Abstract
Leaves of Echinodorus macrophyllus (Kunth) Micheli, Alismataceae, were exposed to different doses of γ-radiation (0.00, 1.00, 3.00, 5.00, 10.00, and 20.00 kGy) and the chemical composition of their essential oils was investigated. The extractive process of the essential oil was more favored when the leaves were irradiated. The essential oil components were identified by correlation between GC-FID data and retention parameters obtained from the Kováts method. Moreover, GC-MS analyses of the essential oils were correlated with fragmentation profiles in the NIST standard mass fragmentation data bank. The essential oil of E. macrophyllus contains biologically active constituents of different chemical classes. Acyclic monoterpenes and sesquiterpenes showed increase in concentration when the leaves were exposed to γ-radiation. On the other hand, the component concentrations of some chemical classes were lightly decreased, i.e., for bicyclic monoterpenes, diterpenes, triterpenes, carboxylic esters, and carotenoid derivatives.
Alismataceae; Echinodorus macrophyllus; essential oil; y-radiation; gas chromatography
Changes in the essential oil composition of leaves of Echinodorus macrophyllus exposed to γ -radiation
Thiago M. SilvaI; Roqueline R. S. MirandaI; Vany P. FerrazI; Márcio T. PereiraII; Ezequias P. de SiqueiraIII; Antônio F. C. AlcântaraI
IDepartamento de Química, ICEx, Universidade Federal de Minas Gerais, Brazil,
IICentro de Desenvolvimento da Tecnologia Nuclear, Belo Horizonte-MG, Brazil,
IIICentro de Pesquisas René Rachou-Fiocruz, Belo Horizonte-MG, Brazil
Correspondence Correspondence: Thiago de Melo Silva Instituto de Ciências Exatas Universidade Federal de Minas Gerais Av. Antônio Carlos, 6627 31270-901 Belo Horizonte-MG, Brazil silvatm@ufmg.br Tel.: +55 31 3409 5728 Fax: +55 31 3409 570
ABSTRACT
Leaves of Echinodorus macrophyllus (Kunth) Micheli, Alismataceae, were exposed to different doses of γ-radiation (0.00, 1.00, 3.00, 5.00, 10.00, and 20.00 kGy) and the chemical composition of their essential oils was investigated. The extractive process of the essential oil was more favored when the leaves were irradiated. The essential oil components were identified by correlation between GC-FID data and retention parameters obtained from the Kováts method. Moreover, GC-MS analyses of the essential oils were correlated with fragmentation profiles in the NIST standard mass fragmentation data bank. The essential oil of E. macrophyllus contains biologically active constituents of different chemical classes. Acyclic monoterpenes and sesquiterpenes showed increase in concentration when the leaves were exposed to γ-radiation. On the other hand, the component concentrations of some chemical classes were lightly decreased, i.e., for bicyclic monoterpenes, diterpenes, triterpenes, carboxylic esters, and carotenoid derivatives.
Keywords: Alismataceae; Echinodorus macrophyllus; essential oil; γ-radiation; gas chromatography
Introduction
The γ-radiation is the safest and the most efficient method of food preservation and microbiological decontamination of plant after harvest (Chatterjee et al., 2012). This method causes structural damage to the DNA molecules and affects the reproducibility of microorganisms, and as a consequence the total population of mycoflora and quantity of mycotoxins are decreased in the irradiated material (Caillet et al., 2009). The reduction of microorganisms in the irradiated material is usually observed at low doses (1.5-3.5 kGy). Some studies report that irradiated materials at doses higher than 10 kGy are microbiologically decontaminated without compromising their nutritional capacities and pharmacological properties (Hanis et al., 1988; Onyenekwe et al., 1997; Migdal & Owczarczyk, 1998; Owczarczyk et al., 2000; Aziz & Moussa, 2002).
