Abstract
Synadenium grantii Hook f., Euphorbiaceae, is popularly known as leitosinha or janaúba. The diluted latex (18 drops/L of water) is commonly used in the south of Brazil to treat gastric disturbances. This study evaluated phytochemical screening and toxicity using Artemia salina Leach of crude bark extract and also latex. The toxicity and the anti-ulcer activity of S. grantii latex were also tested in rats. Phytochemical results showed presence of tannins, terpenes, unsaponificable substances, coumarins and anthraquinones in the crude bark extract and terpenes in the latex. Gas chromatography-mass spectrometry (GC-MS) analysis demonstrated the presence of diterpene tigliane esters in the latex, identified as 12-deoxyphorbol-13-(2-metilpropionate) and phorbol 12,13,20-triacetate. The toxicity results using A. salina presented CL50 26.58μg/mL and CL50 778.66μg/mL, for the latex and the crude bark extract respectively. The toxicological hepatic parameters of the diluted latex were not different to the control group (p<0.05). The eosinophils cells showed an increase in both the diluted and pure latex groups. The pure latex showed gastric protection of 90% (p<0.05) and the diluted latex showed 6% compared to the negative control. Therefore, our data indicate that S. grantii latex, under research conditions presented gastric protection. Pure latex showed more toxicity than the diluted latex.
Synadenium grantii; latex; toxicity; gastric protection
Anti-ulcer activity of Synadenium grantii latex
Larissa L. G. CostaI; Vanessa C. DavidI; Rodrigo M. C. PintoI; Bruno R. MinozzoI; Vitoldo A. Kozlowski JuniorII; Letícia A. CamposII; Rosi Z. SilvaI; Flávio L. BeltrameI,* * Correspondence Flávio Luís Beltrame, Departamento de Farmacia, Universidade Estadual de Ponta Grossa Av. Carlos Cavalcanti, 4748, Uvaranas, 84900-030 Ponta Grossa-PR, Brazil flaviobeltra@uepg.br Tel: 55 42 3220-3782, 55 42 3220-3120
IDepartamento de Ciências Farmacêuticas, Universidade Estadual de Ponta Grossa, Brazil
IIDepartamento de Odontologia, Universidade Estadual de Ponta Grossa, Brazil
ABSTRACT
Synadenium grantii Hook f., Euphorbiaceae, is popularly known as leitosinha or janaúba. The diluted latex (18 drops/L of water) is commonly used in the south of Brazil to treat gastric disturbances. This study evaluated phytochemical screening and toxicity using Artemia salina Leach of crude bark extract and also latex. The toxicity and the anti-ulcer activity of S. grantii latex were also tested in rats. Phytochemical results showed presence of tannins, terpenes, unsaponificable substances, coumarins and anthraquinones in the crude bark extract and terpenes in the latex. Gas chromatography-mass spectrometry (GC-MS) analysis demonstrated the presence of diterpene tigliane esters in the latex, identified as 12-deoxyphorbol-13-(2-metilpropionate) and phorbol 12,13,20-triacetate. The toxicity results using A. salina presented CL50 26.58μg/mL and CL50 778.66μg/mL, for the latex and the crude bark extract respectively. The toxicological hepatic parameters of the diluted latex were not different to the control group (p<0.05). The eosinophils cells showed an increase in both the diluted and pure latex groups. The pure latex showed gastric protection of 90% (p<0.05) and the diluted latex showed 6% compared to the negative control. Therefore, our data indicate that S. grantii latex, under research conditions presented gastric protection. Pure latex showed more toxicity than the diluted latex.
Keywords: Synadenium grantii latex toxicity gastric protection
Introduction
The study of the therapeutic properties of plant species is usually based on ethnopharmacological information and popular knowledge (Chauhan & Chaudhary, 2012). This information often directs clinical and pharmacological studies and also the isolation of substances responsible for therapeutic efficacy (Albuquerque & Andrade, 2002; Gbolade, 2009).
The Euphorbiaceae family contains over 300 genera and 8900 species described worldwide (Dun & Singh, 2007), many of which are used for medicinal purposes. This family is predominantly found in Africa and the Americas, mainly in tropical or arid habitats (Bittner et al., 2001).
