Abstract
The concentrations of phycobiliproteins (phycoerythrin and phycocyanin), chlorophyll-a and total soluble proteins were determined monthly in three strains (red, green and brown) of Gracilaria domingensis (Kützing) Sonder ex Dickie, collected from natural populations on the coast of Rio Grande do Norte, Brazil. In all the strains, pigment and protein concentrations were higher in the months of less sunlight and greater nitrogen availability and decreased gradually with increased sunlight and decreased nutrient concentration. The red strain showed higher concentrations of phycoerythrin and total soluble proteins. The difference in the concentration of biochemical components over the course of the year indicates species acclimation to different environmental conditions.
Gracilaria; strain; pigments; phycobilins; chlorophyll-a; proteins
Seasonal changes in the pigment composition of natural population of Gracilaria domingensis (Gracilariales, Rhodophyta)
Dinaelza Castelo PereiraII; Thiago Gaban TrigueiroI; Pio ColepicoloII; Eliane Marinho-SorianoI,* * Correspondence Eliane Marinho-Soriano Departamento de Oceanografia e Limnologia-Universidade Federal do Rio Grande do Norte Via Costeira, Praia de Mãe Luiza, s/n, 59014-100 Natal-RN, Brazil eliane@ufrnet.br Tel.: 84 3342-4952 Fax: 84 3342-4952
IDepartamento de Oceanografia e Limnologia, Universidade Federal do Rio Grande do Norte, Brazil
IIDepartamento de Bioquimica, Instituto de Química, Universidade de São Paulo, Brazil
ABSTRACT
The concentrations of phycobiliproteins (phycoerythrin and phycocyanin), chlorophyll-a and total soluble proteins were determined monthly in three strains (red, green and brown) of Gracilaria domingensis (Kützing) Sonder ex Dickie, collected from natural populations on the coast of Rio Grande do Norte, Brazil. In all the strains, pigment and protein concentrations were higher in the months of less sunlight and greater nitrogen availability and decreased gradually with increased sunlight and decreased nutrient concentration. The red strain showed higher concentrations of phycoerythrin and total soluble proteins. The difference in the concentration of biochemical components over the course of the year indicates species acclimation to different environmental conditions.
Keywords:Gracilaria, strain, pigments, phycobilins, chlorophyll-a, proteins
Introduction
Marine macroalgae are important source of bioactive compounds to the pharmaceutical, cosmetic and food industries (Cardozo et al., 2007; Carignan et al, 2009; Gressler et al., 2009; Gressler et al., 2010). In the marine environment, seaweeds exhibit a distinct and well-defined vertical distribution pattern. Species that inhabit the intertidal zone are adapted to tidal variations, given that they are exposed during low tide and submersed at high tide. During this variation, the algae are subject to considerable environmental changes. They are gradually uncovered during low tide and, consequently, the incident irradiance also increases gradually. Moreover the incidence of light also varies depending on the water turbidity and the position of the sun during the day, as well as the time of year. Additionally, nutrient concentrations, salinity, temperature, and hydration are other factors that vary during tides (Benson et al., 1983).
The main response mechanisms to sunlight variations occur in the photosynthetic system. High light exposure requires seaweeds to acclimatize, avoiding inhibition of photosynthesis and degradation of the photosynthetic apparatus (Cabello-Pasini et al., 2000). Protection against the harmful effects of superexcitation by supersaturating light intensity is a fundamental survival mechanism of seaweeds in the intertidal zone (Häder et al., 2002; Andersson et al., 2006; Cardozo et al., 2006; Cardozo et al., 2008; Guaratini et al., 2009). On the other hand, during low photon flux conditions the algae have to harvest maximum light. Pigments, carotenoids, and phycobiliproteins act as protection mechanisms against excess light and play a key role in photosynthesis as harvesting pigments, helping in light absorption and radiant energy transfer to the reaction centers.
The genus Gracilaria is commonly found in the intertidal zone, exhibiting a variety of physiological mechanisms in response to environmental alterations (Gómez et al., 2005). Because of this plasticity, color variants often occur, resulting in orange, brown, green, yellow, pink, and purple plants (Guimarães et al., 2003). This type of intraclonal variation takes place because of significant differences in phenotype between the species derived from a number of genes that may result from a single factor or from a combination of effects provoked by several factors. These factors include differences in the surrounding microenvironment during growth, physiological differences and variations in the development of genetically identical species (Santelices et al., 1996; Santelices, 2001).