The γ-radiation also causes damage to plant cell membranes and usually promotes better extraction of their constituents (Byun et al., 1999). The change in concentration of compounds containing sulfuric, acid, alcohol, aldehyde, ester, furan, or ketone groups is observed in plant extracts which were submitted to different doses of γ-radiation. The concentration of phenolic compounds usually decreases in extracts exposed to γ-radiation, due to radioprotective property of these compounds (Gyawali et al., 2006; Silva et al., 2012)
The essential oils (EO) extracted from vegetal species exhibit biological activities against fungi and bacteria and shows important ecological role in plant-insect interactions. Other studies also report the use of EO in food flavoring and perfumery (Perrucci et al., 1995; Lahlou, 2004). The relative proportion of the EO constituents of Curcuma longa, Thymus vulgaris thymoliferum, Eucalyptus radiata, and Lavandula angustifolia did not change when these plants were exposed to γ-radiation (Haddad et al., 2007; Dhanya et al., 2011). However, qualitative and quantitative changes in concentration were observed for EO constituents of some plant species, such as Turnera diffusa (Camargo et al., 2008).
Echinodorus macrophyllus (Kunth) Micheli, Alismataceae, popularly known in Brazil as "chapéu-de-couro", is very used as medicinal plant and exhibits some pharmacological activities, such as astringent, diuretic, antiarrhythmic, anti-inflammatory, and anti-rheumatic agents, in the treatment of atherosclerosis, skin, liver, and urinary tract (lithiasis and nephritis) diseases, and immunosuppressive and cytotoxic effects (Leite et al., 2007; Pinto et al., 2007; Silva et al., 2012). There are few phytochemical reports about E. macrophyllus (Silva et al., 2012) and its EO has not yet been extensively studied (Tanus-Rangel et al., 2010). So, this work describes the identification of the EO components and investigation of the chemical integrity of the leaves of E. macrophyllus when submitted to γ-radiation. The components and composition of the EOs were analyzed by gas chromatography with a flame ionization detector (GC-FID) and gas chromatography-mass spectrometry (GC-MS) methods.
Materials and Methods
Plant material
The leaves of Echinodorus macrophyllus (Kunth) Micheli, Alismataceae, were obtained in April 2011 in the city of Belo Horizonte, Minas Gerais (Brazil). A voucher specimen was deposited in the herbarium of the Instituto de Ciências Biológicas of the Universidade Federal de Minas Gerais, under the code: BHCB 28,557.
Ionizing radiation treatment
Six samples of dried and powdered leaves of E. macrophyllus weighting 250 g each were placed in polyethylene packages. Five samples were submitted to different doses of γ-radiation (1.00, 3.00, 5.00, 10.00, and 20.00 kGy), named samples S1, S3, S5, S10, and S20, respectively. One sample was not exposed to γ-radiation (control sample, named sample S0). All samples were stored at -18 °C until required for experiments. The samples were submitted to γ-radiation apparatus in Gammacell with source 60Co. The dose rate was 2.50 kGy/h and a dose rate error of ±0.02 kGy.
Isolation of the essential oils
The six samples of E. macrophyllus were submitted to hydrodistillation for 5 h using a Clevenger-type apparatus. The aqueous emulsion was concentrated and submitted to extraction with dichloromethane. The solvent was evaporated at room temperature, providing the yellowish viscous essential oils EOS0 (0.0695 g), EOS1 (0.1672 g), EOS3 (0.1159 g), EOS5 (0.1800 g), EOS10 (0.1453 g), and EOS20 (0.1045 g) from S0, S1, S3, S5, S10, and S20, respectively. The EO were immediately analyzed.
TLC analyses
Thin Layer Chromatography was developed using silica gel HF254 glass plate (Merck). The chromatographic profiles were obtained by elution of the plates using pure n-hexane and dichloromethane. The spots were firstly revealed by UV light at 254 and 366 nm and after with a solution of vanillin/sulfuric acid (10%) and plates heated at 100 °C.