Plants belonging to this family have developed various forms of life, including herbs, shrubs and large trees (Dun & Singh, 2007). Of particular note in this family are the Euphorbia, Jatropha, Ricinus, Manihot and Synadenium genera, that present various classes of bioactive molecules such as flavonoids, saponins, diterpenes, phorbol esters (Bagalkotkar et al., 2006; Jassbi, 2006), triterpenes (Uzabakiliho et al., 1987), lecithin (Premaratna et al., 1981; Rogério et al., 2007) and glycoproteins (Rajesh et al., 2006). The Synadenium genus contains nineteen species, and is a small genus of the Euphorbiaceae family (Kinghorn, 1980). Several species of this genus have been pharmacologically evaluated against anti-inflammatory, antitumor, analgesic, immune-regulatory and fibrinolytic experimental models (Premaratna et al., 1981; Jager, 1996; Rajesh et al., 2006; Valadares et al., 2007; Nogueira et al., 2008). Cunha et al. (2009) also evaluated the toxicity of Synadenium. Species belonging to this genus are widely used in folk medicine to treat several diseases like cancer, peptic ulcers and other health problems (Ortêncio, 1997; Souza et al., 2005).
Synadenium grantii Hook f. is a medicinal plant popularly known in southern Brazil as leitosinha and janaúba. In folk medicine, people use its latex to treat neoplasic diseases and gastric disorders such as peptic ulcers and gastritis (Machado, 2008). It is usually prepared by adding eighteen drops of latex to 1 L of water (Ortêncio, 1997). It is usually kept in the refrigerator and drunk three times during the day. Synadenium grantii is a succulent shrub that can reach 5 m in height with oval leaves, short petioles and dark red flowers. When the branches and bark are removed the plant releases a white latex that is highly irritating (Rook, 1965; Kinghorn, 1980).
The literature includes some studies of this species such as Kinghorn (1980) who reported the isolation and identification of diterpene esters presented in the latex; Uzabakiliho et al. (1987) who identified the presence of triterpenes, polyphenolic compounds, organic acids and fatty acids in the latex and Menon et al. (2002) who reported the isolation and characterization of proteolytic enzymes (serineproteases). All the above-mentioned compounds have medicinal properties, such as molluscicidal, (Machado, 2008) fibrinolytic, proteolytic and hemagglutinating (Govindappa et al., 1987; Dayanand & Murthy, 2010).
This study of S. grantii aims to investigate the phytochemical profile and the acute toxicity in vivo (Artemia salina) of the latex and stem bark samples. Anti-ulcer action (indomethacin and ethanol-induced gastric lesions) and toxicity are evaluated in rats using the pure and diluted latex to support the popular use and ethnopharmacological indication of S. grantii.
Materials and Methods
Plant material and extracts
The latex (58 mL) and stem bark of Synadenium grantii Hook f., Euphorbiaceae (334.50 g) were collected in Ponta Grossa (altitude: 975 meters, latitude: 25o 05´38´´ S, longitude: 50o 09´30´´ W), Parana, Brazil, in April 2010. A voucher specimen was identified by Osmar dos Santos Ribas and deposited in the Herbarium of the Botanical Museum of Curitiba (Brazil) under the number 363509. The pure latex (EB1) was extracted through longitudinal incisions in the trunk, after cleaning the trunk with 70% alcohol using a sponge, and the latex was collected in plastic bottles. The crude stem bark extract (EB2, 70% hydroalcoholic, v/v) was prepared by the maceration of dried and powdered material for seven days at room temperature and protected from light. The extract was then filtered and concentrated. The EB2 was lyophilized. To prepare the diluted latex (EB3) 1 L of water was used and eighteen drops (0.7 g) of the latex (Ortêncio, 1997). These extracts were stored in a refrigerator (4 °C) until use.
Phytochemical screening
Phytochemical screening was carried out with extracts EB1 and EB2 (Wagner & Bladt, 1996). The groups of secondary metabolites were assessed by reactions to specific compounds such as terpenes/steroids, alkaloids, flavonoids, anthraquinones, coumarins, saponins and tannins. The intensity of color and/or the appearance of a precipitate in carrying out the reactions were interpreted as responses to the qualitative assays.
Gas chromatography coupled with mass spectrometry (GC/MS)
Apparatus and software
GC was performed on a Varian (Palo Alto, CA) Saturn 2000R model system. The ionization was obtained by electron ionization mode, at 70 eV, for mass analysis and detection. Data acquisition was performed with Analyst 1.4.2 software (Applied Biosystems). The identification of compounds was performed by the respective retention times, and fragmentation mass spectrum was obtained by comparison with mass library NIST (National Institute of Standards and Technology).
GC-MS conditions
The injector temperature was maintained at 250 °C and the trap at 200 °C. Helium 5.0 was used as analytical carrier gas at a flow rate of 1 mL/min The operating conditions were as follows: analyses were performed on a column DB-225-MS. The initial column temperature was 50 °C, which was maintained for 2 min and was then scheduled to increases of 20 °C/min until reaching 90 °C. After 1 min, there were increases of 5 °C/min until reaching a maximum of 280 °C. An amount of 10 mg of each sample was diluted in 1 mL of methanol, and subsequently 0.1 mL of this solution was injected in the column. The compounds were identified by comparison with mass spectra libraries of NIST 05.
