Abstract
Herba Cistanche (Cistanche species) in Traditional Chinese Medicine is used for the treatment of several diseases and symptoms, to include pain. The objective of this study was to evaluate the antinociceptive effect of the hydroethanol extract of Cistanche salsa (C.A.Mey.) Beck, Orobanchaceae, stolons in animal models of pain. Chemical composition of Herba Cistanche was analyzed by HPLC-UV. Mice Swiss Webster (25–30 g, n = 6) were orally pre-treated with Herba Cistanche (10, 30 or 100 mg/kg) and evaluated in the formalin test and in the capsaicin- or glutamate-induced licking response. Kazakh Herba Cistanche is composed mainly by phenylpropanoid glycosides, from which echinacoside, acteoside and tubuloside B are the main constituents. When Herba Cistanche was administered to mice it had an effect in both phases of the formalin test (77% activity at 30 mg/kg for phase 1 and 62% activity at 100 mg/kg for phase 2) suggesting analgesic and anti-inflammatory properties. Kazakh Herba Cistanche was able to reduce the animals licking time after injection of glutamate (81% reduction at 30 mg/kg) and capsaicin (81% reduction at 100 mg/kg). We conclude that phenolics present in the hydroethanol extract of C. salsa could be responsible for its pharmacological profile. In order to source a good quality raw material for Traditional Chinese Medicine we recommended this Kazakh species to be standardized using echinacoside and acteoside as markers.
Keywords
Herba Cistanche; HPLC-UV; Echinacoside; Acteoside; Tubuloside; Standardization
Introduction
Cistanche salsa (C.A. Mey) Beck, Orobanchaceae, is a parasitic plant from the Republic of Kazakhstan where it is used as industrial feedstock (Sarsenbayev et al., 2011Sarsenbayev, K.N., Isabaev, S.O., Kolosov, N.G., 2011. Proceedings of the International Scientific Conference “Modern Ecological State of the Aral Sea Region, the Prospects for Solving Problems” , Kyzylorda, pp. 195–200.; Grudzinskaya and Gemedzhieva, 2012Grudzinskaya, L.M., Gemedzhieva, N.G., 2012. List of Medicinal Plants in Kazakhstan. Publishing House of the Institute of Botany and Phytointroduction of the RK, Almaty.). The scientific value of this herb in Traditional Chinese Medicine relates to the treatment of kidney problems (varying from pain to insufficiency), impotence, female infertility, morbid leucorrhea, profuse metrorrhagia and senile constipation (Jiangsu New Medical College Dictionary of Traditional Chinese Drugs, 1977Jiangsu New Medical College Dictionary of Traditional Chinese Drugs, 1977. 1st ed., Shanghai Scientific & Technologic Publisher, Shanghai.; Chinese Medicinal Herbal, 1988Chinese Academy of Medical Sciences, 1988. Institute of Medicinal Plants, Chinese Medicinal Herbal, vol. 4., 2nd ed. People's Medical Publishing House, Beijing.). The chemical composition of stolons of other species of Cistanche has already been studied in detail by Chinese scientists. The following phenolic compounds were identified: echinacoside, tubuloside, acteoside, besides lignans, iridoids and a complex polysaccharide (Yong and Tu, 2009Yong, J., Tu, P.F., 2009. Analysis of chemical constituents in Cistanche species. J. Chromatogr. A 1216, 1970-1979.; Zhang et al., 2003Zhang, X., Li, X., Rena, K., Du, N.S., 2003. RP-HPLC determination of echinacoside and acteoside in Herba Cistanches cultivated on different parasitic species and habitats. Chin. J. Pharm. Anal. 23, 254-256.; Xie et al., 2005Xie, J.N., Zhao, M.B., Wu, F.W., Tu, P.F., 2005. Chromatographic fingerprint of Cistanche deserticola by HPLC. Chin. Trad. Herb. Drugs 36, 268-271.; Jiang et al., 2009Jiang, Y., Li, S.P., Wang, Y.T., Chen, X.J., Tu, P.F., 2009. Differentiation of Herba Cistanches by fingerprint with high-performance liquid chromatography-diode array detection-mass spectrometry. J. Chromatogr. A 1216, 2156-2162.; Sui et al., 2011Sui, Z.F., Gu, T.M., Liu, B., Peng, S.W., Zhao, Z.L., Le, L., Shi, D.F., Yang, R.Y., 2011. Water-soluble carbohydrate compound from the bodies of Herba Cistanche: isolation and its scavenging effect on free radical in skin. Carbohydr. Polym. 85, 75-79.; Liu et al., 2013Liu, X.M., Li, J., Jiang, Y., Zhao, M.B., Tu, P.F., 2013. Chemical constituents from Cistanche sinensis (Orobanchaceae). Biochem. Syst. Ecol. 47, 21-24.; Zhou et al., 2014Zhou, J., Zhang, Q., Bing Sun, J., Li Sun, X., Zeng, P., 2014. Two-phase hollow fiber liquid phase microextraction based on magnetofluid for simultaneous determination of echinacoside, tubuloside B, acteoside and isoacteoside in rat plasma after oral administration of Cistanche salsa extract by high performance liquid chromatography. J. Pharm. Biomed. Anal. 94, 30-35.).
