Abstract
Amongst other botanical sources, Annona muricata L., Annonaceae, seeds and Piper nigrum L., Piperaceae, fruits are particularly enriched with acetogenins and piperine-related amides, respectively. These crude ethanolic extracts are potent Aedes aegypti bioactives that can kill Aedes aegypti larvae (dengue fever mosquito). A. muricata displayed a 93.48 µg/mL LC50 and P. nigrum an 1.84 µg/mL LC50. An uncommon pharmacognostical/toxicological approach was used, namely different combinations of both extracts to achieve an improved lethal effect on the larvae. The independence test (χ²) was utilized to evaluate the combination of the two crude extracts. All of the tested combinations behaved synergistically and these novel results were attributed to the completely different biochemical mechanisms of the differentiated chemical substances that were present in the two botanical sources. Besides the two above selected plants, Melia azedarach L., Meliaceae, Origanum vulgare L., Lamiaceae, and Ilex paraguariensis A. St.-Hil., Aquifoliaceae, in order of decreasing toxicity, may also be sought as potential extracts for the sake of synergic combinations.
Aedes aegypti; Annona muricata; Piper nigrum; acetogenins; piperine; synergism
The combined action of phytolarvicides for the control of dengue fever vector, Aedes aegypti
Adelia GrzybowskiI; Marcela TiboniI; Mário A. N. da SilvaII; Rodrigo F. ChitolinaII; Maurício PassosI; José D. Fontana* * Correspondence: José D. Fontana Biomass Chemo/Biotechnology Laboratory (LQBB), Department of Pharmacy, Federal University of Parana Av. Pref. Lothario Meissner, 632, 80210-170 Curitiba-PR, Brazil jfontana@ufpr.br Tel/Fax. +55 41 3360 4136 ,I,III
ILaboratório Quimio/Biotecnologia de Biomassas, Departamento de Farmácia, Universidade Federal do Paraná, Brazil
IILaboratório de Entomologia Médico-veterinária, Departamento de Zoologia, Universidade Federal do Paraná, Brazil
IIIDepartamento Acadêmico de Química e Biologia, Universidade Tecnológica Federal do Paraná, Brazil
ABSTRACT
Amongst other botanical sources, Annona muricata L., Annonaceae, seeds and Piper nigrum L., Piperaceae, fruits are particularly enriched with acetogenins and piperine-related amides, respectively. These crude ethanolic extracts are potent Aedes aegypti bioactives that can kill Aedes aegypti larvae (dengue fever mosquito). A. muricata displayed a 93.48 µg/mL LC50 and P. nigrum an 1.84 µg/mL LC50. An uncommon pharmacognostical/toxicological approach was used, namely different combinations of both extracts to achieve an improved lethal effect on the larvae. The independence test (χ2) was utilized to evaluate the combination of the two crude extracts. All of the tested combinations behaved synergistically and these novel results were attributed to the completely different biochemical mechanisms of the differentiated chemical substances that were present in the two botanical sources. Besides the two above selected plants, Melia azedarach L., Meliaceae, Origanum vulgare L., Lamiaceae, and Ilex paraguariensis A. St.-Hil., Aquifoliaceae, in order of decreasing toxicity, may also be sought as potential extracts for the sake of synergic combinations.
Keywords: Aedes aegypti, Annona muricata, Piper nigrum, acetogenins, piperine, synergism
Introduction
Two and half billion people live in areas where there is a risk of transmitting the dengue fever viruses I to IV, a disease that is spread by the mosquito vector Aedes aegypti. Each year, there are 50 million dengue infections that are either estimated or notified, and these infections occur primarily in the Americas, Africa, the Western Mediterranean, South East Asia and the West Pacific. The vector A. aegypti was eradicated in the Americas during the 1960s and early 1970s but the subsequent reinfestation has become apparent by the outbreaks that occurred in 1998, 2002 and 2008. In 2002 alone, more than one million cases of dengue were reported (WHO, 2009).
The causes of this dengue emergency are both social and demographic; the population increase and its circulation in urban areas have resulted in the disordered formation of shantytowns and settlements, which lack nearly all basic sanitation. In addition, dengue outbreaks have resulted from a reduction in epidemiological surveillance and from the progressive resistance of the vector mosquito to several chemical insecticides (Brogdon & McAllister, 1998; Guzman & Kouri, 2003; Guzmán & Kourí, 2004; Lima et al., 2003; Macoris et al., 2007; Melo-Santos et al., 2010). Therefore, alternative methods of vector control have been sought. One such alternative method is the use of plants that produces bioactives or secondary metabolites that have toxic effects on insects.
