popularly known as "canela-branca". It is widespread over the Brazilian Atlantic coast and grows mainly in sandy coastal plains, where it is used as timber-tree. The chemodiversity of Ocotea genus is well recognized and several alkaloids, terpenes, lignans and neolignans have been reported (Dias et al., 2003; Zanin & Lordello, 2007; Barbosa-Filho et al., 2008; Funasaki et al., 2009; Cuca et al., 2009). Nevertheless, only a small number of papers describe the presence of flavonoids in this genus (David et al., 1994; Garcez et al., 1995, 2005). The first published chemical study with O. notata was done by our research group in 2007 (Garrett et al., 2007) and this is the first report on the flavonoid content of this specie.
In the present study, we investigated the antiherpetic activity of a flavonoid fraction obtained from O. notata leaves, the viral inhibition mechanism and also the chemical composition of this fraction.
Materials and methods
Plant material
Aerial parts of Ocotea notata (Nees & Mart.) Mez, Lauraceae, were harvested during spring time (December 2007) in the region located between a periodically flooded forest and open Clusia scrub formation of the Restinga de Jurubatiba National Park sandy coastal plains, Rio de Janeiro state, Brazil. The specimen was identified by Dr. Marcelo G. Santos and a voucher sample (RFFP-10067) was deposited at the herbarium of the Faculdade de Formação de Professores-UERJ.
General experimental procedures
All reagents used for the extraction and isolation process were analytical grade and reagents used for chromatography and mass spectrometry were hplc/spectrum grade. Acyclovir (Sigma) was used as standard compound in the experiments of cytotoxicity and antiviral screening. The flavonoids (+)-catechin and (-)-epicatechin (Fluka); isoquercitrin, quercitrin, quercetin and kaempferol (Aldrich); miquelianin (a gift from LPNBio-NPPN, UFRJ) were used as standards to identify the compounds present in the flavonoid fraction by co-injection in HPLC analyses.
Column chromatography was carried out on Sephadex LH-20 (25-100 µm, Sigma). HPLC-DAD analysis was performed using a Waters Symmetry Shield reversed phase column (4.6 x 250 mm, 5 µm particle size) with a gradient elution of acid water/acetonitrile (1:9; A) and acid water/acetonitrile (2:8; B) from 0 to 50% of B in 55 min. For preparative HPLC a Waters µ-Bondapak column (7.8 x 300 mm, 10 µm particle size) and UV detection at 280 nm were employed with a gradient elution of acid water (A) and acetonitrile (B) from 0 to 10% of B in 40 min. Mass spectra were recorded on a Micromass quadrupole-time-of-flight (Q-TOF) mass spectrometer equipped with an electrospray ion source operating in the negative mode of ionization. Methanol-d4 with TMS as internal standard was used for 1H NMR (400 MHz) and 13C NMR (100 MHz) analyses on a Varian MR-400. GC-MS analyses were recorded on a Shimadzu QP5000 GC-MS instrument (5% phenylmethyl silicone column, 30 m × 0.25 mm ID, 0.25 µm film thickness; programmed column temperature from 110 to 290 ºC, 5 ºC min-1) (Borges et al., 2009).
Extraction and isolation
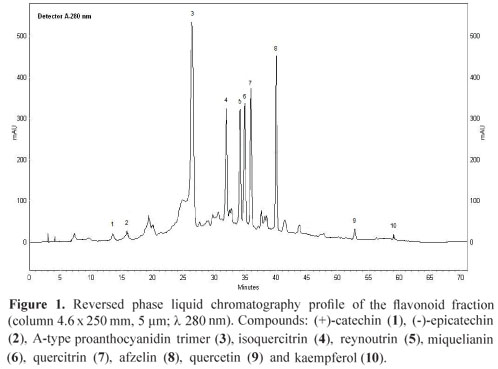
Dried and powdered leaves of O. notata (400 g) were exhaustively extracted by Soxhlet, first with hexane and then with methanol. The methanol extract obtained was partitioned with ethyl acetate and concentrated using a rotary evaporator to provide the flavonoid fraction (3.2 g). This fraction (400 mg) was chromatographed on Sephadex column using ethanol and then methanol to afford 12 mg of the flavonoid quercetin (9), 24 mg of an A-type proanthocyanidin trimer (3) and a mixture of three flavonoids. This mixture was chromatographed in the same conditions described above to afford the 11 mg of isoquercitrin (4), 4 mg of reynoutrin (5) and 8 mg of quercitrin (7). In addition, 22 mg of the flavonoid afzelin (8) and 5 mg of kaempferol (10) were isolated by preparative HPLC. The flavonoids (+)-catechin (1), (-)-epicatechin (2) and miquelianin (6) were identified in the fraction by HPLC and MS analysis and were not isolated. Figure 1 shows the HPLC profile of the flavonoid fraction.