GC-FID analyses
The GC-FID analyses were performed on a HP Gas Chromatograph (HP 5890) with FID detector. The separations were carried out with a capillary column (Equity-5; 30 m x 0.25 mm with a 0.25 µm film thickness), which was purchased from Supelco Analytical. Hydrogen was used as carrier gas at flow rate of 2 mL/min. Exactly 1 µL of sample was injected in the equipment at a temperature programming from 60 to 300 °C (3 °C/min). The injector and detector temperatures were set at 270 and 300 °C, respectively (Pimenta et al., 2006; Radulovic et al., 2010).
The samples were diluted in chloroform (1% p/v) and the EO components were identified using retention parameters in gas chromatography based on the Kováts method, i.e., by comparison of their linear retention indexes with the corresponding value of standards (C10-C18, alkanes).
GC-MS analyses
The GC-MS analyses were carried out on equipment Shimadzu GC-MS (QP5050A) equipped with a capillary column (DB-5; 30 m x 0.25 mm with a 0.25 µm film thickness). Helium was used as carrier gas at flow rate of 2 mL/min, using the same conditions described above for GC-FID analyses.
The spectrometric data were manipulated using the AMDIS (Automated Mass Spectral Deconvolution and Identification System) software. The EO constituents were identified through comparison of the mass spectral fragmentation profile of the sample with the correspondent one available in the NIST standard mass fragmentation data bank (Nist Mass Spectral Search Program for the NIST/EPA/NIH Mass Spectral Library, version 2002, upgraded to 2006).
Results and Discussion
Chemical analyses of the essential oils
EOS0 was qualitatively analysed by TLC. The elution of EOS0 with dichloromethane afforded seven spots revealed with sulphuric vanillin (Rf 0.43, 0.46, 0.67, 0.80, 0.84, 0.87, and 0.99). The TLC analysis indicated that the EO of E. macrophyllus contains constituents of different polarities.
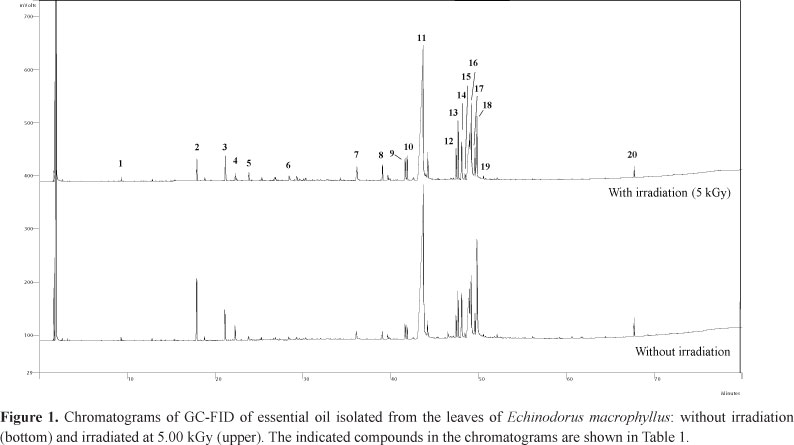
The GC-FID and GC-MS chromatograms of EOS0 show twenty relatively intense peaks, registered at 9.25 to 67.67 min (Figure 1). The chemical attribution of the GC-FID peaks of EOS0 was based on the Kováts method. Moreover, the GC-MS peaks of EOS0 were also chemically attributed through standard mass fragmentation data bank from NIST.
Table 1 shows the chemical class and GC data of the constituents identified in EOS0. The calculated Kováts retention indexes (CKRI) are very close to the corresponding Kováts retention indexes obtained from the literature (LKRI), principally those peaks registered with retention time between 9.25 and 44.16 min, attributed to the compounds 1 to 11, respectively. The peaks on the chromatogram of EOS0 correspond to one acyclic monoterpene (AM; compound 1), three carotenoid derivatives (CD; compounds 2, 8, and 9), one bicyclic monoterpene (BM; compound 3), four sesquiterpenes (ST; compounds 4-7), nine carboxylic esters (CE; compounds 10-13 and 15-19), one diterpene (DT; compound 14), and one triterpene (TT; compound 20). The relative percentage of the GC chromatogram peak areas (PA) of EOS0 indicates that the EO of the leaves of E. macrophyllus is rich in carboxylic esters, mainly 10 (44.28%), 18 (12.67%), 16 (7.94%), and 15 (7.85%). The carotenoid derivative 2 (2.93%), diterpene 14 (2.93%), and bicyclic monoterpene 3 (1.68%) are other important chemical constituents found in the EO obtained from the leaves of the plant.
