Abstract
Gypsophila pilulifera, Boiss & Heldr, Caryophyllaceae, is a perennial medicinal herb that grows in the southwestern region of Turkey. Except for only one report on the isolation of cytotoxic saponins from the underground parts of G. pilulifera, there are no published thorough phytochemical or bioactivity studies on this species. In the present study, the free-radical scavenging activity of extracts and fractions of the stems of G. pilulifera was evaluated, using a slightly modified and more precise version of the 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay, reported here for the first time. The DPPH assay-guided HPLC-PDA-purification of the active solid-phase extraction fraction (50% methanol in water) of the methanolic extract exhibited verbascoside as the main free-radical scavenger present in this species. The structure of this active compound was resolved by spectroscopy, and the free-radical scavenging potential of verbascoside was determined.
Gypsophila pilulifera; Free-radical scavenger; Solid-phase extraction; High-performance liquid chromatography (HPLC); Phenylethanoid; Verbascoside
Introduction
Gypsophila pilulifera Boiss. & Heldr., a wild growing perennial herb from the south-western region of Turkey, is one of the circa 150 species of the genus Gypsophila of the family Caryophyllaceae (Arslan et al., 2012; Yotova et al., 2012). Some species from this genus, growing in China, have been used in the Traditional Chinese Medicine (TCM) to treat fever, consumptive disease, and infantile malnutrition syndrome (Chen et al., 2011; Shun et al., 2011). Several previously studied species of the genus Gypsophila revealed in their phytochemical analysis triterpene saponins; present predominantly in the roots, as main constituents with various pharmacological properties (Hostettmann and Marston, 1995; Gevrenova et al., 2010; Shun et al., 2011). To the best of our knowledge, except for one report on the isolation of cytotoxic triterpenoid saponins from the underground parts of G. pilulifera (Arslan et al., 2012), no other phytochemical or bioactivity study has previously been carried out on this species. As part of our on-going phytochemical and bioactivity studies on medicinal plants from the Turkish flora (Shoeb et al., 2005, 2007a-e; Sarker et al., 2007, 2012; Sauvage et al., 2010; Suntar et al., 2012a,b), we now report on the free-radical scavenging activity of the extracts and fractions of the stems of G. pilulifera. This finding was allowed by DPPH assay-guided isolation and identification of the main free-radical scavenger present in this species, and also a slightly modified and more precise DPPH assay method for determining the RC50 values for extracts, fractions and isolated compounds.
Material and methods
General
Semi-preparative HPLC was performed using a Dionex Ultimate 3000 series coupled with a Dionex Ultimate 3000 photodiode array detector (detection at 220, 254, 280 and 360 nm). The NMR spectroscopic analyses were performed on a Bruker DRX300 NMR spectrometer (300 MHz for1 H, and 75 MHz for 13C). Chemical shifts are given on δ scale (ppm) with TMS as the initial standard. UV-visible spectra were recorded using a Shimadzu UV-1600 spectrophotometer. MS analyses were performed on a Finnigan MAT95 mass spectrometer.
Plant material
The stems of Gypsophila pilulifera Boiss & Heldr, Caryophyllaceae, were collected at the Antalya province, Elmali district, Turkey (dry slopes, 1100 m above the sea level) from May to June 2003. A voucher specimen (Gokturk-GP-0401) were deposited and are kept at the Herbarium of the Biology Department of Akdeniz University, Turkey.
Extraction
The shade-dried and ground stems of G. pilulifera (30 g) were Soxhlet-extracted, successively, using petroleum ether 40-60º (PE), dichloromethane (DCM) and methanol (MeOH), 500 ml each. The extracts were vacuum dried using a rotary evaporator at a temperature not exceeding 45ºC, yielding 0.09, 0.08 and 1.52 g of the PE, DCM and MeOH extracts, respectively.