Cells and viruses
Vero cells (African green monkey kidney cells) (Rio de Janeiro Cell Bank) were grown in Eagle's minimum essential medium (EagleMEM) (Cultilab) supplemented with 2 mM L-glutamine (Sigma), 50 µg/mL garamicin, 2.5 µg/mL fungizon (Gibco), 0.25 mM of sodium bicarbonate solution (Merck), 10 mM of HEPES (Sigma) plus 10% of heat-inactivated fetal bovine serum (FBS) (Cultilab) and maintained at 37 ºC in atmosphere of 5% of CO2. Herpes simplex virus type 1 (HSV-1) was isolated from a typical lip lesion and Herpes simplex virus type 2 (HSV-2) from a typical genital lesion in the Virology Department of the Federal University of Rio de Janeiro (UFRJ), Brazil. Viruses were typed by polymerase chain reaction (PCR) using specific primers for identification (Markoulatos et al., 2001).
Cytotoxicity assay
The cytotoxicity assay was performed prior to antiviral tests by incubating triplicate Vero cell monolayers cultivated in 96-well microplates with two-fold serial dilutions (3.1 to 200 µg/mL) of the flavonoid fraction and acyclovir for 48 h at 37 ºC in a 5% CO2 atmosphere. The morphological alterations of the treated cells were observed in an inverted optical microscope and the maximum non-toxic concentrations (MNTC) were determined (Walker et al., 1971). Cellular viability was evaluated by the neutral red dye-uptake method (Borenfreund & Puerner, 1985). The 50% cytotoxic concentration (CC50) was defined as the compound concentration which caused a 50% reduction in the number of viable cells.
Antiviral activity assay
The antiviral activity of flavonoid fraction and acyclovir was evaluated by the titer reduction. The virus titers were calculated using the Reed and Muench statistical method (Reed & Muench, 1938) and expressed as 50% tissue culture infective dose (TCID50) per mL. Vero cell monolayers were treated with the flavonoid fraction and acyclovir at the MNTC and 100 TCID50/mL of HSV-1 or HSV-2 suspensions were added to treated and untreated cell cultures and incubated at 37 °C for 48 h in a 5% CO2 atmosphere. After incubation, the supernatant was collected and virus titers in treated and untreated cells were determined. The antiviral activity was expressed as percentage inhibition (PI) (Nishimura et al., 1977) using antilogarithmic TCID50 values as follows: PI = [1-(antilogarithmic test value/antilogarithmic control value)]x100. The dose-response curve was established starting from the MNTC, and the 50% effective concentration (EC50) was defined as the concentration required for 50% protection against virus-induced cytopathic effects. The selectivity index (SI) was determined as the ratio of CC50 to EC50. The experiment was performed in triplicate and three times repeated.