Shimoda et al. (2009)Shimoda, H., Tanaka, J., Takahara, Y., Takemoto, K., Shan, S.J., Su, M.H., 2009. The hypocholesterolemic effects of Cistanche tubulosa extract, a Chinese traditional crude medicine, in mice. Am. J. Chin. Med. 37, 1125-1138. showed that this herb possesses hypocholesterolemic effect while Yang et al. (2013)Yang, F.R., Wen, D.S., Fang, B.W., Lou, J.S., Meng, L., 2013. Prevention of Cistanche salsa extract on hepatic fibrosis induced by carbon tetrachloride in rats. Chin. Herb. Med. 5, 199-204. determined its hepatoprotective activity. Nan et al. (2013)Nan, Z.D., Zeng, K.W., Shi, S.P., Zhao, M.B., Jiang, Y., Tu, P.F., 2013. Phenylethanoid glycosides with anti-inflammatory activities from the stems of Cistanche deserticola cultured in Tarim desert. Fitoterapia 89, 167-174. reported the anti-inflammatory activity for its extracts. A complex polysaccharide previously isolated from this plant showed immunomodulatory effects (Wang et al., 2009Wang, X.Y., Qi, Y., Cai, R.L., Li, X.H., Yang, M.H., Shi, Y., 2009. The effect of Cistanche deserticola polysaccharides (CDPS) on marcrophages activation. Chin. Pharmacol. Bull. 25, 787-789.).
Although there is a considerable amount of Cistanche stolons in the Republic of Kazakhstan, there is no popular use for this plant by the Kazakh population although the neighbours in China extensively use it. Due to the fact that there is a considerable problem nowadays in relation to the substitution/adulteration of medicinal plants and the increasing need to standardize medicines for the use in Traditional Chinese Medicine, this study was designed to ascertain the phenolic chemical composition of this raw plant material growing in the neighbouring Kazakhstan as well as to assess its use as antinociceptive to potentially ease pain – which characterizes one of its main use in Traditional Chinese Medicine.
Materials and methods
Plant material
Cistanche salsa (C.A.Mey.) Beck, Orobanchaceae, stolons were collected at the desert of Moinkum, Village of Bakanas, in July 2014 in Almaty. The plant material was identified by Dr. G. Sitpayeva from the Institute of Botany and Phytointroduction, Ministry of Education and Sciences of the Republic of Kazakhstan where it was deposited under the number 01-04/306.
Chemical analysis
Stolons of C. salsa (2 g) were submitted to a microwave extraction. Initially the material was ground to 0.001–2.000 mm and then placed in a hermetic vessel to be extracted for 10 min at 100–1100 °C with ethanol 80% (ratio 1:10). The hydroethanol extract (CSHE) was analyzed by HPLC-MS. Crude extract was dissolved in methanol (9.6 mg/ml) and filtered through a 0.45 mm Teflon membrane prior to the analysis. A liquid chromatograph HP 1100 Series model (company Agilent Technologies, Inc., CA, USA), equipped with a flowing vacuum degasser, a four-channel low-pressure gradient pump, and an automatic injector. Phenolic compounds were chromatographically separated by a column Zorbax Eclipse XDB-C18, 2.1 × 50 mm, filled with octadecylsilyl silica gelpolymer (1.8 µ). Chromatographic analysis was carried out with a mobile phase flow of 0.2 ml/min, eluent operating pressure of 175–200 kPa, column oven temperature of 30 °C, 2 ml sample volume, gradient eluent feed mode: 0–36 min 10% A – 90% B; 36 min – 100% B (eluent A: methanol, eluent B: 0.2% formic acid solution). Detection was performed by UV at the wavelengths of 254, 334, 350, 410, 450 and 550 nm. Comparison of the obtained retention times, UV and mass spectra with those of reference compounds was used for the identification of the chemical compounds in the extract. Quantitative analysis was performed by the use of standard verbascoside and echinacoside analyzed under the same chromatographic conditions. Their calibration curves allowed for the calculation of the quantity of each other phenylpropanoid glycoside in the ethanol extract of this plant. The method was not validated.