Some plants within the rich Brazilian flora are expected to have insecticidal properties like the species Annona muricata L., Annonaceae. This species is known as soursop or as "graviola" in Brazil and it represents a source of acetogenins. These compounds are derived from long chain fatty acids (32 or 34 carbons) and combined with 2-propanol before a terminal unsaturated γ-lactone and various alkane chain modifications, such as hydroxyl and tetrahydrofuran groups, are incorporated (Gu et al., 1995). Acetogenins act as pesticides on several arthopodes what is based on a specific mechanism that blocks energy generation in the mitochondrial complex I. Another species, Piper nigrum L., Piperaceae, which is commonly used as black pepper, contains a series of amides also possessing insecticidal potential such as piperine, pipercide, guineensine, pellitorine, pipgulzarine and pipzorine (Park et al., 2002; Siddiqui et al., 2004; Siddiqui et al., 2003). Ilex paraguariensis A. St.-Hil., Aquifoliaceae, which is known as "erva-mate", biosynthesizes methylxanthines or puric alkaloids which act as defense mechanism against insects and molds (Ashihara et al., 2008; Cardozo Jr et al., 2007; Jacques et al., 2008). Melia azedarach L., Meliaceae, which is known as the Chinaberry tree, "santa-bárbara" or "cinamomo", produces bitter triterpenoids called limonoids and these compounds inhibit chitin biosynthesis and therefore block the development or renewal of the exoskeleton during the insect morphogenetic cycle (Cronquist, 1988; Kumar et al., 1996; Wandscheer et al., 2004). The species Origanum vulgare L., Lamiaceae, is rich in p-cymene and in the phenolic derivatives carvacrol and thymol, the later ones being toxic to Culex pipiens mosquito larvae (Arcila-Lozano et al., 2004).
Our previous research has shown that seed acetogenins from A. muricata can successfully kill pests such as the caterpillars Anticarsia gemmatalis and Pseudaletia sequax (Fontana et al., 1998) of soy (Glycine max (L.) Merr, Leguminosea), and wheat (Triticum vulgare Vill, Poacea), respecetively. We also described the seed limonoids from Melia azedarach as being toxic to A. aegypti larvae (Wandscheer et al., 2004). More recently, our preliminary toxicity bioassays against Artemia salina nauplii have indicated that simple ethanolic extracts from other species of Brazilian flora may kill A. aegypti larvae with similar efficiency or even better. Based on this result, the current study utilizes an approach which has been scarcely reported in the literature, namely, the synergistic effect of a combination of phytopesticides to A. aegypti mosquito, obtaining them from different botanical sources possessing different mechanisms of action.
Materials and Methods
Plant materials
The seeds from the endocarps of the ripe fruits of Annona muricata L., Annonaceae, was provided by the company Brasfrut® (Feira de Santa, Bahia State, Brazil). The ripe and dried fruits of Piper nigrum L., Piperaceae, and the dried leaves from Origanum vulgare L., Lamiaceae, were purchased at the Municipal Market of Curitiba (Parana State, Brazil). The botanical identity of O. vulgare and P. nigrum was confirmed by pharmacognostic analysis and the material deposited at Herboteca Carlos Stelfelld (UFPR), as no 346 and 374, respectively.
Leaves from Ilex paraguariensis A. St.-Hil., Aquifoliaceae, were collected in July 2008 in the Cocho Grande district of the city of Nova Laranjeiras (Parana State, Brazil). The exsicates was deposited in the Municipal Botanical Museum of Curitiba herbarium under the registration numbers MBM 358115.
Ripe Melia azedarach L., Meliaceae, fruits were collected from trees in the urban area of the city of Curitiba-PR-Brazil in May 2008. The fruits were smashed and washed under running water with manual friction to remove the pulp, and the de-pulped seeds were air and sun dried. A Melia azedarach voucher specimen has been previously deposited in the same botanical museum under the number MBM 48708.
Botanical source extraction
All of the botanical materials were triturated in a commercial Waring blender the maximum speed for successive cycles (2 min) and then passed through a coarse screen (12 ABNT/ASTM 10 mesh). Each 250 g of comminuted plant material was extracted with absolute ethanol (five vol.) at the incipient boiling point and was then filtered through Whatman nº 3 paper; the residue was washed with two vol. of warm ethanol. The solvent from each extract was removed in a rotary evaporator (Laborota 4000; Heildolph, Germany). The stock solutions of all of the crude extracts were normalized to 50 mg/mL in ethanol, and further dilutions were made at the time of the bioassays.