2,2-Diphenyl-1-picrylhydrazyl (DPPH) assay
2,2-Diphenyl-1-picrylhydrazyl (DPPH), molecular formula C18H12N5O6, was purchased from Fluka Chemie AG, Bucks. Quercetin was purchased from Avocado Research Chemicals Ltd, Shore road, Heysham, Lancs. The method used by Takao et al. (1994) was used with suitable modifications as outlined by Kumarasamy et al. (2002, 2007). DPPH (8 mg) was dissolved in MeOH (100 ml) for a solution concentration of 80 μg/ml.
Qualitative assay
Testing sample solutions (10 mg/ml) were applied on a TLC plate and sprayed with DPPH solution using an atomiser. They were allowed to develop for 30 min. Colour changes (purple on white) were noted.
Quantitative assay
The PE extract was dissolved in DCM, and the DCM and MeOH extracts were further diluted in MeOH to produce the stock concentration of 10 mg/ml. Further dilutions were made to obtain solution concentrations of 1.0, 0.1, 0.01, 0.001, 0.0001 and 0.00001 mg/ml. Diluted solutions (1 ml each) were mixed with DPPH (1 ml) and allowed to stand for 30 min for any reaction to occur. The absorbance was measured at 517 nm. The experiment was performed in triplicate and the average absorption was noted for each concentration. The same procedure was followed for the positive control, quercetin (stock: 1 mg/ml in MeOH), as well as for the solid-phase extraction fractions (stock: 10 mg/ml). The DPPH inhibitory activity (% inhibition) at each concentration was calculated by using the following formula:
inhibition (%) = (Acontrol-Asample) × 100/Acontrol,
where Acontrol was the control reaction absorbance (containing all reagents except for the test sample), and Asample was the absorbance of the test/reference.
For further precision: From the inhibition values (%) obtained from the quantitative assay outlined above, the concentration range in which 50% inhibition could be detected was assessed. The sample at the higher concentration of the previously cited range was further diluted one-fold over several steps, and the quantitative DPPH assay was performed using the prepared dilutions. Inhibition (%) was calculated as described above. A dose-response plot between concentration vs % inhibition curve was constructed, and from the slope of this curve, the RC50 value, which is the concentration at which 50% inhibition of DPPH absorbance at 517 nm occurs, was calculated for each test sample.
Solid-phase extraction (SPE): fractionation of the MeOH extract
The dried MeOH extract (1 g) was re-suspended in 10 ml of 20% MeOH, and fractionated on a solid-phase extraction cartridge (Strata, 10 g C18 Silica), eluted using 20, 50 and 80% MeOH in water and 100% MeOH (200 ml, each fraction) resulting in four fractions. The fractions were dried using a rotary evaporator at a temperature not exceeding 45ºC.
HPLC-DPPH assay
A portion (100 μl of 10 mg/ml) of the most active SPE fraction (50% MeOH in water) of the MeOH extract, was subjected to reversed-phase HPLC-PDA fractionation on a reversed-phase C18 silica semi-prep column (Luna Phenomenex column, 10 mm × 150 mm, 5 μm), using a gradient elution, 2 ml/min, with 30-100% MeOH-water gradient over 30 min followed by 100% MeOH for 10 min. The chromatogram was monitored at four different wavelengths. Twenty fractions of two minutes were collected. Each fraction (1 ml) was mixed with DPPH (1 ml) in duplicate, kept for 30 min, absorption was recorded, and the % inhibition was calculated as described above. The control was prepared from 1 ml of MeOH and 1 ml of DPPH solution.
Isolation of active compound
The HPLC chromatogram containing various peaks was compared against % inhibition caused by each two min fraction, and the peak corresponding to the highest level of free-radical scavenging activity was identified as the major active compound. Repeated HPLC runs were performed as outlined above with the 50% MeOH-water fraction, and the peak corresponding to the major active compound was collected. Combined collections of the active compound (1) from the repeated runs were dried using a rotary evaporator at a temperature not exceeding 45ºC. The structure of the active compound (1, 4.4 mg) was determined by spectroscopic means (Tokar et al., 2004; Delazar et al., 2005, 2012; Nazemiyeh et al., 2008).