Animals
Female Wistar rats (200 g) were maintained at room temperature with a light-dark cycle of 12/12 h. They were fed a balanced diet and given free access to water. All animals were starved for 18 h before use. They were obtained from the Central Biotery of the Ponta Grossa State University (UEPG). The tests were designed according to the Ethical Principles of Animal Experimentation of the Brazilian Council for Animal Experiments. The experimental protocol (no 15116/2011) was approved by the Ethics Committee on Animal Use (CEUA) of the UEPG.
In vivo assays
Brine shrimp lethality test - Determination of lethal concentration (LC50)
The toxicity of lyophilized crude bark extract (EB2) and the pure latex (EB1) of S. grantti were assessed according to the procedure described by Meyer et al. (1982). Briefly, the cysts of Artemia salina (100 mg) were incubated under artificial light and constant aeration with pH adjusted between 8-9, and without food for 48 h for larvae hatching (nauplii). Extract solutions and latex tubes were prepared in saline (0.38 g/L) at concentrations of 1000, 750, 500, 250, 150, 100, 50, 25, 15, 10, 5 and 1 μg/mL and 10, 7.5, 5, 2.5, 1.25, 1.0, 0.75, 0.5 0.25 and 0.05% (v/v), respectively. The positive control group was prepared by adding 1% potassium dichromate and the negative control was prepared in saline. The larvae (nauplii) of A. saline (10 per vial) were added to each tube and after 24 h of contact the surviving larvae were counted. The data were analyzed using the Probit method (Finney, 1962) and expressed as LC50 (Parra et al., 2001; Lopes et al., 2002).
Evaluation of oral toxicity
The oral toxicity study of S. grantii latex (EB1) and diluted latex (EB3) was performed on rats. In this assay, a single dose of 0.5 mL/animal was administrated orally to a group of six animals after 18 h fast. A group of animals (n=6) received water that served as control. After 6 h of administration, the animals were euthanized. Macroscopic changes in the organs of the rats (liver, kidneys, adrenal glands, spleen, uterus, ovaries, pancreas and stomach) were evaluated and weighed to determine the relative weights (g.100-1 corporal weight). Laboratory evaluation included leukocytes, alanine aminotransferase (ALT), aspartate aminotransferase (AST), albumin, creatinine and C-reactive protein. These tests were carried out to evaluate the biochemical parameters of the animals using their serum (obtained after centrifugation of samples, 2500 rpm/10 min), using a Vitalab Selectra II.
Antiulcerogenic activity assay
Indomethacin-induced gastric lesions
Five different groups of animals (n=6) received an oral administration of pure latex (EB1) and diluted latex (50 µL/100 mL- EB3) of S. grantii; ranitidine (100 mg/kg) and omeprazole (20 mg/kg) as positive controls and water as negative control. Ten minutes before the subcutaneous administration of indomethacin (ulcerogenic agent - 40mg/kg - to induce gastric ulcers) the respective groups of animals received 0.5 mL of samples, positive and negative controls, in the amounts listed above (Hayden et al., 1978; Hiruma-Lima et al., 2000; Ferreira, 2005). After 6 h of the treatment, the animals were euthanized. The stomachs were rapidly removed, opened along the greater curvature, and gently rinsed with 0.9% saline solution and then the lesions were identified, measured, quantified and qualified along their greater length.
Ethanol-induced gastric lesions
The rats (n=6) were treated (by oral administration) with the same samples, positive and negative controls, and volumes used in the indomethacin experiment. After 1 h the rats orally received 0.5 mL of ethanol (PA-Synth) via a stainless steel intubation needle to induce gastric ulcers (Glavin & Szabo, 1992; Mota et al., 2008). The animals were euthanized 1 h after treatment with the ulcerogenic agent. The stomachs were removed, opened along the greater curvature and gently rinsed with 0.9% saline solution, and then the lesions were identified, measured, quantified and qualified along their greater length.
Ulcerative Lesion Index (ULI)
The ulcerative lesions were counted and classified, receiving a score according to their extensive area (large >1 mm and small <1 mm), number of petechial lesions (up to 10 petechiae, 1 point, up to 20, 2 points and up to 30, 3 points), presence of hemorrhagic lesion, loss of folds and loss of color (equal to 1 point). The results were expressed as an index of ulcerative lesions (ULI) applying the formula below (Gamberini, 1992).