The species Gracilaria domingensis is frequently found on the coast of Rio Grande do Norte, Brazil, and exhibits a wide color variation in natural populations, represented most commonly by the colors green, red, and brown. This color variation has been associated with differences in fitness (response to environmental changes). However, studies of the differences in the physiological characteristics of these strains in the field are scarce. In this work, protein, phycobiliproteins and chlorophyll-a were assessed seasonally in natural populations of G. domingensis in the green, red, and brown variants living in the same area and exposed to different environmental conditions during the year.
Materials and Methods
The study was conducted between July, 2007, and April, 2008. Color variants (red, green, and brown) of Gracilaria domingensis Sonder ex Dickie (Gracilariaceae, Rhodophyta) were collected (n = 9) during low tide in the intertidal zone at the Rio do Fogo beach (5º 16'37.54" S; 35º 22'37.34" W), Rio Grande do Norte, Brazil. There are two well-defined seasons in this region: the rainy season from March to July and the dry season from August to February.
The study site is characterized by a wide intertidal zone (~250 m) with many sandstone rocks and shallow tide pools (~15 cm) that form during low tide. There is great species diversity in this zone, mainly of the genus Gracilaria, which remains completely exposed to atmospheric conditions during low tide. After collection, the seaweeds were taken to the laboratory in isothermal boxes containing sea water. They were cleaned of epiphytes and frozen. Before pigment and protein analysis, the seaweeds were lyophilized until constant weight was obtained.
Environmental parameters
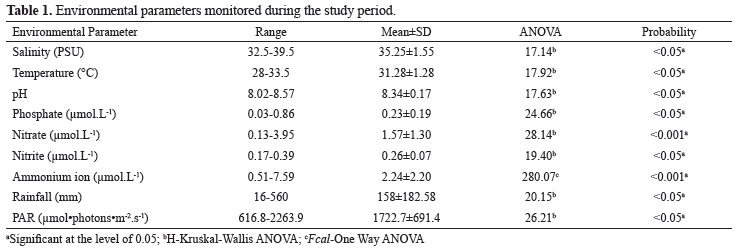
The environmental parameters temperature, salinity, pH and dissolved nutrients (NH4+, NO3-, NO2- and PO43-) were determined simultaneously with seaweed collection. Dissolved nutrient concentrations were measured using the methodology described by Strickland & Parsons (1972). Rainfall and photosynthetically active radiation (PAR) data were obtained from the Meteorological Station of the Brazilian National Institute of Space Research (INPE). Rainfall was obtained by adding the daily values for each month and, for PAR values, a monthly mean was calculated from daily measurements with data collected between 6 and 18 h at intervals of 30 min.
Phycobiliproteins
For each seaweed sample, one fragment (apex) of 90 mg DW was macerated in liquid nitrogen and submersed in 5 mL of 0.1 M phosphate buffer (pH 6.8). The extract with the seaweed was maintained in the dark overnight at 4 °C. It was then centrifuged at 10000 g for 20 min. The supernatant was collected and absorption measurements were performed. Phycoerythrin (PE) and phycocyanin (PC) were calculated according to the formula proposed by Beer & Eshel (1985).
Total soluble proteins
Total soluble proteins and biliproteins were determined in the same extract. The concentrations were measured according to Bradford (1976). Protein content was obtained on a UV-Vis absorption spectrophotometer at 595 nm using bovine serum albumin (BSA) solutions as standard.
Chlorophyll-a
For each seaweed sample, a fragment (apex) of 75 mg DW was macerated in liquid nitrogen and submersed in 1.5 mL of methanol:dimethylformamide. The extract with the seaweed was submitted to ultrasound in an ultrasonic cleaning bath for 15 min (~10 °C) and maintained in the dark overnight at 4 °C. The extract was then centrifuged at 10000 rpm for 5 min. The supernatant was collected and filtered through a 0.45 µm filter. The pigment was separated by high-performance liquid chromatography (HPLC), according to Guaratini et al (2005).