Verbascoside (1): Brown amorphous solid. UV λmax (MeOH) nm: 332, 290 and 220; HR-ESIMS m/z: 642.2392 (calculated 642.2397 for C29H40NO15, [M + NH4]+); 1H NMR (300 MHz, CD3OD) δ 7.60 (1H, d, J = 15.7, H-7'), 7.06 (1H, d, J = 2.0, H-2'), 6.92 (1H, d, J = 2.0, 8.1 Hz, H-6'), 6.78 (1H, d, J = 8.1 Hz, H-5'), 6.69 (1H, d, J = 1.9 Hz, H-2), 6.68 (1H, dd, J = 1.9, 8.0 Hz, H-6), 6.64 (1H, d, J = 8.0 Hz, H-5), 6.28 (1H, d, J = 15.7, H-8'), 5.19 (1H, d, J =1 .5, H-1''', anomeric protons of rhamnose), 4.34 (1H, d, J = 7.9 Hz, H-1'', anomeric protons of glucose), 3.80-4.10 (1H, overlapped peak, H-8), 3.20-3.80 (10H, signal pattern unclear due to overlapping, H-2'', H-3'', H-4'', H-5'', H-6'', H-2''', H-3''', H-4'''and H-5'''), 2.79 (3H, t, J =7.6 Hz, H-7) and 1.09 (3H, d, J =6.0 Hz, H-6'''); 13C NMR (75 MHz, CD3OD): δ 166.8 (C-9'), 148.4 (C-4'), 146.6 (C-7'), 145.4 (C-3'), 144.7 (C-3), 143.2 (C-4), 130.0 (C-1), 126.2 (C-1'), 121.8 (C-6 and C-6'), 115.6 (C-5'), 115.0 (C-5 and C-8'), 114.8 (C-2'), 113.7 (C-2), 102.7 (C-1'''), 101.6 (C-1''), 80.2 (C-3''), 74.7 (C-2''), 74.5 (C-5''), 72.3 (C-4'''), 72.3 (C-8 and C-4'''), 70.9 (C-2'''), 70.6 (C-3'''), 69.4 (C-5'''), 69.1 (C-4''), 60.9 (C-6""), 35.1 (C-7) and 17.0 (C-6'''); all data were in agreement with previously published data (Tokar et al., 2004; Delazar et al., 2005, 2012; Nazemiyeh et al., 2008).
Results and discussion
The Soxhlet successive extraction with PE, DCM and MeOH of the dried and ground stems of G. pilulifera, produced three crude extracts, 0.09 g, 0.08 g and 1.52 g, respectively. The DPPH assay-guided purification of the most active SPE fraction (50% MeOH in water) of the MeOH extract by reversed-phase semi-preparative high performance liquid chromatography (semi-prep-HPLC) coupled with a photodiode array (PDA) detector afforded the isolation of the main free-radical scavenger, which was identified conclusively as verbascoside (1) by spectroscopy (Tokar et al., 2004; Delazar et al., 2005, 2012; Nazemiyeh et al., 2008).
Besides environmental sources, free-radicals may be formed as by-products of normal aerobic cells' metabolism and these free-radicals may adversely interact with biological systems causing cytotoxicity (Kumarasamy et al., 2007). In fact, overproduction of free-radicals has been implicated to cause various chronic diseases, e.g., cancer, atherosclerosis, ageing, diabetes and inflammatory diseases. Therefore, an external supply of free-radical scavengers is often necessary to maintain good health and prevent diseases. The TLC-based qualitative DPPH assay showed no free-radical scavenging activity in the PE extract of G. pilulifera; nevertheless, the DCM and the MeOH extracts were found to be active, indicated by the presence of yellowish-white spots against purple background after the TLC plate was sprayed with DPPH solution and kept for 30 min for the reaction to occur (Table 1) (Takao et al., 1994; Kumarasamy et al., 2002, 2007).