ULI = (3 x A) + (2 x B) + (C)+ (D)+ (E)+ (F)
*A: ulcerative lesion >1 mm; B: ulcerative lesion <1 mm; C: hemorrhagic lesion; D: loss of folds; E: loss of color; and F: n◦ petechial lesions.
The formula used to calculate the percentage of inhibition degree of ulceration (Araújo et al., 2002) in the treated groups was:
*(1): negative group (water); (2): group sample; and (3): ranitidine or omeprazole group
Statistical analysis
The anti-ulcer activity and the biochemical assays were assessed by the difference in experimental statistical significance using analysis of variance (ANOVA), Tukey's test and Student's t-test. The analyses were determined by GraphPad Prism 4.0 (2003). Statistical significance was considered when p<0.05.
Results and Discussion
The phytochemical screening for the main chemical groups of secondary metabolites using specific colorimetric reactions (Wagner & Bladt, 1996) demonstrated the presence of terpenes, phenolic compounds (hydrolysable and condensed tannins, coumarins and anthraquinones) and unsaponificable substances in crude bark extract (EB2) of S. grantii, while in the latex (EB1) only the presence of terpenes was confirmed.
These data are in agreement with the literature, which reports the presence of these groups of secondary metabolites in S. grantii or in another species of the Synadenium genus (Kinghorn, 1980; Uzabakiliho et al., 1987; Bagalkotkar et al., 2006). Phenolic compounds have been identified in S. grantii and other species of the Euphorbiaceae family (Sekar & Francis, 1998). In the same way, terpenes and unsaponificable substances are present in great amounts in plants that contain latex (Uzabakiliho et al., 1987; Machado, 2008; Makkar & Becker, 2009; Melo Reis et al., 2010). Furthermore, terpenes make up the majority of compounds in the S. grantii species (Uzabakiliho et al., 1987). According to Kinghorn (1980), the methanol fraction of latex of S. grantii obtained by a liquid-liquid separation presents diterpenic tigliane esters. As our initial phytochemical screening confirmed the presence of terpenes in the latex, the methanolic fraction was obtained and evaluated by gas chromatography-mass spectrometry (GC-MS) to determine the presence of these kinds of terpenes in the latex. Two phorbol esters, that were identified as 12-deoxyphorbol-13-(2-metilpropionate) and phorbol 12,13,20-triacetate (Figure 1), that have already been reported in S. grantii species were separated by gas chromatography and identified by comparison of mass spectra and data obtained in the NIST library and the literature (Kinghorn, 1980). More than 90% of agreement between the experimental spectra and the NIST spectra was observed.
Based on fragmentation in electronic impact mode, the mass spectrum of 12-deoxyphorbol-13-(2-metilpropionate) presented m/z 41, 71, 83, 109, 312 and 320 and the base peak was m/z 71. In relation to phorbol 12,13,20-triacetate the mass spectrum presented ions m/z 43, 69, 83, 109, 173, 310 and 370 and the base peak was m/z 43 (CH3-C=O) derived from the cleavage of C-O bond from the principal skeleton.
Diterpene tigliane esters are characteristic of the Synadenium genus, and can be indicated as chemotaxonomic markers and can also be responsible for skin irritant proprieties (Evans, 1986; Bagavathi et al., 1988).
Due to the presence of diterpene tigliane esters it was decided to evaluate the toxicological parameters of this species. Therefore, the assessment of acute toxicity of the latex in A. saline assay was performed. This evaluation enabled the determination of the intrinsic toxicity of the plant extract and the effects caused by an acute overdose (Parra et al., 2001).
Briefly, this test was based on the ability of the plant extract to kill laboratory cultures of this microcrustacean, creating a relationship between the degree of toxicity of plant extracts and LC50 against larvae of A. saline. A LC50 greater than 1000 µg/mL indicates that the extract can be considered nontoxic (Parra et al., 2001; Lopes et al., 2002).
The results obtained in the experiment with A. saline and EB2 showed a LC50 778.66 µg/mL, indicating that this material presents a low toxicity. This result is in agreement with Cunha et al. (2009) who studied the ethanolic extract of leaves in rat models and who related that this material did not present toxicity under evaluated conditions.
On the other hand, our study showed that pure latex (EB1) demonstrated a high degree of toxicity using A. saline, showing that this effect is concentration dependent. The LC50 value of 26.58 µg/mL, confirmed the high toxicity of this material.
It is important to note that the literature does not yet contain data evaluating the acute toxicity of latex and crude bark extract of S. grantii in experiments with A. saline. Thus, these data are important for future research because, according to some authors, the toxicity of plant extracts evaluated using A. saline shows a good correlation with antitumor activity, insecticide and anti-Trypanosoma cruzi for substances with LC50<103μg/mL (Meyer et al., 1982; McLauglin et al., 1991; Mackeen et al., 2000; Parra et al., 2001; Ruiz et al., 2005).