Reference bioassay and chromatographic compounds
Because a standard of annonacin is not commercially available, a crude sample of the polar acetogenins (pAG) from the A. muricata seeds was obtained using 3 vol. of acetonitrile for an overnight extraction at 28 ºC in a rotator shaker. This acetogenin extract was further purified with active charcoal and silica gel columns (Fontana et al., 1998). Its LC-MS analysis (negative ion) gave the expected major m/z 594.9 peak, and the subsequent MS-MS mass spectrum revealed the key fragments at m/z 483.4 and 197.2 (Allegrand et al., 2010). The piperine standard (97% purity) and triolein were both purchased from Sigma-Aldrich, St. Louis, MO, USA.
Preliminary brine shrimp lethality test (BSLT)
To perform a preliminary evaluation of the bioactivity and toxicity from all of the selected botanical sources and also for the pAG and piperine samples, the microcrustaceous Artemia salina nauplii were used. A previous eclosion of the eggs (100 mg; Maramar Aquacultura Ltda) was obtained in 100 mL of artificial seawater (26.3 g NaCl, 0.75 g KCl, 1.47 g CaCl2.2H2O, 5.10 g MgCl2.6H2O, 0.08 g NaBr, 0.21 g NaHCO3, 6.20 g MgSO4.7H2O, q.s.p. 1 L distilled water, final pH adjusted to 8.1) with permanent air bubbling and artificial illumination at 25 oC for 24-36 h. Each quadruplicate toxicity bioassay experiment with the nauplii was repeated three times for each dose-response curve, and six progressive concentrations of each extract were tested. The negative and positive controls were run with 1% ethanol and thymol (92.5 µg/mL), respectively, at a level that corresponded to the diagnostic dose (DD) equivalent of twice the concentration of the 99% lethal dose (LD99); this level was determined by previous laboratory tests. The mortality was recorded after the exposition of ten nauplii or 2 mL of bioassay in the aforementioned artificial seawater medium for 24 h at 25 ºC, and the corresponding LC50 were determined by the PROBIT method (Finney, 1981) using version 17.00.0 of the SPSS® Statistic software.
Aedes aegypti larvae bioassay
The "Rockfeller" strain of A. aegypti Linneaus 1762 (Diptera: Culicidae) was kindly provided by the Oswaldo Cruz Institute (Rio de Janeiro State, Brazil) from the original collection at the CDC (Center of Disease Control, Porto Rico, USA), and the mosquito colonies were maintained in the Laboratory of Medical and Veterinary Entomology of the Department of Zoology of UFPR at 25 ºC and 80% relative humidity. Adult female mosquitoes were fed on rat blood in order to stimulate egg oviposition; the eggs were then deposited into filter paper pieces. The layered eggs were placed into plastic trays that contained bottled mineral water (Ouro Fino®) and an artificial diet (Tetramin®), and they were incubated at 28 ºC with a 12 h photoperiod until the eclosion of the 1st instar larvae and their further evolution to the 3rd instar stage had occurred. Dose-response curves for the crude ethanolic extracts from the A. muricata seeds, the P. nigrum fruits and the samples of pAG and piperine were obtained using the standard method for the larvicidal bioassay as recommended by the World Health Organization (WHO, 2005) and employing six progressive concentrations for each extract. For each bioassay, 1 mL of the ethanolic solution from each extract, which had been conveniently prediluted, was added to 150 mL of bottled water in a 320-mL plastic pot; the solution was assayed as four replicates (three sequential repetitions) at 25 ºC for a 24 h period of exposition and a 12 h photoperiod. The negative control was run with 1% ethanol, and the positive controls were run with 0.006 µg/mL Temephos Abate® 500E (BASF), which is a synthetic organophosporated insecticide; the diagnostic dose (DD) was determined for the Rockfeller strain (Lima et al., 2003). These mortality analyses and the determination of the lethal concentrations were also performed using the same above mentioned statistical program.
Chromatographic profiling of the crude extracts
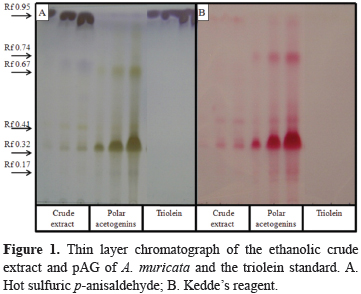
The chromatographic profiles that were derived from the crude ethanolic extracts of A. muricata and P. nigrum were obtained by thin layer chromatography in silica gel 60 chromatoplates (Merck) using hexane: chloroform: nitroethane: ethyl acetate:acetone:methano l:acetonitrile:water (12:2:4:4:1:2:1.6:0.1, v/v) as mobile phase (Fontana et al., 1998). Hot sulfuric p-anisaldehyde was used as the spray for the general constituents of both species, and the specific Kedde´s reagent (alkalinized 3,5-dinitrobenzoic acid) was used for the acetogenins from A. muricata. In the TLC procedure, pAG, the piperine standard and triolein were used as the reference compounds for the acetogenins, piperamides and TAG (triacylglycerols), respectively. All of the samples were applied in increasing volumes of 1, 10 and 20 µL from a stock solution of 10 mg/mL (w/v). The recording of differential colors (rose-wine for the acetogenins with Kedde´s reagent and purple-violet for triolein and the TAG, gray-green for the acetogenins and dark green for piperine with the anisaldehyde reagent) was performed with a Sony CyberShot camera. The plates were also visualized under long-wave UV (365 nm) light in a Chromato-VUE® C-70G UV Viewing System just prior to the heating step.