Free-radical-scavenging property of the extracts and fractions of G. pilulifera determined by the DPPH assay.
The DCM and the MeOH extracts were subjected to a quantitative DPPH assay (Takao et al., 1994; Kumarasamy et al., 2002, 2007) using 10-fold dilutions starting from 10 mg/ml of stock solutions. For the DCM extract, the 50% inhibition of DPPH absorbance at 517 nm was observed between the 1 mg/ ml and 0.1 mg/ml concentrations; while 50% inhibition was achieved by 0.1 mg/ml and 0.01 mg/ml of the MeOH extract. Accordingly, the DCM (1 mg/ml) and the MeOH (0.1 mg/ml) extracts were diluted one-fold to achieve concentrations, 0.1, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7, 0.8 and 0.9 mg/ml of the DCM extract, and 0.01-0.09 mg/ml of the MeOH extract, and were subjected to the quantitative DPPH assay (Takao et al., 1994; Kumarasamy et al., 2002, 2007). These further dilutions allowed construction of better graphs to calculate accurate RC50 values from the slope. The RC50 values of the DCM and the MeOH extracts were calculated as 4.08 × 10-1 and 2.15 × 10-2 mg/ml, respectively (Table 1). As the MeOH extract was clearly the most potent among the extracts, it was subjected to solid-phase extraction (SPE) on a C18 cartridge to obtain four SPE fractions, i.e., 20% (0.68 g), 50%, (0.073 g), 80% (0.064 mg) MeOH in water, and 100% (0.0083 mg) MeOH fractions, which were again subjected to qualitative and quantitative DPPH assay. The test revealed the SPE 50% fraction was the most potent of all (Table 1). This is the first report on the free-radical scavenging activity of G. pilulifera. However, there are only a handful of reports on free-radical scavenging or antioxidant activities of a few other Gypsophila species available to date, and the activity was shown to be associated with phenolic compounds, as flavonoids and their glycosides (Vitcheva et al., 2011; Huang et al., 2012).
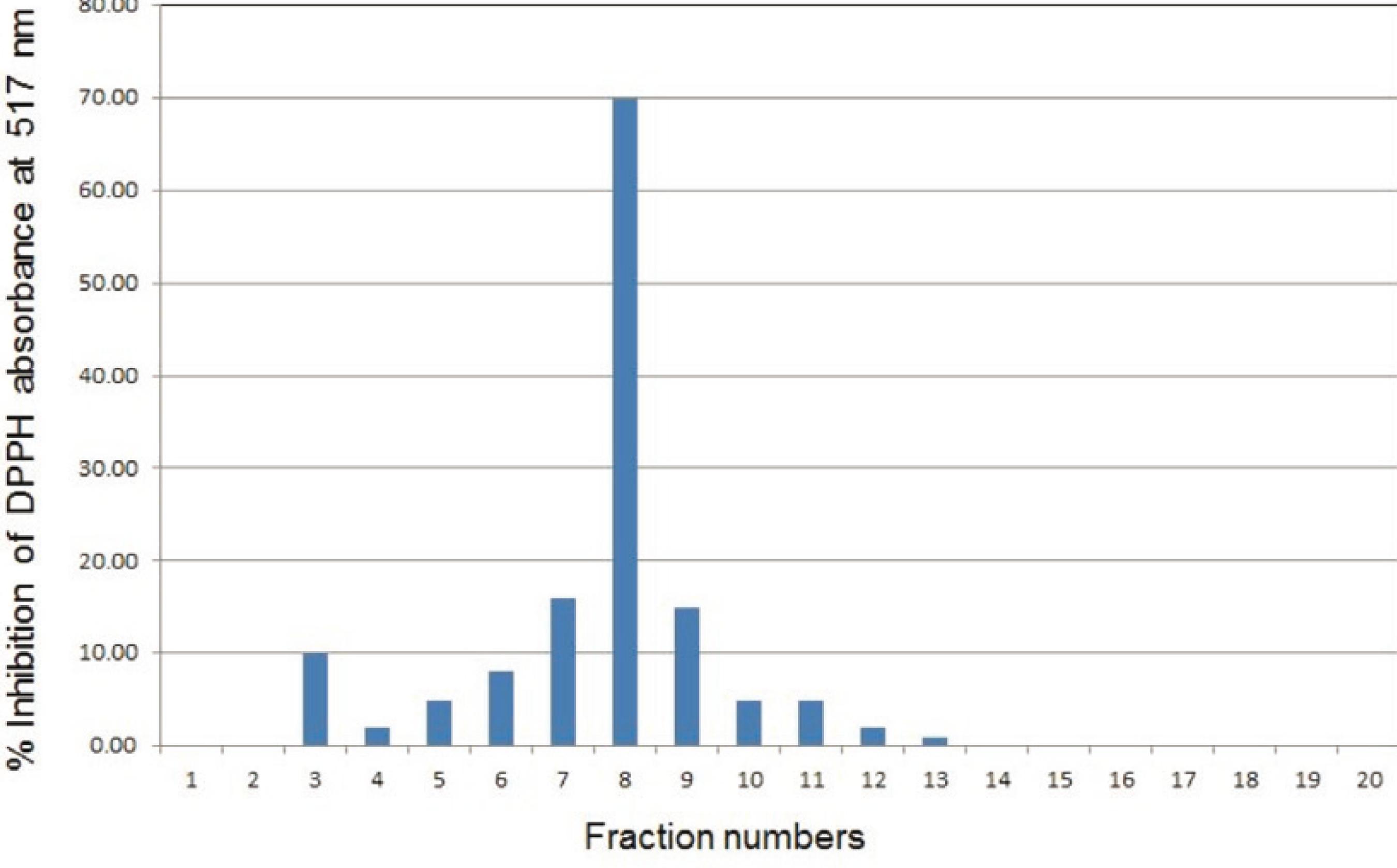