Statistical analyses
The differences in environmental parameter values between the different months of the study were tested using analysis of variance (one-way ANOVA or the corresponding non-parametric Kruskal-Wallis). The differences in the mean concentrations of proteins and pigments between the different strains of G. domingensis and over the different months of the study were tested using analysis of variance (two-way ANOVA), along with the Student Newman-Keuls test for multiple comparisons. The protein and pigment concentrations in each strain were correlated (using multiple regression) with the environmental parameters to estimate the environmental response of each strain studied.
Results
All of the environmental parameters determined varied significantly throughout the year (Table 1). Rainfall and PAR showed inverse tendencies (Figure 1).
Total Soluble Proteins
The highest mean concentration was recorded in the red variant (27.04±1.07 mg.g-1 DW), followed by the brown strain (20.24 mg.g-1 DW); the green strain (0.88 mg.g-1 DW) presented the lowest concentration. The three strains followed the same trend during the year and showed significant statistical differences in the concentrations between months (p<0.001). Protein concentrations in the red, green and brown strains were associated mainly with the temperature (positively) and with the available concentrations of ammonium (positively) and orthophosphate (negative) in the seawater (r = 0.88, p<0.05; r = 0.81; p<0.05; r = 0.81; p<0.05, respectively). These variables accounted for 77% of the variance in the protein concentration in the red strain, 66% in the green strain and 61% in the brown strain.
Phycoerythrin
The phycoerythrin concentrations of the red strain were statistically different from those of the green and brown strains in all months of the study (p<0.001) except April (Figure 2). However, the brown strain did not differ from the green strain in any of the months (p>0.05). The highest concentrations were observed in the red strain (7.69 mg.g-1 DW) and the lowest in the green strain (0.009 mg.g-1 DW). These pigment concentrations also varied between the months (p<0.001).
The phycoerythrin concentration was affected differently in the three strains in each month. There was a highly significant correlation between the months of the year and the strain (p<0.001), which is an indication of differences in the fitness of these colour mutants.
The concentrations of phycoerythrin in the red, green and brown strains correlated with the analyzed nitrogenated nutrients (positive effect) and orthophosphate (negative effect) (r = 0.93; p<0.001; r = 0.83; p<0.05). These nutrients account for 86% (r2 = 0.86) of the effect in the red strain, 68% in the green strain and 84% in the brown strain.
Phycocyanin
Pairwise comparisons showed that the phycocyanin concentrations in the green and brown strains were similar in all months except June, when the green strain differed from both the brown and red strains. In addition, the red strain differed significantly from the other two (p<0.05) only in 6 of the 12 months. The red strain showed the lowest concentration (0.03 mg.g-1 DW).
In the red, green and brown strains, the phycocyanin concentration was directly proportional to the availability of ammonium ion in the seawater (r = 0.73, p<0.001; r = 0.74, p = 0.002; r = 0.88, p<0.001, respectively), affecting phycocyanin concentration by ca. 53% (r2 = 0.53), 55% and 79%, respectively. Only in the brown strain was a significant correlation found with other environmental parameters (nitrate, nitrite and orthophosphate). The correlation with orthophosphate was negative.
Phycocyanin/Phycoerythrin
The Phycoerithrin/Phycocyanin ratio was higher in the red strain during the entire study period. The highest values were registered in October for the red strain (320.9) and the lowest for the brown and green strains (0.75).
Chlorophyll-a
The chlorophyll-a concentration did not differ between the red and green strains (p>0.05), except in May. The green strain showed the highest concentration (0.5 mg•g-1 DW) in May. In March, the red and green strains showed the lowest concentration (0.02 mg.g-1 DW) (Figure 2).
The chlorophyll-a concentration in the red strain was related mainly to water temperature (p<0.001), rainfall (p=0.026) and the available nitrate (p=0.001) and orthophosphate (p=0.005) concentrations in the environment. Temperature, rainfall and nitrate concentration influenced positively, whereas orthophosphate had a negative influence. These variables affected chlorophyll-a in the red strain by up to 70% (r=0.83; r2=0.70).
In the green, red and brown strains, the chlorophyll-a concentration was related (r=0.86; r2= 0.75) to temperature (p=0.0020; p=0.004; p=0.004, respectively) and nitrate concentration (p<0.001; p=0.003; p=0.003, respectively). These parameters acted positively and affected the chlorophyll-a concentration in the green strain by 75% (r2=0.75) and in the red and brown strains by 66% (r2=0.66).