Combination of extracts and interaction analysis
Six combinations in pairs of concentrations in quadruplicates were used to measure the larvicidal effects of the crude ethanolic extracts from A. muricata and P. nigrum (100:0.1; 62.5:0.75; 50:1; 37.5:1.25; 25:1.5 and 75:1.5 µg/mL). The results were evaluated by an independence test (χ2). First, the crude extracts were individually evaluated in different concentrations in order to determine the expected mortality (Mexp) using the following equation where M1 and M2 represent the percentages of the separated observed mortalities for A. muricata and P. nigrum, respectively (Benz, 1971; Koppenhöfer & Fuzy, 2003; Morales-Rodriguez & Peck, 2009):
The results of the expected and observed mortalities from the combinations of two extracts were then applied to the following equation to determine the value of X2:
The χ2 results were compared with the tabulated values assuming that the degrees of freedom = 1. Any result that was larger than the tabulated value indicates that both extracts displayed a non-simple additive effect, which was either antagonistic or synergistic. A simple additive effect is an expected effect when different drugs or extracts are used together. Synergism is defined as an effect more than an additive effect and antagonism is less than an additive effect (Chou, 2010). For each case of non-simple additive effect, the difference between Mobs-Mexp was determined and the effect was:
Additionally, a paired t test was performed between the values for Mobsand Mexpto verify that the differences were statistically significant at p<0.05 (Farenhorst et al., 2010) using the previously described statistics software.
Results and Discussion
Preliminary bioactivity screening with Artemia salina nauplii
A preliminary screen was performed to determine the effect of the crude ethanolic extracts from the nine different botanical sources on Artemia salina nauplii lethality. The overall results revealed the presence of the three following groups of positive responses: a) Piper nigrum and Annona muricata displayed the lowest LC50 at 2.78 (2.31-3.61) and 10.20 (7.52-13.77) µg/mL, respectively; b) Melia azedarach and Origanum vulgare displayed an intermediate LC50 of 71.90 (55.44-92.89) and 122.32 (88.06-170.82) µg/mL, respectively; and c) the LC50 value of Ilex paraguariensis was not considered for the progressive work because at >1000 µg/mL, they were above the normal limit (Meyer, 1982).
In order to proceed with the experiments on A. aegypti larvae lethality and the related chromatographic profiles, we selected the two crude extracts that displayed the greatest activity against Artemia salina, A. muricata and P. nigrum.
Aedes aegypti larvae lethality bioassays and chromatographic profiles of the extracts
The lethal concentrations of the A. muricata crude extracts and pAG on the A. aegypti larvae and Artemia salina nauplii are shown in Table 1. The observed effects were expressed as LC (lethal concentration) and were obtained by a Probit analysis within a 95% confidence limit.
Two previous reports measured an LC50 of 60 (50-70) and 900 (380-1300) µg/mL, respectively, for A. muricata extracts toward the 4th instar A. aegypti larvae (Bobadilla et al., 2005; Henao et al., 2007). Further, if the extract was initially defatted with hexane, the LC50 was found to be 74.68 µg/mL, and the instar stage of the mosquito larvae was irrelevant (Morales et al., 2004). Therefore, our results are more similar to the results of Bobadilla (2005) and Morales (2004). However, the activity of the A. muricata ethanol extracts against Artemia salina resulted in an LC50 of 0.49 µg/mL for the leaves and 210 µg/mL for the seeds (Luna et al., 2006; Rojas et al., 2004). Therefore, our seed preparation was 21 times more potent than the published values. Annona squamosa is richer in squamocins (Araya et al., 2002), i.e., acetogenins other than annonacin, which is the major acetogenin from A. muricata. The crude methanol extracts that were prepared from the different botanical parts of Annona squamosa were previously evaluated, and an LC50 of 6.35, 1.49 and 0.15 were found for the stem bark, leaves and seeds, respectively (Pisutthanan et al., 2004).