The SPE 50% MeOH-water fraction was initially analyzed by the reversed-phase HPLC coupled with a PDA detector followed by the DPPH assay, resulting in the most free-radical scavenging activity found in the fraction collected between 14-16 min (Fig. 1), and corresponding to the peak with the retention time (tR) of 15.3 min. UV-visible spectral analysis and the peak shape indicated that the peak might represent a single compound. Repetitive HPLC runs on this sample, and multiple collections of this peak afforded verbascoside (1).
Free-radical scavenging property of the HPLC fractions obtained from the active SPE fraction (50% MeOH in water). Each fraction collected refers to two minutes 1 = 0-2 min, 2 = 2-4 min, and 20 = 38-40 min. Fraction 8 (14 -16 min) contains verbascoside (1).
To the best of our knowledge, this is the first report on the occurrence of the phenylethanoid glycoside, verbascoside (1), in any species of the genus Gypsophila. The free-radical scavenging activity of verbascoside (1) was determined by the DPPH assay (RC50 1.25 × 10-3 mg/ml), and it was found to be more potent than the positive control, quercetin (RC50 2.50 × 10-3 mg/ml) (Table 1). Verbascoside (1) is a well known bioactive compound with a variety of reported bioactivities. Although it may not be within the scope of this article to review its various bioactivities (including anti-oxidant properties) previously reported, a brief summary of bioactivities of this compound published during the year 2013 are presented in Chart 1.
The results obtained in this study revealed that the significant free-radical scavenging activity of G. pilulifera was predominantly, if not exclusively, associated with the polar extract, e.g. MeOH, and verbascoside (1), a well-known bioactive natural product, was the major free-radical scavenger present in this plant. The results also established that G. pilulifera could be used as a new source for this bioactive compound (1).