Animals
Swiss Webster mice (20–25 g, two months old) were used in this study (donated by Instituto Vital Brazil, Niterói, RJ, Brazil). The animals were kept in standard conditions (light-dark cycle of 12 h, 22 ± 2 °C and 70–80% humidity. Food and water ad libitum). Animals received only water in order to avoid food interference with substance absorption 12 h prior to the onset of the experiments. Acclimatization to the laboratory conditions happened for at least 1 h before each test. All protocols were conducted in accordance with the Guidelines on Ethical Standards for Investigation of Experimental Pain in Animals and followed the principles and guidelines adopted by the National Council for the Control of Animal Experimentation (CONCEA), approved by the Biomedical Science Institute/UFRJ, Ethical Committee for Animal Research, and received the number DFBCICB015-04/16. All experimental protocols were performed during the light phase. Animal numbers per group were kept at a minimum and according to rules from CONCEA. At the end of each experiment mice were killed by ketamine/xylazine overdose.
Treatments
In this study CSHE was evaluated at 10, 30 and 100 mg/kg. The extract was dissolved in dimethyl sulfoxide (DMSO, Fisher Biotech) in order to prepare a stock solution at 100 mg/ml. PBS was used as diluent for the preparation of the different doses. Solutions containing 10, 30 and 100 mg/kg of the hydroethanol extract of C. salsa were prepared. The standard drugs used were morphine 2.5 mg/kg (Merck, diluted in phosphate buffer saline (PBS)), acetylsalicylic acid 200 mg/kg (Sigma Aldrich, dissolved with 5 M of sodium hydroxide (NaOH) in 0.9% saline) and capsazepine 10 nMol per paw. Saline plus DMSO (at the same concentration as in the highest treatment with extract) was given to the negative control group. All treatments (tested extract and standards) were administered by oral route. The only exception was capsazepine which was administered by intraplantar injection.
Formalin-induced acute pain
A solution of 2.5% formalin (37% formaldehyde) was injected (20 µl) in the plantar region of the right hind paw of mice 60 min after treatment (hydroethanol extract of C. salsa or acetylsalicylic acid 200 mg/kg or morphine 2.5 mg/kg) (Matheus et al., 2005Matheus, M.E., Berrondo, L.F., Vieitas, E.C., Menezes, F.S., Fernandes, P.D., 2005. Evaluation of the antinociceptive properties from Brillantaisia palisotii Lindau stems extracts. J. Ethnopharmacol. 102, 377-381.). The animals were individually placed in a transparent glass chamber and the duration of time in seconds that they spent licking their paw after injection of formalin was recorded and analyzed over two separate periods, early phase-neurogenic pain (0–5 min after injection) and late phase-inflammatory pain (15–30 min after injection).
Nociception induced by capsaicin
This test was based on the method described by Sakurada et al. with some modifications (Sakurada et al., 1992Sakurada, T., Katsumata, K., Tanno, K., Sakurada, S., Kisara, K., 1992. The capsaicin test in mice for evaluating tachykinin antagonists in the spinal-cord. Neuropharmacology 31, 1279-1285.). Capsaicin (20 µl) C18H27NO3 (Galena, Campinas, SP) was injected in the plantar region of the right hind paw of the mice (1.6 µg/paw) one hour after treatment (hydroethanol extract of C. salsa or capsazepine 10 nMol/paw). The animals were individually placed in a glass chamber and paw-licking duration (s) was recorded between 0 and 5 min after the capsaicin injection and then analyzed.
Glutamate-induced nociception
In the method described by Beirith et al. (2002)Beirith, A., Santos, A.R.S., Calixto, J.B., 2002. Mechanisms underlying the nociception and paw oedema caused by injection of glutamate into the mouse paw. Brain Res. 924, 219-228., glutamate solution in PBS (20 µl) (L-glutamic acid, Sigma–Aldrich, 3.7 ng/paw) was injected in the plantar region of the right hind paw of the mice one hour after treatment (hydroethanol extract of C. salsa or morphine 2.5 mg/kg). The animals were placed individually in a glass chamber and paw-licking duration (seconds) was recorded between 0 and15 min after the glutamate injection and then analyzed.
Statistical analysis
The chemical data is presented as mean ± SD of five experiments. All pharmacological experimental groups consisted of a minimum of six mice. Analysis of one-way variance (ANOVA) followed by Dunnett's test allowed the visualization of statistical significance between groups using GraphPad Prism 5.0 software. p values were considered significant when they were less than 0.05 (p < 0.05).