Some biological activities which are described for the individual EO components of E. macrophyllus (Chart 1) can also be observed in the species E. macrophyllus. For example, EO containing linalool (compound 1) exhibit anti-inflammatory and anticancer properties (Kamatou & Viljoen, 2008), while EO containing dihydroedulan (compound 2) exhibits cytotoxic activity (Skaltsa et al., 2003; Sarikurkcu et al., 2008; Conforti et al., 2009; Stojković et al., 2011). A detailed information about each of the EO components of E. macrophyllus is listed in Chart 1.
The combination among biological properties of E. macrophyllus and biological activities of the majority components of its EO suggests that the anti-inflammatory and anti-rheumatic activities of the plant is mainly related to compound 15, followed by compounds 1, 4, 5, 12, 13, and 14. The anticancer activity of the plant is mainly related to compounds 1, 2, 4, 5, 7, 14, 17, and 20. Its diuretic property is mainly related to compounds 14 and 20. All EO components exhibit antioxidant and antimicrobial activities. However, the literature does not report these properties for the plant.
Effect of γ -radiation in the essential oils
The quantities of the EO obtained from the hydrodistillation of the irradiated leaves (EOS1, EOS3, EOS5, EOS10, and EOS20) were higher than EOS0 (see section Isolation of the essential oils). These results confirm that γ-radiation causes damage to the leaf cell membranes (Dhanya et al., 2011), and as a consequence the extractive process of the EO has become more favored when the plant material was submitted to radiation. However, the quantity of EOS1 (0.1673 g) is higher than the quantity of EOS3 (0.1159 g) and lower than EOS5 (0.1800 g), i.e. the EO quantity is not directly proportional to the radiation dose.
Table 2 shows the relative proportion of the constituents of EOS1, EOS3, EOS5, EOS10, and EOS20 based on CG-FID analyses (see Figure 1). The change in concentration of some essential oil components can be attributed to extraction efficiency and chemical and structural stabilities of these components when the leaves are exposed to γ-radiation.
Compounds 1, 5, 7-9, 11, 12, and 16 show higher relative proportions when the samples were exposed to γ-radiation. On the other hand, compounds 2, 4, 10, 18, and 20 show lower relative proportions when the samples were exposed to γ-radiation. The samples submitted to γ-radiation did not show significant change in relative proportion for the other compounds (3, 6, 13-15, 17, and 19).
The effect of the γ-radiation on the EO can be also observed when their chemical constituents are grouped in the chemical classes (Table 2). The concentration of acyclic monoterpene and sesquiterpene derivatives is increased when the plant was exposed to γ-radiation. On the other hand, the component concentrations of the other chemical classes were lightly decreased, i.e. for triterpene, diterpene, esters, and carotenoid derivatives. However, the effect of the γ-radiation is not the same for the different compounds in each chemical class. Examples are observed to carboxylic esters: the concentration of the compounds 11 and 16 increases when the radiation dose was higher, while the concentration of the compounds 10 and 18 decreases under the same conditions. As result, the effects of γ-radiation are intrinsic and specific for certain compounds. Similar compounds can exhibit different effects with γ-radiation. Moreover, the change in concentration of the EOs' components is not dependent of γ-radiation dose.