Additionally, we evaluated the oral toxicity of the pure latex (EB1) and diluted latex (EB3) in female rats. The animals were treated with a single dose of 0.6 g/rat and the organs of the animals of the control group and of the treated groups with EB1 and EB3 were analyzed. Significant macroscopic alterations were not demonstrated. Furthermore, the relative weights of these organs did not present statistical differences.
These results are in disagreement with Cunha et al. (2009). These authors relate that latex doses (50-200 mg/kg) demonstrated tissue alterations with toxicity effects.
The results obtained for the biochemical parameters, albumin, creatinine and C-reactive protein did not demonstrate significant differences when the data between groups was compared using Student's t-test. The toxicological hepatic parameters AST and ALT in the pure latex group showed higher enzymatic activity than the diluted latex and control groups, respectively (Table 1). These results are in agreement with data obtained by Cunha et al. (2009), although these authors studied the ethanolic extract of leaves. It is plausible to suggest that the biological effect of S. grantii latex may be associated with potential sensibilization and allergic effect mediated by increased level of eosinophils in the blood of the female rats verified in this study. This biological response was independent of latex concentration and was accompanied by a drop in the number of lymphocytes (Table 2).
As latex is popularly used in the south of Brazil in diluted form (18 drops/L of water) for the treatment of peptic ulcers (Ortêncio, 1997), an evaluation of this diluted latex (EB3) and pure latex (EB1) was carried out with relationship to potential protective effects in rats. Two different models of the production of peptic ulcers (ethanol and indomethacin) (Hayden et al., 1978; Glavin & Szabo, 1992) were used, with the intention of checking the traditional use of latex. The results using the gastric lesion model induced by the oral administration of ethanol demonstrated that the formation of ulcerations and petechiae were induced in different percentages in the mucous of the stomach. The evaluation of the negative control presented ulcerative lesion index (ULI) of 23.33±3.15 and the pre-treatment of the animals with ranitidine and omeprazole reduced ULI significantly (51.78% and 67.85% respectively) when compared with the negative control (water). In the same way, the latex (EB1) of S. grantii induced significant protective effect (Figure 2).
The use of diluted latex (EB3) provided a 6% reduction in the formation of the ulcerative lesions and petechiae formation, and did not indicate significant differences when compared with the negative control. The pure latex presented significantly reduced values of ULI (**p< 0.01), when obtained by the Tukey test, presenting a proportional value of gastric protection of 90.01% in relation to the negative control group.
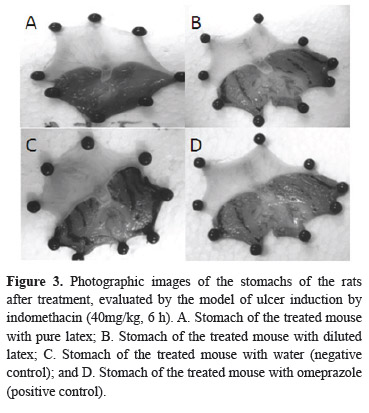
To confirm the results that demonstrated the gastric protective effect of the EB1 and EB3 samples against the ethanol model, the indomethacin model (a medicine that produces ulcers in laboratory animals) was carried out (Morimoto et al., 1991; Borrelli & Izzo, 2000). Regarding the application of the referred experimental model, it was verified that the sample of the latex exhibited high antiulcerogenic activity, impeding the formation of ulcerative lesions in the stomach of the rats treated with this material (Figure 3).
The obtained results were statistically similar to the results verified for the positive control groups (ranitidine and omeprazole), and did not present significant differences among these groups (Figure 4).
The diluted latex (EB3) showed a slight gastric protective action (6%) with the ethanol model, but when compared with the control group and also with the indomethacin model it was not statistically significant (p>0.05, Figure 4).
As previously indicated, in spite of the popular use of diluted latex of S. grantii for the treatment of gastric ulcers, no scientific study has been made until now to evaluate such a therapeutic indication. In the experiments to induce ulcers, the data obtained with relationship to a gastric protective effect of the diluted latex demonstrated that this material, in single dose shows a slight anti-ulcerative effect indicating that it is necessary to conduct further experiments using repeated doses.
However, when the results obtained with the pure latex were appraised a gastric protective effect was verified. This could indicate that the vegetable material, when in contact with the stomach content, can generate the formation of a protective layer that impedes the damage caused by aggressive agents (ethanol, for example). Also, this latex may contain chemical substances that can act to promote antiulcerogenic action (Figure 5).