Mechanism of action studies
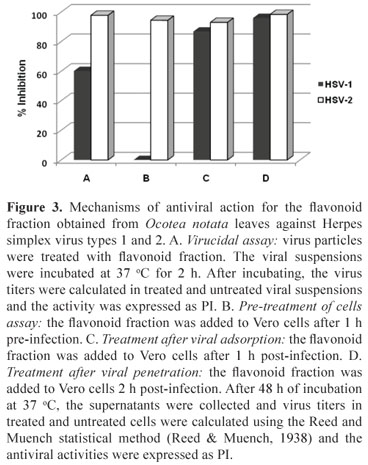
Virucidal assay
HSV-1 and HSV-2 suspension (100 µL) (105 TCID50/mL) were added to 900 µL of the flavonoid fraction at the MNTC and MEM-Eagle without serum (virus control), according to Chen et al. (1988) and incubated at 37 °C for 2 h. After incubating, the virus titers were calculated in treated and untreated viral suspensions using the Reed and Muench statistical method (Reed & Muench, 1938) and the activity was expressed as PI.
Pre-treatment of cells
The flavonoid fraction was added to Vero cell monolayers before infection (pre-treatment) in order to evaluate their effect on cell receptors. Vero cell monolayers were pre-treated with the flavonoid fraction for 1 h at 4 °C. After this time, the cells were washed three times with MEM-Eagle for removing sample, and the treated and untreated cells were inoculated with 100 TCID50/mL of HSV-1 or HSV-2. After incubating the cells at 37 °C for 48 h, the supernatant was collected and virus titers in treated and untreated cells were determined and the activity was expressed as PI.
Treatment after viral adsorption
Vero cell monolayers were inoculated with 100 TCID50/mL of HSV-1 or HSV-2 and incubated for 1 h at 4 °C. After this period, the monolayers were washed with culture medium and the flavonoid fraction (at the MNTC) was added. The cultures were immediately shifted to 37 °C to allow the penetration of the particles into the cells for another hour. After incubation, the monolayer was washed, MEM-Eagle was added, and the cultures incubated at 37 °C for 48 h. After incubating, the supernatant was collected and virus titers in treated and untreated cells were determined and the activity was expressed as PI.
Treatment after viral penetration
Vero cell monolayers were inoculated with 100 TCID50/mL of HSV-1 or HSV-2 and incubated at 37 °C for 2 h. The cells were washed and the flavonoid fraction at concentration of 100 µg/mL or MEM-Eagle (control) was added and the cultures incubated at 37 °C for 16 h. After incubation, the cells were washed to remove the flavonoid fraction before releasing viral particles. Then, MEM-Eagle was added and the cultures incubated for 32 h at 37 °C. After incubating, the supernatant was collected and virus titers in treated and untreated cells were determined and the activity was expressed as PI.
Results and Discussion
Flavonoid fraction
Different methods were used to identify the compounds present in the flavonoid fraction. Compounds 1, 2 and 6 were not isolated. They were directly identified in the flavonoid fraction by HPLC co-injection with standards and through the negative ions observed in the direct infusion ESI-MS of the flavonoid fraction. Quasi-molecular ions at m/z 289 (compounds 1 and 2) and m/z 477 (compound 6; MS/MS 477, 301) were observed in the negative ESI mass spectra of the flavonoid fraction (Charrouf et al., 2007; Cavaliere et al., 2008). Compound 3 was isolated from the flavonoid fraction and showed an ion at m/z 863. The MS/MS study of 3 revealed product ions at m/z 711, 573, 531, 451, 411 and 289. Scheme 1 shows a chemical structure proposed for the compound 3 and the suggested fragmentation pathway. The m/z ion 711 is in agreement with a neutral loss of 152 Da from the A-type proanthocyanidin trimer rings E and F by a retro-Diels-Alder (RDA) fission. The loss of a lower epi (catechin) unit (290 Da) from 3 by a quinone methide (QM) fission generates the m/z ion 573, which corresponds to the upper and middle units of the proanthocyanidin with an A-type ether interflavan linkage. The m/z ion 451 is produced through the A ring loss from m/z 573 by heterocyclic ring (HRF) fission. The presence of the (epi) catechin units is confirmed by the m/z ion 289 (Foo et al., 2000; Zhang et al., 2003; He et al., 2007, Li & Deinzer, 2007, 2008). A-type