Conclusion
In conclusion, the essential oil of E. macrophyllys is rich in carboxylic esters, carotenoid derivatives, and terpenes. These chemical classes are represented on the essential oil by compounds that exhibit a large spectrum of the biological activities. The concentration of some essential oil components is changed when the leaves are exposed to γ-radiation. As a consequence of the use of γ-radiation to the biological decontamination of the leaves of E. macrophyllus, some alterations in the biological properties of the plant can certainly occur. Compound 16 is the main component among those that exhibited increase in concentration when leaves of E. macrophyllus were subjected to radiation. According to the literature data, this compound exhibits antioxidant, antimicrobial, and cytotoxic properties. As a consequence of changes in its concentration can modify the biological activities of the essential oil.
On the other hand, compounds 2, 10, and 18 are the main components among those that exhibited decrease in concentration when leaves of E. macrophyllus were subjected to radiation. These compounds exhibit antioxidant, antimicrobial, antifungal, acaricidal, hypoglycemic, nematicidal, insect repellent, disturbance in the neuroendocrine system, and promotion of the protein synthesis and hypopharyngeal gland development in honey properties and changes in their concentration can also modify the biological activities of the essential oil.
Acknowledgments
The authors thank CNPq, CAPES, and FAPEMIG for their financial support.
Authors'contributions
TMS (PhD student) contributed in collecting plant, essential oil isolation, analysis the GC-FID and GC-MS data, and draft the paper. RRSM contributed to critical reading of the manuscript. VPF contributed to chromatographic analysis (GC-FID). MTP contributed in gamma radiation of the samples. EPS contributed to chromatographic analysis (GC-MS). AFCA supervised the laboratory work and contributed to critical reading of the manuscript. All the authors have read the final manuscript and approved the submission.
Received 5 Mar 2013
Accepted 26 Jun 2013
- Abdel-Rahman FH, Alaniz NM, Saleh MA 2013. Nematicidal activity of terpenoids. Molecules 48: 16-22.
- Abreu VGC, Takahashi JA, Duarte LP, Piló-Veloso D, Junior PAS, Alves RO, Romanha AJ, Alcântara AFC 2011. Evaluation of the bactericidal and trypanocidal activities of triterpenes isolated from the leaves, stem, and flowers of Lychnophora pinaster. Rev Bras Farmacogn 21: 615-621.
- Ali MR, Young MJ, Gyawali R, Mosaddik A, Ryu YC, Cho SK 2012. Mango (Mangifera indica L.) peel extracts inhibit proliferation of HeLa human cervical carcinoma cell via induction of apoptosis. J Korean Soc Appl Biol Chem 55: 397-405.
- Arruda DC, D'Alexandri FL, Katzin AM, Uliana SRB 2005. Antileishmanial activity of the terpene nerolidol. Antimicrob Agents Chemoter 49: 1679-1687.
- Aziz NH, Moussa LAA 2002. Influence of gamma-irradiation on mycotoxin producing moulds and mycotoxins in fruits. Food Control 13: 281-288.
- Byun MW, Yook HS, Kim KS, Chung CK 1999. Effect of gamma irradiation on physiological effectiveness of Korean medicinal herbs. Radiat Phys Chem 54: 291-300.
- Caillet S, Ursachi L, Shareck F, Lacroix M 2009. Effect of gamma radiation and oregano essential oil on murein and ATP concentration of Staphylococcus aureus. J Food Sci 74: 499-508.
- Camargo EES, Telascrea M, Vilegas W 2008. Effect of the decontamination using gamma irradiation on the essential oil of Turnera diffusa Wild. Rev Bras Farmacogn 18: 356-359.
- Chatterjee S, Variyar PS, Sharma A 2012. Post-irradiation identification of papaya (Carica papaya L.) fruit. Radiat Phys Chem 81: 352-353.
- Conforti F, Statti GA, Menichini F 2007. Chemical and biological variability of hot pepper fruits (Capsicum annuum var. acuminatum L.) in relation to maturity stage. Food Chem 102: 1096-1104.