Results
C. salsa hydroethanol extract (two different dilutions) chromatogram is shown in Fig. 1. Table 1 shows the determination of the phenolic compounds together with their respective retention times in the chromatogram.
Effect of CSHE on formalin-induced acute pain
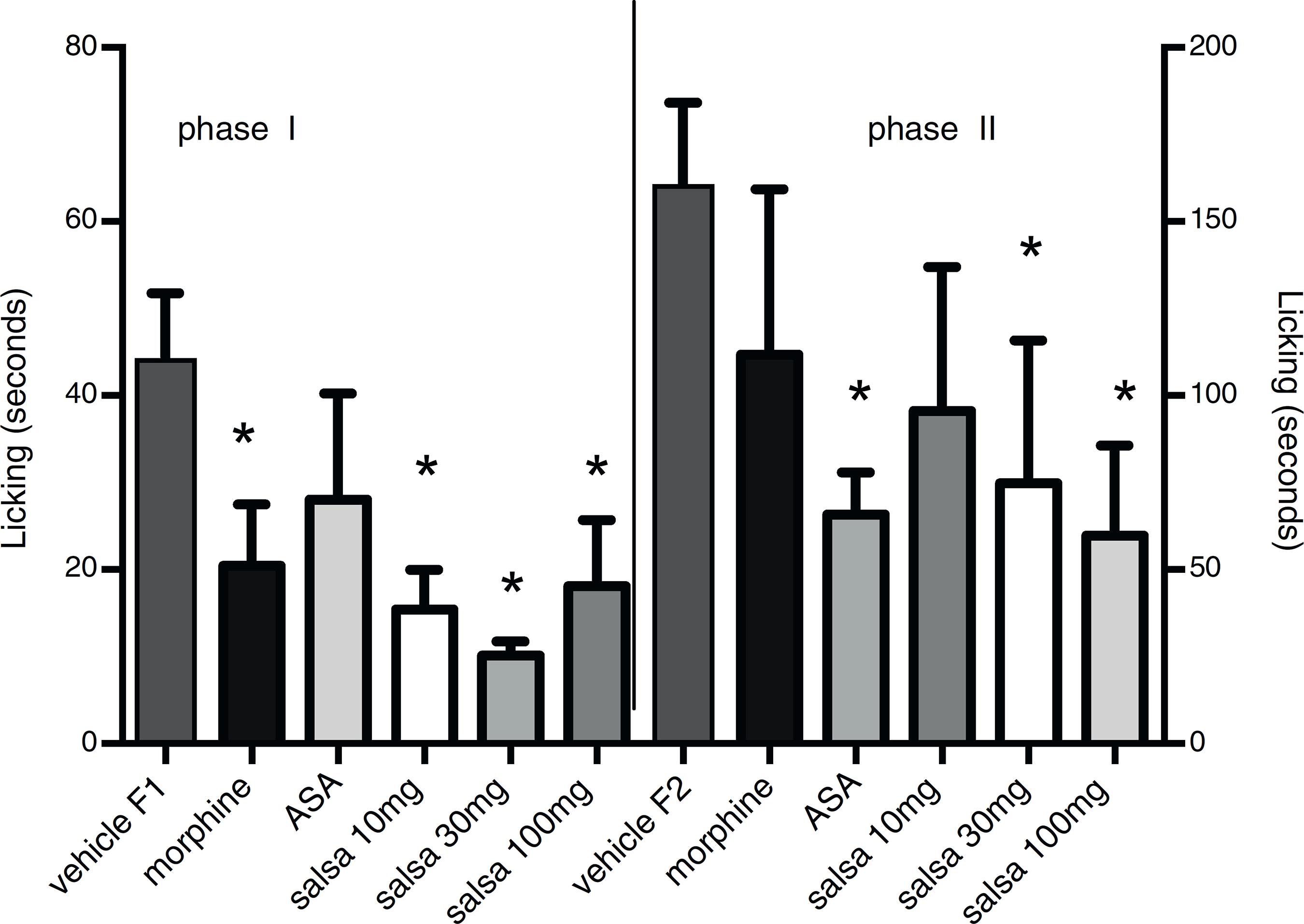
In the formalin-induced acute pain test, the CSHE at 10, 30 and 100 mg/kg were able to decrease paw-licking in the first phase of the test known as the neurogenic pain phase, these reductions were 60, 77 and 58%, respectively. In addition, they were also effective in reducing inflammatory pain induced during the second phase of the test, the percentage inhibition of inflammatory pain were 42, 46 and 62%, respectively (Fig. 2). Morphine (2.5 mg/kg) and acetylsalicylic acid (200 mg/kg) presented the following results: 55%/33% and 31%/54% for the first and second phases, respectively.