proanthocyanidins molecular ions displays two mass units lower regarding the B-types thus providing evidence for the presence of the additional ether linkage between C2 and C7 (Li & Deinzer, 2007, 2008). These considerations allowed the proposal of a A-type proanthocyanidin trimer (epi)catechin-A-(epi)catechin-(epi)catechin structure for compound 3. Additional work is needed to elucidate the structure of this compound. Compounds 4, 5 and 7-10 were isolated from the flavonoid fraction. The co-injection with standards on HPLC (compounds 4, 7, 9 and 10), the negative ESI mass spectra at m/z 463 (4; MS/MS 463, 301), m/z 433 (5; MS/MS 433, 301), m/z 447 (7; MS/MS 477, 301), m/z 431 (8; MS/MS 431, 285), m/z 301 9, m/z 285 10 and the comparison of 1H NMR spectra of 4, 5 and 7 (Lu & Foo, 1997; Lee et al., 2004) and 1H NMR, COSY and HSQC spectra of 8 with literature (Min et al., 2003; Gohar et al., 2009) allowed the identification of these compounds in the flavonoid fraction. Furthermore, the flavonoid glycosides were acid hydrolyzed and the resulting monosaccharides were reduced and acetylated (Borges et al., 2009). The alditol acetates of glucose, xylose and rhamnose were identified by direct comparison with authentic samples using GC-MS.
Cytotoxicity
Changes in the cellular morphology were observed when 100 and 200 µg/mL of the flavonoid fraction were used (MNTC 50 µg/mL), although more than 50% of cells remained viable at the highest concentration used (CC50 > 200 mg/mL) (Table 1).
Antiviral activity
The antiviral experiments were performed with the flavonoid fraction at concentration of 50 µg/mL and acyclovir at 200 µg/mL. The EC50 values calculated from the dose-response curve (Figure 2) and the SI values were: EC50 35.8 µg/mL and SI > 5.5 to HSV-1 and EC50 23.5 µg/mL and SI > 8.5 to HSV-2. Acyclovir presented EC50 0.8 µg/mL and SI > 250 to HSV-1 and EC50 1.38 µg/mL and SI > 144.9 to HSV-2 (Table 1).
The flavonoid fraction showed antiherpes activity against both HSV-1 and HSV-2. Moreover, this fraction was more active against HSV-2 than HSV-1. All the compounds identified in the fraction had already demonstrated antiviral properties in literature. For instance, afzelin, quercitrin and kaempferol showed anti-HSV-1 activity (Almeida et al., 1998; Lyu et al., 2005) whereas quercetin and isoquercitrin showed activity against both HSV-1 and HSV-2 (Chiang et al., 2003; Gomes et al., 2008). A medicinal preparation from the plant extract Rhododendron ungernii containing the flavonoids quercetin, isoquercitrin, quercitrin, (+)-catechin, (-)-epicatechin and others received the approval from the Georgia government to treat HSV-1 disorders in the oral cavity (Kemertelidze et al., 2007).
The mechanisms of antiviral action of the flavonoid fraction against the herpesvirus types 1 and 2 were evaluated and the inhibition of different steps of the virus replication cycle was observed (Figure 3). The PIs obtained for HSV-2 were higher than 90% in all performed experiments. Differently, for HSV-1, the flavonoid fraction had no effect in pre-treatment of cells and showed 60% of inhibition in virucidal assay.
Despite the drug acyclovir be the first choice for treating the HSV infections, new antiviral agents exhibiting different mechanisms of action are urgently needed. The flavonoid fraction had its major compounds and some minor compounds identified, showed different viral inhibition mechanisms and low toxicity and thus could be used as complement in the HSV-1 and HSV-2 infections treatment.
Acknowledgment
The authors thank CNPq and CAPES for their financial support, ICMBio-MMA and The Restinga de Jurubatiba National Park.
Received 1 Mar 2011
Accepted 30 Aug 2011
- Amaral ACF, Kuster RM, Gonçalves JLS, Wigg MD 1999. Antiviral investigation on the flavonoids of Chamaesyce thymifolia. Fitoterapia 70: 293-295.