- Conforti F, Menichini F, Formisano C, Rigano D, Senatore F, Arnold NA, Piozzi F 2009. Comparative chemical composition, free radical-scavenging and cytotoxic properties of essential oils of six Stachys species from different regions of the Mediterranean area. Food Chem 116: 898-905.
- Dhanya R, Mishra BB, Khaleel KM 2011. Effect of gamma irradiation on curcuminoids and volatile oils of fresh turmeric (Curcuma longa). Radiat Phys Chem 80: 1247-1249.
- Dziri S, Hosni K 2012. Effects of cement dust on volatile oil constituents and antioxidative metabolism of Aleppo pine (Pinus halepensis) needles. Acta Physiol Plant 34: 1669-1678
- Faizi S, Fayyaz S, Bano S, Iqbal EY, Lubna, Siddiqi H, Naz A 2011. Isolation of nematicidal compounds from Tagetes patula L. yellow flowers: structure-activity relationship studies against cyst Nematode Heterodera zeae infective stage larvae. J Agric Food Chem 59: 9080-9093.
- Gertsch J, Leonti M, Raduner S, Racz I, Chen JZ, Xie XQ, Altmann KH, Karsak M, Zimmer A 2008. Beta-caryophyllene is a dietary cannabinoid. PNAS 105: 9099-9104.
- Gyawali R, Seo HY, Lee HJ, Song HP, Kim DH, Byun MW, Kim KS 2006. Effect of γ-irradiation on volatile compounds of dried Welsh onion (Allium fistulosum L.). Radiat Phys Chem 75: 322-328.
- Haddad M, Herent MF, Tilquin B, Quentin-Leclercq J 2007. Effect of gamma and e-beam radiation on the essential oils of Thymus vulgaris thymoliferum, Eucalyptus radiate, and Lavandula angustifolia. J Agric Food Chem 55: 6082-6086.
- Hanis T, Mnukova J, Jelen P, Klir P, Perez B, Pesek M 1988. Effect of gamma irradiation on survival of natural microflora and some nutrients in cereal meals. Cereal Chem 65: 381-383.
- Hua KF, Hsu HY, Su YC, Lin IF, Yang SS, Chen YM, Chao LK 2006. Study on the antiinflammatory activity of methanol extract from seagrass Zostera japonica. J Agric Food Chem 54: 306-311.
- Innocent E, Joseph CC, Gikonyo NK, Nkunya MH, Hassanali A 2010. Constituents of the essential oil of Suregada zanzibariensis leaves are repellent to the mosquito, Anopheles gambiae s.s. J Insect Sci 10: 1-8.
- Jegadeeswari P, Nishanthini A, Muthukumarasamy S, Mohan VR 2012. GC-MS analysis of bioactive components of Aristolochia krysagathra (Aristolochiaceae). J Curr Chem Pharm Sc 2: 226-232.
- Jeong JG, Kim YS, Min YK, Kim SH 2008. Low concentration of 3-Carene stimulates the differentiation of mouse osteoblastic MC3T3-E1 subclone 4 Cells. Phytother Res 22: 18-22.
- Huang WY, Cai YZ, Xing J, Corke H, Sun M 2007. A potencial antioxidant resource: endophytic fungi from medicinal plants. Econ Bot 61: 14-30.
- Kamatou GP, Viljoen AM, Gono-Bwalya AB, van Zyl RL, van Vuuren SF, Lourens AC, Baser KH, Demirci B, Lindsey KL, van Staden J, Steenkamp P 2005. The in vitro pharmacological activities and a chemical investigation of three South African Salvia species. J Ethnopharmacol 102: 382-390.
- Kamatou GPP, Viljoen AM 2008. Linalool - A review of a biologically active compound of commercial importance. Nat Prod Commun 3: 1183-1192.
- Lahlou M 2004. Methods to study the phytochemistry and bioactivity of essential oils. Phytother Res 18: 435-448.