Effect of the CSHE on formalin-induced licking in mice. Animals were pre-treated with the CSHE 10 mg/kg, 30 mg/kg or 100 mg/kg (p.o.), acetylsalicylic acid 200 mg/kg (p.o.), morphine 2.5 mg/kg (p.o.) or vehicle (DMSO). Results are presented as mean ± S.D. (n = 6). One-way ANOVA with Dunnett's multiple comparison with vehicle, post-test (*p < 0.05). GraphPad Prism version 5.1.
Effect of CSHE on glutamate-induced nociception
The CSHE reduced the licking induced by glutamate at the three doses tested, 10, 30 and 100 mg/kg by 76, 81 and 53%, respectively (Fig. 3). Morphine at 2.5% resulted in 76% reduction.
Effect of CSHE on glutamate-induced nociception in mice. Animals were pre-treated with the CSHE 10 mg/kg, 30 mg/kg or 100 mg/kg (p.o.), morphine 2.5 mg/kg (p.o.) or vehicle (DMSO). Results are presented as mean ± S.D. (n = 6). One-way ANOVA with Dunnett's multiple comparison with vehicle, post-test (*p < 0.05). GraphPad Prism version 5.1.
Effect of CSHE on capsaicin-induced nociception
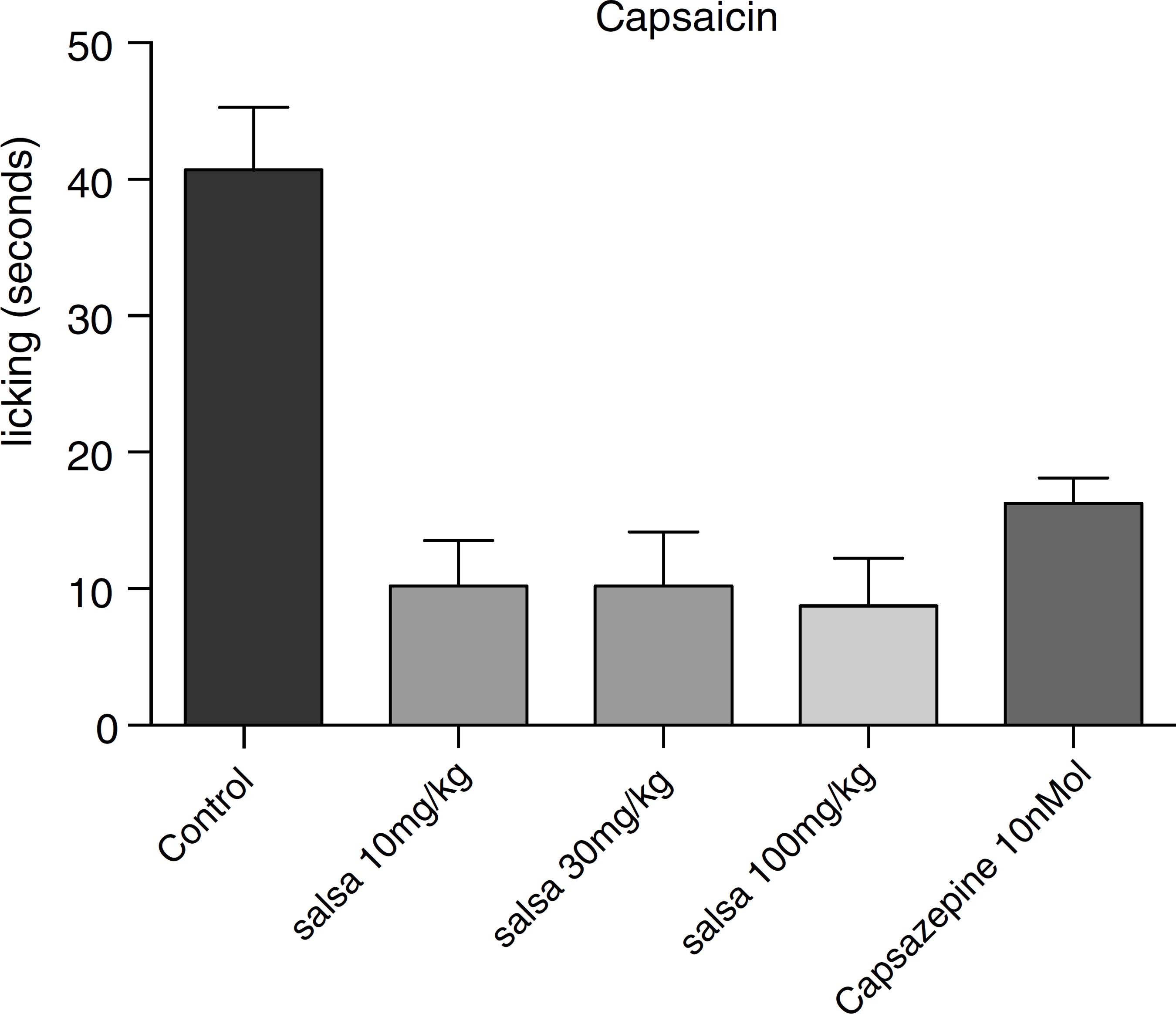
In order to verify if the CSHE would affect nociception through TRPV1 receptors, it was tested in a model of pain induced by capsaicin. The antinociceptive effect through this model was observed for the CSHE in the three tested doses, by 76, 79 and 81%, respectively (Fig. 4). Capsazepine 10 nMol/paw resulted in 60% reduction.