Figure 1 shows the chromatographic profiles of the crude extract from A. muricata and the purified sample of pAG. The chromatoplate A (left) shows the general profile of the constituents. This clearly revealed predominant purple-violet TAG spots at Rf 0.95 (which was nearly absent in the sample of pAG that was previously purified in active charcoal and on silica gel columns) and lesser amounts of the acetogenin family members, which have a gray-greenish color at Rf 0.67, 0.41, 0.32 and 0.17; the third band is annonacin because this acetogenin is the major form in A. muricata seeds (Champy et al., 2002; Champy et al., 2005). Plate B (right) used Kedde's reagent for the specific detection of unsaturated lactones. The acetogenins, which showed a strong rose-wine reaction, confirmed the aforementioned interpretations, and due to the selectivity of the reagent, the sample overload facilitated the detection of the minor pAG contaminants with the other acetogenins (for example, one was present at Rf 0.74).
By comparing the bioactivities of the crude ethanolic extract from A. muricata and the pAG sample, it is clear that the activities increased for both A. aegypti and Artemia salina shrimp. The pAG LC50, which is the standard parameter of lethality, only increased 11 fold. The increase in pAG activity is due to the enrichment in acetogenins as observed in the chromatographic profile.
The efficient mechanism of action of acetogenins in several cell and tissue models is explained by the specific inhibition of mitochondrial respiration at complex I. Therefore, the inhibition of NADH: ubiquinone oxyreductase blocks the mitochondrial oxidative phosphorylation; the blocking of the electron transport prevents the conversion of ADP and Pi into ATP (Carmen Zafra-Polo et al., 1998; Fontana et al., 1998; Gu et al., 1995).
The lethal concentrations of P. nigrum crude extracts and piperine on A. aegypti larvae and Artemia salina nauplii at a 95 % confidence limit are shown in Table 2.
The ethanol crude extract from the unripe P. nigrum fruits has previously been tested against the 3rd instar larvae of A. aegypti that are resistant to the pyrethroid cypermethrin with an LC50 value of 0.98 µg/mL (Simas et al., 2007). The ethanolic extracts of three other species of Piperaceae were tested against the A. aegypti 4th instar larvae, and the LC50 results were higher than those found for P. nigrum. The results were as follows: P. longum, 2.23 (2.11-2.37); P. ribesoides, 8.13 (7.84-8.42); and P. sarmentosum, 4.06 (3.68-4.43) µg/mL (Chaithong et al., 2006).
Figure 2 shows the chromatographic profiles for the crude extracts of P. nigrum and the piperine standard. Both plates were treated with hot sulfuric p-anisaldehyde to obtain the general profile of the constituents. Plate A corresponds to the immediate UV-365 nm observation after the anisaldehyde spray, and plate B was visualized after the subsequent heating step.
Plate A (left) revealed the strong green fluorescence of piperine (Rf 0.60) in the crude ethanolic extract of P. nigrum. This same color pattern was observed for the Rf 0.46 component, which is possibly another piperamide. Other components that had higher and lower Rf were also visible, but they displayed different fluorescent colors, such as orange, blue and red. In plate B (right), the violet spots that are related to the high-Rf (0.95 and 0.87) lipids (e.g., TAG) were visible after heating. Piperine was the main component and displayed a green-brown color and, again, the component at Rf 0.46 displayed the same color hue as that of piperine.
Regarding the larvicidal bioactivities from the crude ethanolic extract of P. nigrum and the piperine standard, we noticed that the heterogeneous extract, i.e., piperine along with several other minor components, was more active than the pure piperine standard (3.6-fold and 2-fold for the two bioassay models, respectively). Therefore, because the Rf 0.46 component reacted much like piperine to both sprays, the above referred minor components may explain this unforeseen result of the higher larvicidal effect. For example, because piperine is an amide that lacks the isobutyl substituent. the previous studies have suggested the presence of several other bioactive compounds in P. nigrum; such compounds could include the isobutylamides retrofractamide A, pipercide, guineensine and pellitorine (Park et al., 2002).
The mechanism of the action of piperine, which is similar to piperamides, is exerted at the level of the enzyme cytochrome P450 at the MDP (methylenedioxyphenyl) group, and its amide group is essential for the neurotoxin activity (Scott et al., 2003,; 2008).
When both of crude extracts from A. muricata and P. nigrum were compared and based on the LC50 for the A. aegypti 3rd instar larvae, the former was 51 times more potent than the latter (Tables 1 and 2); this effect was reduced to 16-fold in the case of LC10 and increased to 407-fold for LC99. It is noteworthy that the LC50 for the P. nigrum crude extract was only four times more potent than the A. muricata crude extract when both were assayed against Artemia salina.
Based on these overall results, the combination of the crude ethanolic extracts from Piper nigrum and Annona muricata resulted in the increased mortality of the A. aegypti larvae.