-
Authors' contributionsNKC (MPharm student) carried out the main laboratory work as part of her final year research project. RRTM obtained the NMR data of verbascoside and contributed to its identification. SC collected the plant materials, identified it properly, and prepared the herbarium sample for deposition. LN and SDS are the supervisors of this project, provided intellectual input and prepared the manuscript. All the authors have read the final manuscript and approved the submission.
Acknowledgment
Mass spectroscopic analysis on the isolated compound was performed at the EPSRC National Mass Spectrometry Service Centre in Swansea, Wales, UK.
REFERENCES
- AbouZid, S.F., Sameh, F., Wahba, H.M., Elshamy, A., Cos, P., Maes, L., Apers, S., Pieters, L., Shahat, A.A., 2013. Antimicrobial activity of some Clerodendrum species in Egypt. Nat. Prod. Res. 27, 1032-1036.
- Arslan, I., Celik, A., Chol, J.H.A., 2012. Cytotoxic triterpenoid saponin from under-ground parts of Gypsophila pilulifera Boiss.& Heldr. Fitoterapia 83, 699-703.
- Brandao, G.C., Kroon, E.G., Souza, D.E.R., Souza, J.D., Oliveira, A.B., 2013. Chemistry and antiviral activity of Arrabidaea pulchra (Bigoniaceae). Molecules 18, 9919-9932.
- Carrillo-Ocampo, D., Bazaldua-Gomez, S., Bonilla-Barbosa, J.R., Abuto-Amar, R., Rodriguez-Lopez, V., 2013. Anti-inflammatory activity of iridoids and verbascoside isolated from Castilleja tebuiflora. Molecules 18, 12109-118.
- Cespedes, C.L., Munoz, E., Salazar, J.R., Yamaguchi, L., Werner, E., Alarcon, J., Kubo, I., 2013. Inhibition of cholinesterase activity by extracts, fractions and compounds from Calceilaria talcana and C. integrifolia (Calceolariaceae: Scrophulariaceae). Food Chem. Toxicol. 62, 919-926.
- Chen, Q., Luo, J-G., Kong, L-Y., 2011. New triterpenoid saponins from the roots of Gypsophila perfoliata Linn. Carbohyd. Res. 346, 2206-2212.
- Delazar, A., Gibbons, S., Kumarasamy, Y., Nahar, L., Shoeb, M., Sarker, S.D., 2005. Antioxidant phenylethanoid glycosides from the rhizomes of Eremostachys glabra (Lamiaceaea). Biochem. Syst. Ecol. 33, 87-90.
- Delazar, A., Delnavazi, M-R., Yassa, N., Parkhideh, S., Delazar, N., Nahar, L., Sarker, S.D., 2012. Essential oil composition and isolation of free-radical-scavenging phenolic glycosides from the aerial parts of Ajuga chamaepitys (L.) Schreb. (Lamiaceae) growing in Iran. Rev. Bras. Farmacogn. 22, 299-305.
- Gevrenova, R., Stancheva, T., Voynikova, Y., Laurain-Mattar, D., Henry, M., 2010. Root in vitro cultures of six Gypsophila species and their saponin contents. Enzyme Microb. Tech. 47, 97-104.
- Hostettmann, K., Marston, A., 1995. Saponins. In Chemistry and Pharmacology of Natural Products; Phillipson, J.D., Ayres, D.C., Baxter, H. Eds. Cambridge University Press: Cambridge, p. 326-327.
- Huang, Q.F., Zhang, S.J., Zheng, L., Liao, M., He, M., Huang, R.B., Zhuo, L., Lin, X., 2012. Protective effect of isoorientin 2''-O-αarabinopyranosyl isolated from Gypsophila elegans on alcohol induced hepatic fibrosis in rats. Food Chem. Toxicol. 50, 1992-2001.
- Kumarasamy, Y., Fergusson, M., Nahar, L., Sarker, S.D., 2002. Biological activity of moschamindole from Centaurea moschata. Pharm. Biol. 40: 307-310.