Our preliminary phytochemical results indicated the presence of secondary metabolites (phenolic compounds/unsaponificable substances) in S. grantii that suggest that the antiulcerogenic effect of the latex in relation to the indomethacin and ethanol models, may be related to the presence of those substances in the appraised material (Alcaraz & Hoult, 1985; Aguwa, 1985; Bronner & Landry, 1985; Scarlat et al., 1985; Aguwa & Okunji, 1986; Beil et al., 1995; Matsuda et al., 1998; Araújo et al., 2002). However, it should be noted that the pure latex presented high values of CL50 when evaluated using the sharp toxicity with A. saline experiment and it presented biochemical alterations in relation to the ALT and AST parameters. Therefore, further studies should be undertaken seeking to isolate and to identify compounds responsible for this protective action of the stomach wall and also to determine the possible mechanism of action of the latex. Thus, it will be possible to evaluate the viability of the use of the latex of S. grantii for the treatment of pathological problems such as stomach disorders or to allow the identification of new substances with the desired pharmacological properties.
Furthermore, due to the toxicity of this material its continuing popular use in Brazil requires care on the part of the user. It is necessary to carry out further studies to determine its toxicity, specifically, because this study indicated a potential sensibilization with allergic effects associated with the increase of eosinophils in pure and diluted latex.
It can be concluded from the present study that the preliminary phytochemical analysis of Synadenium grantii indicated the presence of several phytochemical groups that allowed us to infer that the antiulcerogenic effect determined might be related to the presence of these phenolic and unsaponifiable compounds in this vegetable material. The analysis by gas chromatography showed the presence of phorbol esters that, according to the literature, present anti-cancerous actions. Such results should be evaluated against anti-cancerous models because this plant is also used popularly in Brazil for the treatment of cancer. The data obtained using a single dose in the anti-ulcer experiments suggest that the use of S. grantii latex may potentially present a gastroprotective effect in relation to the appraised models. However it is necessary tocarry out a prior treatment of the vegetable material to diminish the toxicity effect and to increase the anti-ulcerogenic effect.
Acknowledgement
This work received financial support from the Araucaria Foundation. We would also like to thank Professor Fábio André dos Santos for help in some experiments and Zilda Mara Consalter for support.
Received 19 Jan 2012
Accepted 27 Feb 2012
- Aguwa CN 1985. Gastrointestinal effects of the extracts of Rhigio caryaracemifera (Menispermaceae). Gen Pharmacol 16: 387-90.
- Aguwa CN, Okunji CO 1986. Gastrointestinal studies of Pyrena canthastaudtii leaf. J Ethnopharmacol 15: 45-55.
- Albuquerque UP, Andrade LHC 2002. Conhecimento botânico tradicional e conservação em uma área de caatinga no estado de Pernambuco, nordeste do Brasil. Acta Bot Bras 16: 263-285.
- Alcaraz MJ, Hoult JR 1985. Actions of flavonoids and the novel anti-inflammatory flavone, hypolaetin-8-glucoside, on prostaglandin biosynthesis and inactivation. Biochem Pharmacol 34: 2477-2482.
- Araújo CEP, Rodrigues RFO, Oliveira F, Schreiner L 2002. Análise preliminar da atividade antiulcerogênica do extrato hidroalcoólico de Solanum cernuum Vell. Acta Farm Bonaer 21: 283-286.
- Bagalkotkar G, Sagineedu SR, Saad MS, Stanslas J 2006. Phytochemicals from Phyllanthus niruri Linn. and their pharmacological properties-a review. J Pharm Pharmacol 8: 1559-1570.
- Bagavathi R, Sorg B, Hecher E 1988. Tigliane-type diterpene esters from Synadenium grantii. Planta Med 54: 506-510.
- Beil W, Birkholz C, Sewing KF 1995. Effects of flavonoids on parietal cell acid secretion, gastric mucosal prostaglandin production and Helicobacter pylori growth. Arzneimittel Forschung 45: 697-700.
- Bittner M, Alarcón J, Aqueveque P, Becerra J,Hernández V, Hoeneisen M, Silva M 2001. Estudio quimico de especies de La família Euphorbiaceae em Chile. Bol Soc Chil Quim 46: 1-15.
- Borrelli F, Izzo AA 2000. The plant kingdom as a source of anti-ulcer remedies. Phytother Res 14: 581-591.
- Bronner C, Landry Y 1985. Kinetics of the inhibitory effect of flavonoids on histamine secretion from mast cells. Agents Actions 16: 147-151.
- Chauhan B, Chaudhary AK 2012. Memory enhancing activity of methanolic extract of Pterocarpus marsupium Roxb. Phytopharmacology 2: 72-80.