Analysis of the synergism between the combined extracts from A. muricata and P. nigrum
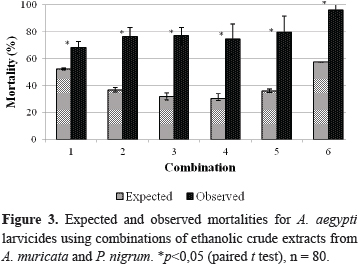
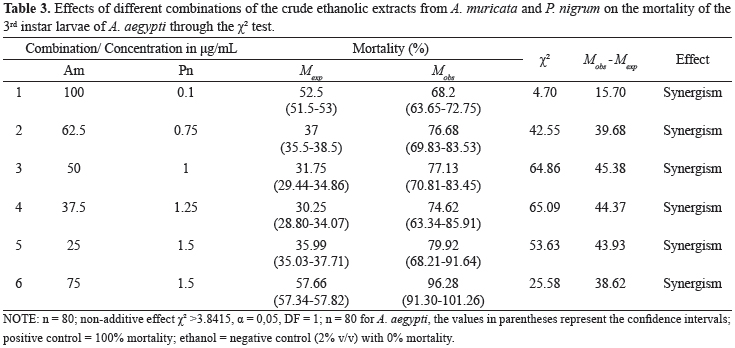
The crude ethanolic extracts from A. muricata (Am) and P. nigrum (Pn) were combined as six variants of Am:Pn (100:0,1; 62,5, 0,75; 50:1; 37,5, 1,25; 25:1,5 and 75:1,5). The relative proportion of each extract in respect to its counterpart was measured in µg/mL in each combination. This proportion, in addition to the expected and observed larvicidal mortalities at these combinations, their confidence intervals, their arithmetic differences and the interpreted results following the application of the χ2 test, are presented in Table 3.
Because the presented values of χ2 were larger than the tabulated ones, all of the results supported the existence of a non-additive simple effect. All of the combinations resulted in an increased expected mortality; this was especially true for combinations 3 and 4, which showed a higher χ2 of approximately 65. All of the differences between the mortalities that were observed and expected were positive, which confirmed the synergism between the crude ethanolic extracts from A. muricata and P. nigrum on the A. aegypti larvae.
Figure 3 displays the expected and observed mortalities for the combinations of the larvicides on the A. aegypti larvae. The existence of a statistical difference (p<0.05) between the expected and observed mortalities in all of the assayed combinations was verified using a paired t test; there was a 2.4-fold increase in combinations 3 and 4.
In summary, our results supported the initially proposed innovation because all combinations of the ethanolic extracts from the Annona muricata seeds and the Piper nigrum fruits displayed synergistic effects on the lethality of Aedes aegypti larvae. These positive results may be explained by the reliance of the primary toxic components of these two plants on different mechanisms of action; Annona muricata acetogenins block the mitochondrial energy generation, and piperine and the related amides of Piper nigrum have general neurotoxic effects.
Conclusion
The sinergystic combination of acetogenins from Annona muricata and piperamides from Piper nigrum has proven to be a novel and efficient strategy to kill the vector for dengue fever, the mosquito Aedes aegypti in its probably more sensitive morphogenetic step, namely, the larva. This finding counsels a deeper exploration of other botanical sources toxic for harmful insects and pragues since the obtained synergism most probably arises from the distinct mechanism of action of the two herein explored phytolarvicidal chemicals.
Acknowledgments
The authors thank the National Research Council for Scientific and Technological Development, CAPES for providing a PVNS fellowship to the corresponding author and for providing graduate fellowships for the co-authors MT and AG. We also thank UGF-SETI-PR for financial support and BRASFRUT for providing the A. muricata seeds. We also thank Dr. Francinete Ramos Campos, who collaborated with us to perform LC-MS in the annonacin sample, and Fernanda Gaensly for critically reviewing this work. The procedure described here has been subjected as part of a Brazilian patent request (INPI, PI protocol number 0000221109573019, November 7, 2011).
Received 23 Aug 2011
Accepted 30 Dec 2011
- Allegrand J, Touboul D, Schmitz-Afonso I, Guérineau V, Giuliani A, Ven JL, Champy P, Laprévote O 2010. Structural study of acetogenins by tandem mass spectrometry under high and low collision energy. Rapid Commun Mass Sp 24: 3602-3608.
- Araya H, Sahai M, Singh S, Singh AK, Yoshida M, Hara N, Fujimoto Y 2002. Squamocin-O1 and squamocin-O2, new adjacent bis-tetrahydrofuran acetogenins from the seeds of Annona squamosa. Phytochemistry 61: 999-1004.