- Almeida AP, Miranda MMFS, Simoni IC, Wigg MD, Lagrota MHC, Costa SS 1998. Flavonol monoglycosides isolated from the antiviral fractions of Persea americana (Lauraceae) leaf infusion. Phytother Res 12: 562-567.
- Bacon TH, Levin MJ, Leary JJ, Sarisky RT, Sutton D 2003. Herpes simplex virus resistance to acyclovir and penciclovir after two decades of antiviral therapy. Clin Microbiol Rev 16: 114-128.
- Barbosa-Filho JM, Cunha RM, Dias CS, Athayde-Filho PF, Silva MS, da Cunha EVL, Machado MIL, Craveiro AA, Medeiros IA 2008. GC-MS Analysis e cardiovascualr activity of the essential oil of Ocotea duckei. Rev Bras Farmacogn 18: 37- 41.
- Benencia F, Courreges MC 2000. In vitro and in vivo activity of eugenol on human herpesvirus. Phytother Res 14: 495-500.
- Borenfreund E, Puerner J 1985. Toxicity determined in vitro by morphological alterations and neutral red absorption. Toxicol Lett 24: 119-124.
- Bourne KZ, Bourne N, Reising SF and Stanberry LR 1999. Plant products as topical microbicide candidates: assessment of in vitro and in vivo activity against herpes simplex virus type 2. Antiviral Res 42: 219-226.
- Borges RM, Tinoco LW, Souza Filho JD, Barbic NS, da Silva AJR 2009. Two new oleanane saponins from Chiococca alba (L.) Hitch. J Braz Chem Soc 20: 1738-1741.
- Brady RC, Bernstein, DI 2004. Treatment of herpes simplex virus infections. Antivir Res 61: 73-81.
- Cavaliere C, Foglia P, Gubbiotti R, Sacchetti P, Samperi R, Laganà A 2008. Rapid-resolution liquid chromatography/mass spectrometry for determination and quantitation of polyphenols in grape berries. Rapid Commun Mass Sp 22: 3089-3099.
- Charrouf Z, Hilali M, Jauregui O, Soufiaoui M, Guillaume D 2007. Separation and characterization of phenolic compounds in argan fruit pulp using liquid chromatografy-negative electrospray ionization tandem mass spectroscopy. Food Chem 100: 1398 -1401.
- Chen M, Griffith P, Lucia HL, Hsiung GD 1988. Efficacy of S26308 against guinea pig cytomegalovirus infection. Antimicrob Agents Chemother 32: 678-683.
- Chiang LC, Chiang H, Liu MC, Lin CC 2003. In vitro antiviral activities of Caesalpinia pulcherrima and its related flavonoids. J Antimicr Chemoth 52: 194-198.
- Chiang LC, Chiang W, Chang MY, Ng LT and Lin CC 2002. Antiviral activity of Plantago major extracts and related compounds in vitro. Antiviral Res 55: 53-62.
- Cuca LE, Leon P, Coy ED 2009. A bicyclo[3.2.1]octanoid neolignan e toxicity of the ethanol extract from the fruit of Ocotea heterochroma. Chem Nat Compd 45: 179-181.
- David JM, Yoshida IM, Gottlieb OR 1994. The chemistry of Brazilian Lauraceae 103. Phenylpropanoid-catechins from bark of Ocotea porosa. Phytochemistry 35: 545-546.
- De Clercq E 2004. Antiviral drugs in current clinical use. J Clin Virol 30: 115-133.
- De Clercq E 2005. Recent highlights in the development of new antiviral drugs. Curr Opin Microbial 8: 552-560.
- Dias CS, Silva IG, Cunha EVL, Silva MS, Braz-Filho R, Barbosa-Filho JM 2003. Isolamento e identificação de novos alcalóides de Ocotea duckei Vattimo (Lauraceae). Rev Bras Farmacogn 13: 62-63.
- Foo YL, Lu Y, Howell AB, Vorsa N 2000. A-Type proanthocyanidin trimers from cranberry that inhibit adherence of uropathogenic P-fimbriated Escherichia coli. J Nat Prod 63: 1225-1228.