Effect of CSHE on capsaicin-induced nociception in mice. Animals were pre-treated with CSHE 10 mg/kg, 30 mg/kg or 100 mg/kg (p.o.) or vehicle (DMSO). Results are presented as mean ± S.D. (n = 6). One-way ANOVA with Dunnett's multiple comparison with vehicle, post-test (*p < 0.05). GraphPad Prism version 5.1.
Discussion
A total of ten phenylpropanoid glucosides were identified in the C. salsa (growing in Kazakhstan) hydroethanol extract. Echinacoside (10.98 mg/g), acteoside (9.44 mg/g) and tubuloside B (7.94 mg/g) were the major compounds identified. The obtained data was correlated with the available literature for species of Cistanche growing in different regions of Asia (Zhou et al., 2014Zhou, J., Zhang, Q., Bing Sun, J., Li Sun, X., Zeng, P., 2014. Two-phase hollow fiber liquid phase microextraction based on magnetofluid for simultaneous determination of echinacoside, tubuloside B, acteoside and isoacteoside in rat plasma after oral administration of Cistanche salsa extract by high performance liquid chromatography. J. Pharm. Biomed. Anal. 94, 30-35.; Xie et al., 2005Xie, J.N., Zhao, M.B., Wu, F.W., Tu, P.F., 2005. Chromatographic fingerprint of Cistanche deserticola by HPLC. Chin. Trad. Herb. Drugs 36, 268-271.; Jiang et al., 2009Jiang, Y., Li, S.P., Wang, Y.T., Chen, X.J., Tu, P.F., 2009. Differentiation of Herba Cistanches by fingerprint with high-performance liquid chromatography-diode array detection-mass spectrometry. J. Chromatogr. A 1216, 2156-2162.). Echinacoside and acteoside are the main components, which form the basis for standardization of the officinal species C. deserticola and C. tubulosa, listed in the Chinese Pharmacopoeia in 2005. We propose that these compounds could also be used for the standardization of C. salsa stolons from Kazakhstan, as they are the major ones in the studied hydroethanol extract.
According to the pharmacological results of this study it is possible to suggest that the CSHE is an effective agent against neurogenic pain and inflammation as observed in the formalin test. To the best of our knowledge C. salsa has never been tested in relation to its antinociceptive actions. A study from 2002 with C. deserticola (Lin et al., 2002Lin, L.W., Hsieh, M.T., Tsai, F.H., Wang, W.H., Wu, C.R., 2002. Anti-nociceptive and anti-inflammatory activity caused by Cistanche deserticola in rodents. J. Ethnopharmacol. 83, 177-182.) has shown that this plant presented antinociceptive and anti-inflammatory activities when assayed in models such as total writhing, formalin and paw oedema. Although there was no attempt to investigate the main constituents in the extracts that were active, they deemed the butanol extract and the water layer as the active ones. Not surprisingly these solvents are at the scale of polarity that matches the phenylpropanoid glycosides found in C. salsa. Moreover, Lin and co-workers established that the antinociceptive action of C. deserticola extracts was not due to the action of the compound in the opiate receptor or related to the immune system. In our study, the phenylpropanoid-rich extract showed activity in both phases of the formalin test. The activity observed was due in fact to the pharmacological target investigated and not to any possible motor alteration as evidenced by the rota rod test. Formalin test results indicate potential for neurogenic pain as well as pain induced by inflammatory mediators. Initially we decided to investigate the neurogenic pain route. Because similar compounds from other species previously studied did not show any opiate receptor activity, we decided to test other models, such as Glutamate and capsaicin models.