- Arcila-Lozano CC, Loarca-Piña G, Lecona-Uribe S, Mejía EG 2004. El orégano: propiedades, composición y actividad biológica de sus componentes. Arch Latinoam Nutr 54
- Ashihara H, Sano H, Crozier A 2008. Caffeine and related purine alkaloids: biosynthesis, catabolism, function and genetic engineering. Phytochemistry 69: 841-856.
- Benz G 1971. Synergism fo micro-organisms and chemical insecticides. In Burges HD, Hussey NW (org.) Microbial control of insects and mites. New York: Academic Press, p. 327-355
- Bobadilla M, Zavala F, Sisniegas M, Zavaleta G, Mostacerco J, Taramona L 2005. Larvicidal evaluation of aqueous suspensions of Annona muricata Linnaeus (custard apple) against Aedes aegypti Linnaeus (Diptera, Culicidae). Rev Peruana Biol 12: 145-152.
- Brogdon W, McAllister J 1998. Insecticide resistance and vector control. Emer Infect Dis 4: 605-613.
- Cardozo Jr EL, Ferrarese-Filho O, Cardozo Filho L, Ferrarese MdLL, Donaduzzi CM, Sturion JA 2007. Methylxanthines and phenolic compounds in mate (Ilex paraguariensis St. Hil.) progenies grown in Brazil. J Food Compos Anal 553-558.
- Carmen Zafra-Polo M, Figadère B, Gallardo T, Tormo J, Cortes D 1998. Natural acetogenins from annonaceae, synthesis and mechanisms of action. Phytochemistry 48: 1087-1117.
- Chaithong U, Choochote W, Kamsuk K, Jitpakdi A, Tippawangkosol P, Chaiyasit D, Champakaew D, Tuetun B, Pitasawat B 2006. Larvicidal effect of pepper plants on Aedes aegypti (L.) (Diptera : Culicidae). J Vector Ecol 31: 138-144.
- Champy P, Hoglinger G, Feeger J, Gleye C, Hocquemiller R, Hirsch EC 2002. Annonacin, the major acetogenin of Annona muricata (Annonaceae) induces neurodegeneration and astrogliosis in rats. Movement Disord 17: S59-S60.
- Champy P, Melot A, Guerineau V, Gleye C, Fall D, Hoglinger GU, Ruberg M, Lannuzel A, Laprevote O, Laurens A, Hocquemiller R 2005. Quantification of acetogenins in Annona muricata linked to atypical parkinsonism in Guadeloupe. Movement Disord 20: 1629-1633.
- Chou T-C 2010. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res 70: 440-446.
- Cronquist RGB 1988. The evolution and classification of flowering plants. New York: New York Botanical Garden.
- Farenhorst M, Knols BGJ, Thomas MB, Howard AFV, Takken W, Rowland M, N'Guessan R 2010. Synergy in efficacy of fungal entomopathogens and permethrin against West African insecticide-resistant Anopheles gambiae mosquitoes. PLoS One 5: e12081.
- Finney DJ 1981. Probit Analysis. New Delhi: Ed. S. Chand & Company Ltda.
- Fontana JD, Lancas FM, Passos M, Cappelaro E, Vilegas J, Baron M, Noseda M, Pomilio AB, Vitale A, Webber AC, Maul AA, Peres WA, Foerster LA 1998. Selective polarity-and adsorption-guided extraction purification of Annona sp. polar acetogenins and biological assay against agricultural pests. Appl Biochem Biotech 70-2: 67-76.
- Gu Z-M, Zhao G-S, Oberlies NH, Zeng L, McLaughlin JL 1995. Annonaceous acetogenins-potent mitochondrial inhibitors with diverse applications. In al. JTAe (org.) Phytochemistry of Medicinal Plants New York: Plenum Press, p. 249-310
- Guzman MG, Kouri G 2003. Dengue and dengue hemorrhagic fever in the Americas: lessons and challenges. J Clin Virol 27: 1-13.
- Guzmán MG, Kourí G 2004. Dengue diagnosis, advances and challenges. Int J Infect Dis 8: 69-80.
- Henao GJP, Pajón CMG, Torres JMC 2007. Actividad insecticida de extractos vegetales sobre Aedes aegypti (Diptera: Culicidae) vector del dengue en Colombia. Rev CES Med 21: 47-54.
- Jacques RA, Dariva C, de Oliveira JV, Caramao EB 2008. Pressurized liquid extraction of mate tea leaves. Anal Chim Acta 625: 70-76.
- Koppenhöfer AM, Fuzy EM 2003. Steinernema scarabaei for the control of white grubs. Biol Control 28: 47-59.
- Kumar CSSR, Srinivas M, Yakkundi S 1996. Limonoids from the seeds of Azadirachta indica. Phytochemistry 43: 451-455.