Discussion
The capacity of a genotype to modify its phenotype as a function of environmental alterations is considered to be crucial for the adaptation of an organism to the spatial and temporal dynamics of the environment it inhabits (Barros et al., 2003; Monro & Poore, 2005). Seaweeds have been described as highly mutable organisms (Lobban & Harrrison, 1997; Collado-Vides, 2002) and these mutations can affect a wide variety of phenotypes, including morphology, pigmentation, chemical composition and expression of sexual characters (Poore & Fagerstrõm, 2000).
The analyses of phycoerithrin and the proteins of the red, green and brown strains pointed to significant differences only between the red and green strains (p<0.001), a finding that suggests different acclimatization strategies. The red strain had the highest concentration and the green strain the lowest. These strains also showed a slight difference in phycocyanin and chlorophyll-a concentrations in some months. These results corroborate those of earlier studies using color variants of other species of Rhodophyceae (Kursar et al., 1983; Dawes, 1992; Guimarães et al., 2003; Plastino et al., 2004; Yokoya et al., 2007).
The total soluble proteins and the pigments showed a tendency to decrease at the beginning of the study. The highest concentrations were recorded in the months with lowest sunlight (July, August, May and June) and the lowest in the months with elevated sunlight levels (December, January and February). These variations are similar to those described by other authors, who reported an increase in total soluble proteins and phycobiliproteins in winter and a drop in summer (Kosovel & Talarico, 1979; Rosenberg & Ramus, 1982; Campbell et al., 1999; Aguilera et al., 2002; Orduña-Rojas et al., 2002). In this study, the association between high protein and pigment concentrations, lower solar irradiance and high nitrogen concentrations was clear.
The decrease in phycobiliprotein, chlorophyll-a and protein can be associated with nitrogen availability and light conditions. The significantly reduction in chlorophyll-a and protein during high light months indicates photo-oxidative damage and a decrease in photosystem number. This hypothesis was confirmed by the bleaching observed in the summer months in the strains, noticeably in the red and brown strains. This observation also indicates a higher capacity of photoprotection in the green strain than the others, which is probably related to the higher concentration of zeaxanthin.
The winter in Northeast Brazil is characterized by very abundant rainfall, a reduction in radiation and an increase in water turbidity and nitrogen concentration. These environmental conditions are the inverse of those observed in the summer months and result in strategic alterations of the pigment levels (increase) in order to optimize and maximize the harvesting of light and, consequently, the photosynthetic rates. According to Lapointe (1981), the alterations in pigment concentration are determined by the interaction between two factors, light intensity and nutrient availability. This interaction determines whether the pigment concentration should increase or decrease in order to increase the photosynthetic capacity and avoid photodamage (Sigaud-Kutner et al., 2002; Pinto et al., 2003).
The green strain exhibited a different organization of the phycoerythrin/phycocyanin ratio in the phycobilisomes. This reorganization results in a different light absorption spectrum, given that phycoerythrin absorbs light in the green and blue regions of the visible spectrum and phycocyanin in the red (Glazer, 1985; López-Figueroa, 1991). Therefore, it can be surmised that the green strain increased its phycocyanin/phycoerythrin ratio in order to modify its response to incident light, thereby optimizing and preserving the functioning of the photosynthetic apparatus. This hypothesis agrees with that of Yokoya et al. (2007), who concluded that the green strain of Hypnea musciformis is better adapted to environments with high irradiance levels.
The seven-fold higher phycoerythrin concentration registered in the red strain than in the green strain is apparently not a deficiency, but rather a new strategy of the photosynthetic apparatus since this strain was readily found during all months of study. Moreover, it was observed that, during the high light months, the green strain predominated in the environment and showed the better conditions. This suggests that the green strain has a greater ability to protect the photosynthetic apparatus under conditions of high light.
Acknowledgements
This work was supported by FAPESP, CAPES, CNPq, Ministério da Saúde, Ministério de Ciência e Tecnologia, NAP-USP de Biodiversidade Marinha and CNPq-INCT-Redoxoma.