- Kumarasamy, Y., Byres, M., Cox, P.J., Jaspars, M., Nahar, L., Sarker, S.D., 2007. Screening seeds of some Scottish plants for free-radical scavenging activity. Phytother. Res. 21, 615-621.
- Munoz, E., Avila, J.G., Alarcon, J., Kubo, I., Werner, E., Cespedes, C.L., 2013. Tyrosinase inhibitors from Calceolaria integrifolia s.l.: Calceolaria talcana aerial parts. J. Agric. Food Chem. 61, 4336-4343.
- Nazemiyeh, H., Rahman, M.M., Gibbons, S., Nahar, L., Delazar, A., Ghahramani, M-A., Talebpour, A-H., Sarker, S.D., 2008. Assessment of the antibacterial activity of phenylethanoid glycosides from Phlomis lanceolata against multiple-drugresistant strains of Staphylococcus aureus. J. Nat. Med. 62, 91-92.
- Oyourou, J.N., Combrinck, S., Regnier, T., Marston, A., 2013. Purification, stability and antifungal activity of verbascoside from Lippia javanica and Lantana camara leaf extracts. Ind. Crop. Prod. 43, 820-826.
- Quirantes-Pine, R., Herranz-Lopez, M., Funes, L., Borras-Linares, I., Micol, V., Segura-Carretero, A., Fernandez-Gutierrez, A., 2013. Phenylpropanoids and their metabolites are the major compounds responsible for blood-cell protection against oxidative stress after administration of Lippia citriodora in rats. Phytomedicine 20, 1112-1118.
- Sanchez, P.M., Villareal, M.L., Herrera-Ruiz, M., Zamilpa, A., Jimenez-Ferrer, E., Trejo-Tapia, G., 2013. In vivo anti-inflammatory and anti-ulcerogenic activities of extracts from wild growing and in vitro plants of Castilleja tenuiflora Benth. (Orobanchaceae). J. Ethnopharmacol. 150, 1032-1037.
- Sarker, S.D., Shoeb, M., Celik, S., Jaspars, M., Nahar, L., KongThoo-Lin, P., MacManus, S.M., 2007. Extracts of Centaurea bornmuelleri and Centaurea huber-morathii inhibit the growth of colon cancer cells in vitro. Oriental Pharm. Experimental Med. 7, 336-340.
- Sarker, S.D., Nahar, L., Gujja, S., Begum, S., Celik, S., 2012. Bioactivity of Centaurea persica Boiss. (Asteraceae). Arch. Biol. Sci. 64, 517-523.
- Sauvage, S., Granger, M., Samson, E., Majumdar, A., Nigam, P., Nahar, L., Celik, S., Sarker, S.D., 2010. Assessment of freeradical-scavenging and antibacterial activities, and brine shrimp toxicity of Scutellaria pinnatifida (Lamiaceae). Oriental Pharm. Experimental Med. 10, 304-309.
- Shoeb, M., Celik, S., Jaspars, M., Kumarasamy, Y., MacManus, S.M., Nahar, L., Thoo-Lin, P.K., Sarker, S.D., 2005. Isolation, structure elucidation and bioactivity of schischkiniin, a unique indole alkaloid from the seeds of Centaurea schischkini. Tetrahedron 61, 9001-9006.
- Shoeb, M., MacManus, S.M., Jaspars, M., Kong-Thoo-Lin, P., Nahar, L., Celik, S., Sarker, S.D., 2007a. Bioactivity of two Turkish Centaurea species, and their major constituents. Rev. Bras. Farmacogn. 17, 155-159.
- Shoeb, M., Jaspars, M., MacManus, S.M., Celik, S., Nahar, L., KongThoo-Lin, P., Sarker, S.D., 2007b. Anti-colon cancer potential of phenolic compounds from the aerial parts of Centaurea gigantea (Asteraceae). J. Nat. Med. 61, 164-169.