- Lima JBP, Da-Cunha MP, Da Silva Júnior RC, Ribeuri Galardo AK, Da Silva Soares S, Braga IA, Ramos RP, Valle D 2003. Resistance of Aedes aegypti to organophorphates in several municipalities in the state of Rio de Janeiro and Espírito Santo, Brazil. Am J Trop Med Hyg 68: 329-333.
- Luna JD, De Carvalho JM, De Lima MRF, Bieber LW, Bento ED, Franck X, Sant'ana AEG 2006. Acetogenins in Annona muricata L. (annonaceae) leaves are potent molluscicides. Nat Prod Res 20: 253-257.
- Macoris MdLdG, Andrighetti MTM, Otrera VCG, Carvalho LRd, Júnior ALC, Brogdon WG 2007. Association of insecticide use and alteration on Aedes aegypti susceptibility status. Mem I Oswaldo Cruz 102: 895-900.
- Melo-Santos MAV, Varjal-Melo JJM, Araújo AP, Gomes TCS, Paiva MHS, Regis LN, Furtado AF, Magalhaes T, Macoris MLG, Andrighetti MTM, Ayres CFJ 2010. Resistance to the organophosphate temephos: Mechanisms, evolution and reversion in an Aedes aegypti laboratory strain from Brazil. Acta Trop 113: 180-189.
- Meyer BNF, Ferrigni NR, Putnam JE, Jacobsen LB, Nichols DE, McLaughlin JL 1982. Brine Shrimp: A convenient general biossay for active plant constituents. Planta Med 45: 31-34.
- Morales-Rodriguez A, Peck DC 2009. Synergies between biological and neonicotinoid insecticides for the curative control of the white grubs Amphimallon majale and Popillia japonica. Biol Control 51: 169-180.
- Morales CA, González R, Aragón R 2004. Evaluación de la actividad larvicida de extractos polares y no polares de acetogeninas de Annona muricata sobre larvas de Aedes aegypti y Anopheles albimanus (Diptera:Culicidae). Rev Colomb Entomol 30: 187-192.
- Park K, Lee S-G, Shin S-C, Park J-D, Ahn Y-J 2002. Larvicidal activity of isobutylamides identified in Piper nigrum fruits against three mosquito species. J Agric Food Chem 50: 1866-1870.
- Pisutthanan S, Plianbangchang P, Pisutthanan N, Ruanruay S, Muanrit O 2004. Brine shrimp lethality activity of Thai medicinal plants in the family Meliaceae. Naresuan University J 12: 13-18.
- Rojas I, Santiago R, Arvizu G, Muñoz D, Pérez D, Sucilla M 2004. Análisis químico y biológico preliminar de las semillas de Annona muricata L. (Annonaceae). Investigación Universitaria Multidisciplinaria 3: 7-12.
- Scott I, Jensen H, Philogène B, Arnason J 2008. A review of Piper spp. (Piperaceae) phytochemistry, insecticidal activity and mode of action. Phytochem Rev 7: 65-75.
- Scott IM, Jensen H, Scott JG, Isman MB, Arnason JT, Philogène BJR 2003. Botanical insecticides for controlling agricultural pests: piperamides and the Colorado potato betle Leptinotarsa decemlineatta say (Coleoptera: Chrysomelidae). Arch Insect Biochem 54: 212-225.
- Siddiqui BS, Gulzar T, Begum S, Afshan F, Sattar FA 2004. Two new insecticidal amide dimers from fruits of Piper nigrum Linn. Helv Chim Acta 87: 660-666.
- Siddiqui BS, Gulzar T, Begum S, Rasheed M, Saftar FA, Afshan F 2003. Two new insecticidal amides and a new alcoholic amide from Piper nigrum Linn. Helv Chim Acta 86: 2760-2767.
- Simas NK, Lima EDC, Kuster RM, Lage CLS, de Oliveira AM 2007. Potential use of Piper nigrum ethanol extract against pyrethroid-resistant Aedes aegypti larvae. Rev Soc Bras Med Tro 40: 405-407.
- Wandscheer CB, Duque JE, Silva MANd, Fukuyama Y, Wohlke JL, Adelmann J, Fontana JD 2004. Larvicidal action of ethanolic extracts from fruit endocarps of Melia azedarach and Azadirachta indica against the dengue mosquito Aedes aegypti. Toxicon 44: 829-835.
- WHO 2005. Guidelines for laboratory and field testing of mosquito larvicides. WHO/CDS/WHOPES/ GCDPP/2005.13
- WHO 2009. Dengue: guidelines for diagnosis, treatment, prevention and control. WHO/HTM/NTD/DEN/2009.1
Publication Dates
-
Publication in this collection
2012 -
Date of issue
June 2012
History
-
Received
23 Aug 2011 -
Accepted
30 Dec 2011