Received 25 Nov 2011
Accepted 3 Jan 2012
- Aguilera J, Bischof K, Karsten U, Hanelt D, Wiencke C 2002. Seasonal variation in the ecophysiological patterns in macroalgae from an Artic fjord. II. Pigment accumulation and biochemical defence systems against high light stress. Mar Biol 140: 1087-1095.
- Andersson M, Schubert H, Pedersén M 2006. Different patterns of carotenoid composition and photosynthesis acclimation in two tropical red algae. Mar Biol 149: 653-665.
- Beer S, Eshel A 1985. Determining phycoerythrin and phycocyanin concentrations in aqueous crude extracts of red algae. Aust J Mar Fresh Res 36: 785-792.
- Barros MP, Pedersén M, Colepicolo P, Snoeijs P 2003. Self-shading phytoplankton communities against H2O2-induced oxidative damage. Aquat Microb Ecol 30: 275-282.
- Benson EE, Rutter JC, Cobb AH 1983. Seasonal variation in frond morphology and chloroplast physiology of the intertidal alga Codium fragile (Suringar) Hariot. New Phytol 95: 569-580.
- Bradford M 1976. A rapid and sensitive method for the quantitation of microgram quantities utilizing the principle of protein dye binding. Anal Biochem 72: 248-254.
- Cabello-Pasini A, Aguirre-von-Wobeser E, Figueroa F 2000. Photoinhibition of photosynthesis in Macrosystis pyrifera (Phaeophyceae), Chondrus crispus (Rodophyceae) and Ulva lactuca (Chlorophyceae) in outdoor culture systems. J Photoch Photobio B 57: 169-178.
- Cardozo KHM, Guaratini T, Barros MP, Falcão VR, Tonon AP, Lopes NP, Campos S, Torres MA, Souza AO, Colepicolo P, Pinto E 2007. Metabolites from algae with economical impact. Comp Biochem Phys C 146: 60-78.
- Cardozo KHM, Carvalho VM, Pinto E, Colepicolo P 2006. Fragmentation of mycosporine-like amino acids by hydrogen/deuterium exchange and electrospray ionisation tandem mass spectrometry. Rapid Commun Mass Sp 20: 253-258.
- Cardozo KHM, Vessecchi R, Carvalho VM, Pinto E, Gates PJ, Colepicolo P, Galembeck SE, Lopes NP 2008. A theoretical and mass spectrometry study of the fragmentation of mycosporine-like amino acids. Int J Mass Spectrom 273: 11-19.
- Carignan MO, Cardozo KHM, Oliveira-Silva D, Colepicolo P, Carreto JI 2009. Palythine-threonine, a major novel mycosporine-like amino acid (MAA) isolated from the hermatypic coral Pocillopora capitata. J Photoch Photobio B 94: 191-200.
- Collado-Vides L 2002. Clonal architeture in marine macroalgae: ecologial and evolutionary perspectives. Evol Ecol 15: 531-545.
- Campbell SJ, Bité JS, Burridge TR 1999. Seasonal patterns in the photosynthetic capacity, tissue pigment and nutrient content of developmental stages of Undaria pinnatifida (Phaeophyta: Laminariales) in Port Phillip bay, South-Eastern Australia. Bot Mar 42: 231-241.
- Dawes CJ 1992. Irradiance acclimation of the cultured Philippine seaweed, Kappaphycus alvarezii and Euchema denticulatum. Bot Mar 35: 189-195.
- Glazer A 1985. Light harvesting of phycobilisomes. Ann Revs Biophys Biophys Chem 14: 47-77.
- Gómez I, López-Figueroa F, Huovinen P, Ulloaa N, Morales V 2005. Photosynthesis of the red alga Gracilaria chilensis under natural solar radiation in an estuary in southern Chile. Aquaculture 244: 369-382.
- Gressler V, Colepicolo P, Pinto E 2009. Useful strategies for algal volatile analysis. Curr AnalChem 5: 271-292.
- Gressler V, Yokoya NS, Fujii MT, Colepicolo P, Mancini J, Torres RP, Pinto E 2010. Lipid, fatty acid, protein, amino acid and ash contents in four Brazilian red algae species. Food Chem 120: 585-590.
- Guaratini T, Vessecchi R, Pinto E, Colepicolo P, Lopes NP 2005. Balance of xanthophylls molecular and quasi-molecular ions in electrospray ionization. J Mass Spectrom 40: 963-968.