Glutamate is an excitatory neurotransmitter that has an important role in modulating pain throughout the peripheral and central nervous system. This action is mediated by ligand-gated ionotropic glutamate receptors (iGluRs) and metabotropic glutamate receptors. The iGluRs can be divided into N-methyl-D-aspartate (NMDA) and α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) (Kolber, 2015Kolber, B.J., 2015. mGluRs head to toe in pain. Prog. Mol. Biol. Transl. 131, 281-324.). Research has shown that when antagonizing NMDA and AMPA receptors with ketamine and kainate, respectively, an antinociception effect is observed. However, antagonists targeting these receptors so far have induced a substantial adverse effect and for this reason a new research focus on mGluRs hoping that medicine mediated through this receptor would cause less side effect has been carried out (Palazzo et al., 2014Palazzo, E., Marabese, I., de Novellis, V., Rossi, F., Maione, S., 2014. Supraspinal metabotropic glutamate receptors: a target for pain relief and beyond. Eur. J. Neurosci. 39, 444-454.). The results of testing the CSHE showed that this treatment was effective in reducing pain induced by glutamate. However, it is still unknown if this effect is mediated through ionotropic and/or metabotropic receptors. Morphine is one of the drugs available that has an effect on glutamatergic transmission (Deyama et al., 2007Deyama, S., Yamamoto, J., Machida, T., Tanimoto, S., Nakagawa, A.T., Kaneko, S., Satoh, M., Minami, M., 2007. Inhibition of glutamatergic transmission by morphine in the basolateral amygdaloid nucleus reduces pain-induced aversion. Neurosci. Res. 59, 199-204.). It is reported in the literature that glutamate receptor activation increases TRPV1 responses (Szteyn et al., 2015Szteyn, K., Rowen, M.P., Gomez, R., Du, J., Carlton, S.M., Jeske, N.A., 2015. A-kinase anchoring protein 79/150 coordinates metabotropic glutamate receptor sensitization of peripheral sensory neurons. Pain 156, 2364-2372.). For that reason, it is possible that the effect observed for CSHE could be due to activity on TRPV1 and not necessarily through a direct response on glutamate receptors.
The next step of this research was to carry out a paw-licking test using capsaicin as the algesic agent, which is a TRPV1 receptor agonist. The results showed that indeed CSHE decreased pain through TRPV1 receptor.
In the case of CSHE, almost in all the tested methodologies, we could observe that the dose of 30 mg/kg achieved better results than 100 mg/kg. This can be due to the saturation of the solution leading to precipitated compounds and effectively less amount of drugs being bioavailable.
Confirming the established chemical composition for the Kazakh extract CSHE was an important step because the major constituents present in it have already had their antinociceptive/anti-inflammatory actions somehow confirmed. For example, echinacoside was established as one of the active principles responsible for the antinociceptive action of Echinaceae (Hostettmann, 2003Hostettmann, K., 2003. History of a plant: the example of Echinacea. Forsch Komp. Klas. Nat. Suppl. 1, 9-12.). Also, a previous study by Schapoval and co-workers (Schapoval et al., 1998Schapoval, E.E.S., Vargas, M.R.W., Chaves, C.G., Bridi, R., Zuanazzi, J.A., Henriques, A.T., 1998. Antiinflammatory and antinociceptive activities of extracts and isolated compounds from Stachytarpheta cayennensis. J. Ethnopharmacol. 60, 53-59.) pointed out acteoside as one of the main active principles in an ethanol extract prepared with Stachytarpheta cayennensis as assessed by paw oedema and hot plate models. Backhouse and co-workers (Backhouse et al., 2008Backhouse, N., Delporte, C., Apablaza, C., Farias, M., Goïty, L., Arrau, S., Negrete, R., Castro, C., Miranda, H., 2008. Antinociceptive activity of Buddleja globosa (matico) in several models of pain. J. Ethnopharmacol. 119, 160-165.) also deemed acteoside to be the active principle of Buddleja globosa using several models of pain assessment, including formalin test. New formulations are now being developed using the state of the art knowledge to increase stability and prolong the antinociceptive action of acteoside (Isacchi et al., 2016Isacchi, B., Bergonzi, M.C., Iacopi, R., Ghelardini, C., Galeotti, N., Bilia, A.R., 2016. Liposomal formulation to increase stability and prolong antineuropathic activity of verbascoside. Planta Med. 83, 412-419.).