- Fritz D, Venturi CR, Cargnin S, Schripsema J, Roehe PM, Montanha JA, Poser GLV 2007. Herpes virus inhibitory substances from Hypericum connatum Lam., a plant used in southern Brazil to treat oral lesions. J Ethnopharmacol 113: 517-520.
- Funasaki M, Lordello ALL, Viana AM, Santa-Catarina C, Floh EIS, Yoshida M, Kato MJ 2009. Neolignans and sesquiterpenes from leaves and embryogenic cultures of Ocotea catharinensis (Lauraceae). J Braz Chem Soc 20: 853-859.
- Garcez WS, Yoshida M, Gottlieb OR 1995. Benzylisoquinoline alkaloids e flavonols from Ocotea vellosiana. Phytochemistry 39: 815-816.
- Garcez WS, Garcez FR, da Silva LMGE, Shimabukuro AA 2005. Indole alkaloid and other constituents from Ocotea minarum. J Braz Chem Soc 16: 1382-1386.
- Gomes MMR, Cerqueira DM, Falcão DQ, Menezes FS, Wigg MD, Mendes GS, Martins FO, Silva JFM, Kuster RM, Romanos MTV 2008. In vitro anti-HSV-2 activity of isoquercetin from Hyptis fasciculata Benth. Virus Rev Res 13: 1-15.
- Garrett R, Gattuso M, Santos MG, Rocha L 2007. Atividade antibacteriana do óleo essencial de Ocotea notata guiada pelo ensaio de toxidade sobre Artemia salina Leach. Bol Latinoamer Caribe Pl Med Arom 6: 334-335.
- Gohar A, GedarA SR, Baraka HN 2009. New acylated flavonol glycoside from Ceratonia siliqua L. seeds. J Med Pl Res 3: 424-428.
- Grotewold E 2006. The science of flavonoids. New York: Springer Science, 274p.
- He M, Wang P, Xiang Y, Qi Y, Sun H, Phillips J 2007. Profiling and characterization of polyphenol polymers from cinnamon using an ion trap mass spectrometer. Application note 392 Thermo Fisher Scientific. <http://www.thermo.com/eThermo/CMA/PDFs/Articles/articlesFile_2464.pdf>
- Hayashi K, Hayashi T, Otsuka H, Takeda Y 1997. Antiviral activity of 5,6,7-trimethoxyflavone and its potentiation of the antiherpes activity of acyclovir. J Antimicr Chemoth 39: 821-824.
- Harborne JB, Williams CA 2000. Advances in flavonoid research since 1992. Phytochemistry 55: 481-504.
- Hill JM, Ball MJ, Neumann DM, Azcuv AM, Bhattacharjee PS, Bouhanik S, Clement C, Lukiw WJ, Foster TP, Kumar M, Kafman HE, Thompson HW 2008. The high prevalence of Herpes Simplex virus type 1 DNA in human trigeminal Ganglia is not a function of age or gender. J Virol 85: 8230-8234.
- Hook EW, Cannon RO, Nahmias AJ, Lee FF, Campbell Jr CH, Glasser D, Quinn TC 1992. Herpes simplex virus infection as a risk factor for human immunodeficiency virus infection in heterosexuals. J Infect Dis 165: 251-255.
- Kemertelidze EP, Shalashvili KG, Korsantiya BM, Nizharadze NO, Chipashvili NS 2007. Therapeutic effect of phenolic compounds isolated from Rhododendron ungernii leaves. Pharm Chem J-USSR 41: 10-13.
- Kleymann G 2003. Novel agents and strategies to treat herpes simplex infections. Expert Opin Inv Drug 12: 165-183.
- Kutluay SB, Doroghazi J, Roemer ME, Triezenberg SJ 2008. Curcumin inhibits Herpes simplex virus immediate-early gene expression by a mechanism independent of p300/CBP histone acetyltransferase activity. Virology 373: 239-247.
- Lee JH, Ku CH, Baek N, Kim S, Park SW, Kim DK 2004. Phytochemical constituents from Diodia teres. Arch Pharm Res 27: 40-43.