- Cunha LC, Azeredo FS, Mendonça ACV, Vieira MS, Pucci LL, Valadares MC, Freitas HOG, Sena AAS, Junior RSL 2009. Avaliação da toxicidade aguda e subaguda, em ratos, do extrato etanólico das folhas e do látex de Synadenium umbellatum Pax. Rev Bras Farmacogn 19: 403-411.
- Dayanand CD, Murthy NK 2010. Evidence of fibrinolytic protease in the latex of Synadenium grantii Hoof. F.. Int J Biotechnol Biochem 6: 645-655.
- Dun D, Singh BSMP 2007. The family Euphorbiaceae in India: a synopsis of its profile, taxonomy and bibliography/N.P. Balakrishnan: T. Chakrabarty.
- Evans FJ 1986. Naturally occurring phorbol esters. Boca Raton, Flórida: CRC Press.
- Ferreira AL 2005. Atividade Antiulcerogênica da espécie Anacardium humile St. Hil. (Anacardiaceae). Campinas, Dissertação de Mestrado, Programa de Pós-graduação em Farmacologia, Universidade Estadual de Campinas.
- Finney DJ 1962. Probit Analysis. Cambridge: Cambridge University Press.
- Gamberini MT 1992. Atividades anti-úlcera e antiácida do extrato aquoso e frações obtidas da Baccharis trimera Mart. (carqueja). São Paulo, Dissertação de Mestrado, Instituto de Farmácia, Universidade de São Paulo.
- Gbolade AA 2009. Inventory of antidiabetic plants in selected districts of Lagos States, Nigeria. J Ethnopharmacol 121: 135-139.
- Glavin GB, Szabo S 1992. Experimental gastric mucosal injury: laboratory models reveal mechanisms of pathogenesis and new therapeutic strategies. FASEB J 6: 825-831.
- Govindappa T, Govardhan L, Jyothy PS, Veerabhadrappa PS 1987. Purification and characterization of acetylcholinesterase isozymes from the latex of Synadenium grantii Hook F. Indian J Biochem Bio 24: 209-217.
- Hayden L, Thomas G, West GB 1978. Inhibitors of gastric lesions in the rat. J Pharm Pharmacol 30: 244-246.
- Hiruma-Lima CA, Gracioso JS, Rodriguez JA, Haun M, Nunes DS, Souza Brito ARM 2000. Gastroprotective effect of essential oil from Croton cajucara Benth (Euphorbiaceae). J Ethnopharmacol 69: 229-234.
- Jager AK 1996. Screening of Zulu medicinal plants for prostaglandin-synthesis inhibitors. J Ethnopharmacol 52: 95-100.
- Jassbi AR 2006. Chemistry and biological activity of secondary metabolites in Euphorbia from Iran. Phytochemistry 67: 1977-1984.
- Kinghorn DA 1980. Major skin-irritant principle from Synadenium grantii. J Pharm Sci 69: 1446-1447.
- Lopes WB, Moroni FT, Brandeburgo MIH, Hamaguchi A 2002. Desenvolvimento de um método alternativo ao uso de animais de laboratório para avaliação da toxicidade de extratos vegetais. Rev Eletr Hor Científico 1: 1-11.
- Machado AA 2008. Caracterização fitoquímica e avaliação da citotoxicidade de Synadenium carinatum Boiss (Euphorbiaceae). Curitiba, Dissertação de Mestrado em Ciências Farmacêuticas, Programa de Mesrado em Ciências Farmacêuticas/UFPR.
- Mackeen MM, Ali AM, Lajis NH, Kawazu K, Hassan Z, Amran M, Habsah M, Mooi LY, Mohamed SM 2000. Antimicrobial, antioxidant, antitumour- promoting and cytotoxic activities of different plant part extracts of Garcinia atroviridis Griff. Ex.T. Anders. J Ethnopharmacol 72: 395-402.
- Makkar HPS, Becker K 2009. Jatrophacurcas, a promising crop for the generation of biodiesel and value-added coproducts. Eur J Lipid Sci Tech 111: 773-787.
- Matsuda H, Li Y, Murakami T, Yamahara J, Yoshikawa M 1998. Protective effects of oleanolic acid oligoglycosides on etanol- or indomethacin-induced gastric mucosal lesion in rats. Life Sci 63: 245-50.
- McLauglin JL, Chang CJ, Smith DL, Bench top "bioassay for the discovery of bioactive natural products: an update". In: Rahman AU 1991. Studies in Natural Products Chemistry. Karachi: Elsevier, p. 383-409.