- Leandro LM, Vargas FS, Barbosa PCS, Neves JKO, Silva JA, Veiga-Junior VF 2012. Chemistry and biological activities of terpenoids from copaiba (Copaifera spp.) oleoresins. Molecules 17: 3866-3889.
- Leite JPV, Pimenta DS, Gomes RSDL, Dantas-Barros AM 2007. Contribution to the pharmacobotanical study of Echinodorus macrophyllus (Kunth) Micheli (chapéu-de-couro) - Alismataceae. Rev Bras Farmacogn 17: 242-248.
- Legault J, Pichette A 2007. Potentiating effect of beta-caryophyllene on anticancer activity of alpha-humulene, isocaryophyllene and paclitaxel. J Pharm Pharmacol 59: 1643-1647.
- Maua JL, Ko PT, Chyau CC 2003. Aroma characterization and antioxidant activity of supercritical carbon dioxide extracts from Terminalia catappa leaves. Food Res Int 36: 97-104.
- Migdal W, Owczarczyk B 1998. The effect of ionizing radiation on microbiological decontamination of medical herbs and biologically active compounds. Radiat Phys Chem 52: 91-94.
- Miyazawa M, Yamafuji C 2005. Inhibition of acetylcholinesterase activity by bicyclic monoterpenoids. J Agric Food Chem 53: 1765-1768.
- Olufunke DM, Oladosu IA, Adeleke O, Ali MS 2009. Chemical composition and anti-inflammatory activity of the essential oil of the aerial part of Mezoneuron benthamianum Baill. (Caesalpinoideae). European J Appl Sci 1: 30-33.
- Onyenekwe PC, Ogbadu GH, Hashimoto S 1997. The effect of gamma radiation on the microflora and essential oil of Ashanti pepper (Piper guineense) berries. Postharvest Biol Technol 10: 161-167.
- Owczarczyk HB, Migdal W, Kedzia B 2000. The pharmacological activity of medicinal herbs after microbiological decontamination by irradiation. Radiat Phys Chem 57: 331-335.
- Pacheco AG, Oliveira PM, Piló-Veloso D, Alcântara AFC 2009. 13C NMR data of diterpenes isolated from Aristolochia esperanzae species. Molecules 14: 1245-1262.
- Palma M, Taylor LT 1999. Fractional extraction of compounds from grape seeds by supercritical fluid extraction and analysis for antimicrobial and agrochemical activities. J Agric Food Chem 47: 5044-5048.
- Peana AT, De Montis MG, Nieddu E, Spano MT, D'Aquila PS, Pippia P 2004. Profile of spinal and supra-spinal antinociception of (-)-linalool. Eur J Pharmacol 485: 165-174.
- Perrucci S, Macchioni G, Cioni PL, Flamini G, Morelli I 1995. Structure/activity relationship of some natural monoterpenes as acaricides against Psoroptes cuniculi. J Nat Prod 58: 1261-1264.
- Pimenta DS, Figueiredo MR, Kaplan MAC 2006. Essential oil from two populations of Echinodorus grandiflorus (Cham. & Schltdl.) Micheli (Chapéu de couro). An Acad Bras Cienc 78: 623-628.
- Pinto AC, Rego CG, Siqueira AM, Cardoso CC, Reis PA, Marques EA, Coelho MG, Sabino KC 2007. Immunosuppressive effects of Echinodorus macrophyllus aqueous extract. J Ethnopharmacol 111: 435-439.
- Radulovic N, Dordevic N, Markovic M, Palic R 2010. Volatile constituents of Glechoma hirsuta Waldst. & Kit. and G. hederacea L. (Lamiaceae). Bull Chem Soc Ethiop 24: 67-76.
- Rajeswari G, Murugan M, Mohan VR 2012. GC-MS analysis of bioactive components of Hugonia mystax L. (Linaceae). RJPBCS 3: 301-308.
- Sahin F, Güllüce M, Daferera D, Sökmen A, Sökmen M, Polissiou M, Agar G, Özer H 2004. Biological activities of the essential oils and methanol extract of Origanum vulgare ssp. vulgare in the Eastern Anatolia region of Turkey. Food Control 15: 549-557.