- Guaratini T, Cardozo KHM, Pinto E, Colepicolo P 2009. Comparison of diode array and electrochemical detection in the C30 reverse phase HPLC analysis of algae carotenoids. J Brazil Chem Soc 9: 1609-1616.
- Guimarães M, Plastino EM, Destombe C 2003. Green mutant frequency in natural population of Gracilaria domingensis (Gracilariales, Rhodophyta) from Brazil. Eur J Phycol 38: 165-169.
- Häder DP, Lebert M, Sinha RP, Barbieri ES, Helbling, EW 2002. Role of protective and repair mechanisms in the inhibition of photosynthesis in marine macroalgae. Photoch Photobio Sci 1: 809-814.
- Kosovel V, Talarico L 1979. Seasonal variation of phorosynthetic pigments in Gracilaria verrucosa (Huds.) Papenfuss (Florideophyceae-Gigartinales). Boll Soc Adriat Sci 63: 5-15.
- Kursar TA, Van Der Meer JP, Albert RS 1983. Light-harvesting system of red alga Gracilaria tikvahiae 1. Biochemical analysis of pigment mutation. Plant Physiol 73: 353-360.
- Lapointe B 1981. The effects of light and nitrogen on growth, pigment content, and biochemical composition of Gracilaria foliifera v. agustissima (Giagartinales, Rhodophyta). J Phycol 17: 90-95.
- Lobban C, Harrison PJ 1997. Seaweed Ecology and Physiology Cambridge: Cambridge University Press.
- López-Figueroa F 1991. Red, Green and Blue light photoreceptors controlling chlorophyll-a, biliprotein and total protein synthesis in the red alga Chondrus crispus. Brit phycol J 26: 383-393.
- Monro K, Poore AGB 2005. Light quantity and quality induce shade-avoiding plasticity in a marine macroalgae. J Evolution Biol 18: 426-435.
- Orduña-Rojas J, Robledo D, Dawes C J 2002. Studies on the tropical agarophyte Gracilaria cornea J.Agardh (Rhodophyta, Gracilariales) from Yucatán, Mexico. I. Seasonal physiological and biochemical responses. Bot Mar 45: 453-458.
- Pinto E, Nieuwerburgh LV, Barros MP, Pedersén M, Colepicolo P, Snoeijs P 2003. Density-dependent patterns of thiamine and pigments in production in Nitzschia microcephala. Phytochemistry 63: 155-163.
- Plastino EM, Ursi S, Fujii MT 2004. Color inheriritance, pigment characterization and growth of a rare light green strain of Gracilaria birdiae (Gracilariales, Rhodophyta). Phycol Res 52: 45-52.
- Poore AG, Fagerström T 2000. Intraclonal variation in macroalgae: causes and evolutionary consequences. Selection 1: 123-133.
- Rosenberg G, Ramus J 1982. Ecological growth strategies in the seaweeds Gracilaria foliifera (Rhodophyceae) and Ulva sp. (Chlorophyceae): photosynthesis and antena composition. Mar Ecol-Prog Ser 8: 233-241.
- Santelices B, Correa JA, Meneses I, Aedo D, Varela D 1996. Sporeling coalescence and intraclonal variation in Gracilaria chilensis. J Phycol 32: 313-322.
- Santelies B 2001. Advances in the study of intraclonal variation in Gracilaria chilensis (Rhodophyta). Cah Biol Mar 42: 39-44.
- Sigaud-Kutner TCS, Pinto E, Okamoto OK, Latorre LR, Colepicolo P 2002. Changes in superoxide dismutase activity and photosynthetic pigment content during growth of marine phytoplankters in batch-cultures. Physiol Plantarum 114: 566-572.
- Strickland JH, Parsons TR 1972. A practical handbook of seawater analysis. Ottawa: Fisheries Research Board of Canada.
- Yokoya NS, Necchi O, Martins AP, Gonzalez SF, Plastino EM 2007. Growth responses and photosynthetic characteristics of wild and pycoerythrin-deficient strains of Hypnea musciformis (Rhodophyta). J Appl Phycol 19: 197-205.
Publication Dates
-
Publication in this collection
05 June 2012 -
Date of issue
Aug 2012
History
-
Received
25 Nov 2011 -
Accepted
03 Jan 2012