- Lu Y, Foo LY 1997. Identification and quantification of major polyphenols in apple pomace. Food Chem 59: 187-194.
- Li H, Deinzer ML 2007. Tandem mass spectrometry for sequencing proanthocyanidins. Anal Chem 79: 1739-1748.
- Li H, Deinzer ML 2008. The mass spectral analysis of isolated hops A-type proanthocyanidins by electrospray ionization tandem mass spectrometry. J Mass Spectrom 43: 1353-1363.
- Lyu SY, Rhim JY, Park WB 2005. Antiherpetic activities of flavonoids against Herpes simplex virus type 1 (HSV-1) and type 2 (HSV-2) in vitro. Arch Pharmacol Res 28: 1293-1301.
- Ma SC, But PP, Ooi VE, HE Y, Lee SH, Lee S, Lin R 2001. Antiviral amentoflavone from Selaginella sinensis. Biol Pharm Bull 24: 311-312.
- Markoulatos P, Georgopoulou A, Siafakas N, Plakokefalos E, Tzanakaki G, Kourea-Kremastinou J 2001. Laboratory diagnosis of common herpes virus infections of the central nervous system by a multiplex PCR assay. J Clin Microbiol 39: 4426-4432.
- Martins LRR, Brenzan MA, Nakamura CV, Dias Filho BP, Nakamura TU, Cortez LER, Cortez DAG 2011. In vitro antiviral activity from Acanthospermum australe on herpesvirus and poliovirus. Pharm Biol 49: 26-31.
- Mehnert DU, Candeias JAN 2005. Herpesvírus. In: Trabulsi LR, Alterthum F (org.) Microbiologia, São Paulo: Atheneu, p. 599-606.
- Min BS, Lee SY, Kim JH, Lee JK, Kim TJ, Kim DH, Kim YH, Joung H, Lee HK, Nakamura N, Miyashiro H, Hattori M 2003. Anti-complement activity of constituents from the stembark of Juglans mandshurica. Biol Pharm Bull 26: 1042-1044.
- Mundinger TA, Efferth T 2008. Herpes simplex virus: drug resistance and new treatment options using natural products (review). Mol Med Rep 1: 611-616.
- Nishimura T, Toku K, Fukuyasu H 1977. Antiviral compounds. XII. Antiviral activity of aminohydrazones of alkoxyphenil substituted carbonyl compounds against influenza virus in eggs and mice. Kitasato Arch Exp Med 50: 39-46.
- Nohara T 2004. Search for functions of natural oligoglycosides - Solanaceae and Leguminosae origin glycosides. Yakugaku Zasshi 124: 183-205.
- Reed LJ, Muench H 1938. A simple method of estimating fifty percents endpoints. Am J Hyg 27: 493-497.
- Sacks SL, Griffiths PD, Corey L Cohen C, Cunningham A, Dusheiko GM, Self S, Spruance S, Stanberry LR, Wald A, Whitley RJ 2004. HSV-2 transmission. Antivir Res 63: S27-S35.
- Schnitzler P, Neuner A, Nolkemper S, Zundel C, Nowack H, Sensch KH, Reichling J 2009. Antiviral activity and mode of action of propolis extracts and selected compounds. Phytother Res 24: S20-S28.
- Walker WE, Waisbren BA, Martins RR, Batayias GE 1971. A method for determining sensitivities of antiviral drugs in vitro for possible use as clinical consultation. Am J Clin Pathol 56: 687-692.
- Zanin SMW, Lordello ALL 2007. Aporphine alkaloids in Ocotea species (Lauraceae). Quim Nova 30: 92-98.
- Zhang CF, Sun QS, Wang ZT, Hattori M 2003. One new A-type proanthocyanidin trimer from Lindera aggregate (Sims) Kosterm. Chinese Chem Lett 14: 1033-1036.
Publication Dates
-
Publication in this collection
06 Jan 2012 -
Date of issue
Apr 2012
History
-
Received
01 Mar 2011 -
Accepted
30 Aug 2011