- Sarikurkcu C, Tepe B, Daferera D, Polissiou M, Harmandar M 2008. Studies on the antioxidant activity of the essential oil and methanol extract of Marrubium globosum subsp. globosum (Lamiaceae) by three different chemical assays. Bioresource Technol 99: 4239-4246.
- Satyal P, Paudel P, Poudel A, Setzer WN 2012. Chemical composition and biological activities of essential oil from leaf and bark of Nyctanthes arbor-tristis L. from Nepal. OAJMAP 3: 1-4.
- Silva TBC, Alves VL, Mendonça LVH, Conserva LM, Rocha EMM, Andrade EHA, Lemos RPL 2004. Chemical constituents and preliminary antimalarial activity of Humiria balsamifera. Pharm Biol 42: 94-97.
- Silva TM, Dias MD, Pereira MT, Takahashi JA, Ferraz VP, Piló-Veloso D, Alcântara AFC 2012. Effect of the γ-radiation on phenol fractions obtained from the leaves of Echinodorus macrophyllus Mich. Radiat Phys Chem 81: 22-26.
- Sinensky M, McLain T, Fantle K 1994. Expression of prelamin A but not mature lamin A confers sensitivity of DNA biosynthesis to lovastatin on F9 teratocarcinoma cells. J Cell Sci 107: 2215-2218.
- Singh S, Majumdar DK 1997. Evaluation of antiinflammatory activity of fatty acids of Ocimum sanctum fixed oil. Indian J Exp Biol 35: 380-383.
- Skaltsa HD, Demetzos C, Lazari D, Sokovic M 2003. Essential oil analysis and antimicrobial activity of eight Stachys species from Greece. Phytochemistry 64: 743-752.
- Stojković D, Soković M, Glamočlija J, Džamić A, Ćirić A, Ristić M, Grubiši D 2011. Chemical composition and antimicrobial activity of Vitex agnus-castus L. fruits and leaves essential oils. Food Chem 128: 1017-1022.
- Tanus-Rangel E, Santos SR, Lima JC, Lopes L, Noldin V, Monache FD, Cechinel-Filho V, Martins DT 2010. Topical and systemic anti-inflammatory effects of Echinodorus macrophyllus (Kunth) Micheli (Alismataceae). J Med Food 13: 1161-1166.
- Tundis R, Loizzo MR, Menichini F, Bonesi M, Conforti F, Statti G, de Luca D, de Cindio B, Menichini F 2011. Comparative study on the chemical composition, antioxidant properties and hypoglycaemic activities of two Capsicum annuum L. cultivars (Acuminatum small and Cerasiferum). Plant Foods Hum Nutr 66: 261-269.
- Tunón H, Thorsell W, Bohlin L 1994. Mosquito repelling activity of compounds occurring in Achillea millefolium L. (Asteraceae). Econ Bot 48: 111-120.
- Vlad PF 2006. Synthetic investigations in the field of drimane sesquiterpenoids. In: Atta-ur-Rahman. Bioactive Natural Producs. London: Elsevier 33rd ed, p. 393-432.
- Wang YN, Wang HX, Shen ZJ, Zhao LL, Clarke SR, Sun JH, Du YY, Shi GL 2009. Methyl palmitate, an acaricidal compound occurring in green walnut husks. J Econ Entomol 102: 196-202.
- Werner J, Laposata M, Fernandez - Del Castillo C, Saghir M, Iozzo RV, Lewandrowski KB, Warshaw AL 1997. Pancreatic injury in rats induced by fatty acid ethyl ester, a nonoxidative metabolite of alcohol. Gastroenterology 113: 286-294.
 Correspondence:
Correspondence: Publication Dates
-
Publication in this collection
02 Aug 2013 -
Date of issue
Aug 2013
History
-
Received
05 Mar 2013 -
Accepted
26 June 2013