- Shoeb, M., Jaspars, M., MacManus, S.M., Celik, S., Nahar, L., Kong-Thoo-Lin, P., Sarker, S.D., 2007c. Two salonitenolide derivatives from the aerial parts of Centaurea gigantea inhibit the growth of colorectal cancer cells in vitro. Nat. Prod. Comm. 2, 121-125.
- Shoeb, M., MacManus, S.M., Celik, S., Jaspars, M., Kong-Thoo-Lin, P., Nahar, L., Sarker, S.D., 2007d. Bioactivity of the extracts and the isolation of lignans and a sesquiterpene from the aerial parts of Centaurea pamphylica (Asteraceae). DARU 15, 118-122.
- Shoeb, M., MacManus, S.M., Jaspars, M., Nahar, L., Kong-Thoo-Lin, P., Celik, S., Sarker, S.D., 2007e. Lignans and flavonoids from the seeds of Centaurea bornmuelleri Hausskn. Ex. Bornm. and Centaurea huber-morathii Wagenitz. Pol. J. Chem. 81, 39-44.
- Shun, Y., Jian-Guang, L., Li, M., Ling-Yi, K., 2011. Two new triterpenoid saponins from the roots of Gypsophila paniculata with potent α-glucosidase inhibition activity. Chinese J. Nat. Med. 9, 401-406.
- Si, C.L., Liu, S.C., Hu, H.Y., Jiang, J.Z., Yu, G.J., Ren, X.D., Xu, G.H., 2013. Activity-guided screening of the antioxidants from Paulownia tomentosa var. tomentosa bark. Bioresources 8, 828-637.
- Suntar, I., Akkol, K., Keles, H., Yesilada, E., Sarker, S.D., Arroo, R., Baykal, T., 2012a. Efficacy of Daphne oleoides subsp. kurdica used for wound healing: identification of active compounds through bioassay guided isolation technique. J. Ethnopharmacol. 141, 1058-1070.
- Suntar, I., Akkol, K., Keles, H., Yesilada, E., Sarker, S.D., Baykal, T., 2012b. Comparative evaluation of traditional prescriptions from Cichorium intybus L. for wound healing: Stepwise isolation of an active component by in vivo bioassay and its mode of activity. J. Ethnopharmacol. 143, 299-309.
- Takao, T., Watanabe, N., Yagi, I., Sakata, K., 1994. A simple screening method for antioxidants and isolation of several antioxidants produced by marine bacteria from fish and shellfish. Biosci. Biotech. Bioch. 58, 1780-1783.
- Tokar, M., Klimek, B., 2004. Isolation and identification of biologically active compounds from Forsythia viridissima flowers. Acta Pol. Pharm. 61, 191-197.
- Vitcheva, V., Simeonova, R., Krasteva, I., Yotova, M., Nikolov, S., Mitcheva, M., 2011. Hepatoprotective effects of saponarin, isolated from Gypsophila trichotoma Wend. on cocaine-induced oxidative stress in rats. Redox Rep. 16, 56-61.
- Xiong, W.T., Gu, L., Wang, C., Sun, H.X., Liu, X., 2013. Antihyperglycemic and hypolipidemic effects of Cistanche tubulosa in type 2 diabetic db/db mice. J. Ethnopharmacol. 150, 935-945.
- Yotova, M., Krasteva, I., Jenett-Siems, K., Zdraveva, P., Nikolov, S., 2012. Triterpenoids in Gypsophila trichotoma Wend. Phytochem. Lett. 5, 752-755.
- Zhao, X.H., Yue, H.L., Li, P., Zeng, X., Zhang, G., 2013. Evaluation of the antitumor activity by CdTe QDs with verbascoside. Nano 8, DOI: 10.1142/S1793292013500318.
» https://doi.org/10.1142/S1793292013500318
Publication Dates
-
Publication in this collection
Jan-Feb 2014
History
-
Received
31 Oct 2013 -
Accepted
08 Mar 2014