- Melo Reis PR, Andrade LS, Silva CB, Araújo LMM, Pereira MS, Mrue F, Chen-Chen L 2010. Angiogenic activity of Synadenium umbellatum. Braz J Biol 70: 189-194.
- Menon M, Vithayathil PJ, Raju SM, Ramadoss CS 2002. Isolation and characterization of proteolytic enzymes from the latex of Synadenium grantii Hook f. Plant Sci 163: 131-139.
- Meyer BN, Ferrigni NR, Putnam JE, Jacobsen LB, Nichols DE, McLaughlin JL 1982. Brine shrimp: a convenient general bioassay for active plant constituents. Planta Med 45: 31-34.
- Morimoto Y, Shimohara K, Oshira S, Takayuki S 1991. Effects of the new anti-ulcer agent KB 5492 on experimental gastric mucosal lesions and gastric mucosal defensive factors, as compared to those of trepenone and cimetidine. Jpn J Pharmacol 57: 495-505.
- Mota KSL, Pita JCLR, Estevam EC, Medeiros VM, Tavares JF, Agra MF, Diniz MFFM, Silva MS, Batista LM 2008. Evaluation of the toxicity and antiulcerogenic activity of the ethanol extract of Maytenus obtusifolia Mart. Leaves. Rev Bras Farmacogn 18: 441-446.
- Nogueira IAL, Leão ABB, Vieira MS, Benfica PL, Cunha LC, Valadares MC 2008. Antitumoral and antiangiogenic activity of Synadenium umbellatum Pax. J Ethnopharmacol 120: 474-478.
- Ortêncio WB 1997. Medicina popular do Centro-Oeste Brasília: Thesaurus.
- Parra AL, Yhebra RS, Sardiñas IG, Buela LI 2001. Comparative study of the assay of Artemia salina L. and the estimate of the medium lethal dose (LD50 value) in mice, to determine oral acute toxicity of plant extracts. Phytomedicine 8: 395-400.
- Premaratna A, Shadaksharaswamy M, Nanjappa SS 1981. Isolation, purification and properties of a lectin from the latex of Synadenium grantii Hook F.. Indian J Biochem Bio 18: 32-35.
- Rajesh R, Nataraju A, Gowda CDR, Frey BM, Frey FJ, Vishwanath BS 2006, Purification and characterization of 34-kDa, heat stable glycoprotein from Synadenium grantii latex: action on human fibrinogen and fibrinclot. Biochemistry 88: 1313-1322.
- Rogerio AP, Cardoso CR, Fontanari C, Souza MA, Afonso-Cardoso SR, Silva EVG, Koyama NS, Basei FL, Soares EG, Calixto JB, Stowell SR, Baruffi MD, Faccioli LH 2007. Anti-asthmatic potential of a D-galactose-binding lectin from Synadenium carinatum latex. Glycobiology 17: 795-804.
- Rook AJ 1965. Unrecorded irritant plant Synadenium grantii. Br J Dermatol 77: 284.
- Ruiz ALT, Magalhães EG, Magalhães AF, Faria AD, Amaral MCF, Serrano DR, Zanotti-Magalhães EM, Magalhães LA 2005. Avaliação da atividade tóxica em Artemia salina e Biomphalaria glabrata de extratos de quatroespécies do gênero Eleocharis (Cyperaceae). Rev Bras Farmacogn 15: 98-102.
- Scarlat M, Sandor V, Tamas M, Cuparencu B 1985. Experimental anti-ulcer activity of Veronica officinalis L. extracts. J Ethnopharmacol 13: 157-63.
- Sekar T, Francis K 1998. Some plant species screened for energy, hydrocarbons and phytochemicals. Bioresource Technol 65: 257-259.
- Souza MA, Pereira FA, Cardoso CRB, Silva AG, Silva EG, Andrade LR, Pena JDO, Lanza H, Afonso-Cardoso SR 2005. Isolation and partial characterization of D-galactose-binding lectin from the latex of Synadenium carinatum Braz Arch Biol Tech 48: 705-716.
- Uzabakiliho B, Largeau C, Casadevall E 1987. Latex constituents of Euphorbia candelabrum, E. grantii, E. tirucalli and Synadenium grantii. Phytochemistry 26: 3041-3045.
- Valadares MC, Castro NC, Cunha LC 2007. Synadenium umbellatum: citotoxicidade e danos ao DNA de células da medula óssea de camundongos. Rev Bras Cien Farm 43: 631-638.
- Wagner H, Bladt S 1996. Plant drug analysis - A thin layer cromatography atlas. Berlin: Springer.
Publication Dates
-
Publication in this collection
17 Apr 2012 -
Date of issue
Oct 2012
History
-
Received
19 Jan 2012 -
Accepted
27 Feb 2012