Conclusion
Ten substances of phenolic nature were identified in the stolons of the Kazakh C. salsa by HPLC/MS analysis. These compounds could be responsible for the antinociceptive activity observed in the experimental models performed herein. Taking into consideration that on the territory of Kazakhstan the genus Cistanche is represented mainly by species C. salsa, we can recommend its harvesting and standardization using echinacoside and acteoside as standard compounds, to source a good quality raw material for Traditional Chinese Medicine.
Ethical disclosures
Protection of human and animal subjects
The authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of data
The authors declare that no patient data appear in this article.
Right to privacy and informed consent
The authors declare that no patient data appear in this article.
Acknowledgements
The authors from Brazil thank Mr. Alan Minho for technical assistance and the Instituto Vital Brazil for donations of animals used. They also want to acknowledge the grants from CNPq and Fundação Carlos Chagas Filho de Apoio à Pesquisa do Estado do Rio de Janeiro. The authors from Ireland wish to acknowledge the High Education Authority's Programme for Research in Third-Level Institutions Cycle 5's funding support for TBSI and to reinforce the importance of SFI programme ISCA-Brazil (grant no. SFI/13/ISCA/2843) that contributed to the collaborative work between Brazil and Ireland. The authors from Kazakhstan want to acknowledge KazNMU for the financial support allowed to the execution of this work.
References
- Backhouse, N., Delporte, C., Apablaza, C., Farias, M., Goïty, L., Arrau, S., Negrete, R., Castro, C., Miranda, H., 2008. Antinociceptive activity of Buddleja globosa (matico) in several models of pain. J. Ethnopharmacol. 119, 160-165.
- Beirith, A., Santos, A.R.S., Calixto, J.B., 2002. Mechanisms underlying the nociception and paw oedema caused by injection of glutamate into the mouse paw. Brain Res. 924, 219-228.
- Chinese Academy of Medical Sciences, 1988. Institute of Medicinal Plants, Chinese Medicinal Herbal, vol. 4., 2nd ed. People's Medical Publishing House, Beijing.
- Deyama, S., Yamamoto, J., Machida, T., Tanimoto, S., Nakagawa, A.T., Kaneko, S., Satoh, M., Minami, M., 2007. Inhibition of glutamatergic transmission by morphine in the basolateral amygdaloid nucleus reduces pain-induced aversion. Neurosci. Res. 59, 199-204.
- Grudzinskaya, L.M., Gemedzhieva, N.G., 2012. List of Medicinal Plants in Kazakhstan. Publishing House of the Institute of Botany and Phytointroduction of the RK, Almaty.
- Hostettmann, K., 2003. History of a plant: the example of Echinacea. Forsch Komp. Klas. Nat. Suppl. 1, 9-12.
- Isacchi, B., Bergonzi, M.C., Iacopi, R., Ghelardini, C., Galeotti, N., Bilia, A.R., 2016. Liposomal formulation to increase stability and prolong antineuropathic activity of verbascoside. Planta Med. 83, 412-419.
- Jiang, Y., Li, S.P., Wang, Y.T., Chen, X.J., Tu, P.F., 2009. Differentiation of Herba Cistanches by fingerprint with high-performance liquid chromatography-diode array detection-mass spectrometry. J. Chromatogr. A 1216, 2156-2162.
- Jiangsu New Medical College Dictionary of Traditional Chinese Drugs, 1977. 1st ed., Shanghai Scientific & Technologic Publisher, Shanghai.
- Kolber, B.J., 2015. mGluRs head to toe in pain. Prog. Mol. Biol. Transl. 131, 281-324.
- Lin, L.W., Hsieh, M.T., Tsai, F.H., Wang, W.H., Wu, C.R., 2002. Anti-nociceptive and anti-inflammatory activity caused by Cistanche deserticola in rodents. J. Ethnopharmacol. 83, 177-182.
- Liu, X.M., Li, J., Jiang, Y., Zhao, M.B., Tu, P.F., 2013. Chemical constituents from Cistanche sinensis (Orobanchaceae). Biochem. Syst. Ecol. 47, 21-24.
- Matheus, M.E., Berrondo, L.F., Vieitas, E.C., Menezes, F.S., Fernandes, P.D., 2005. Evaluation of the antinociceptive properties from Brillantaisia palisotii Lindau stems extracts. J. Ethnopharmacol. 102, 377-381.
- Nan, Z.D., Zeng, K.W., Shi, S.P., Zhao, M.B., Jiang, Y., Tu, P.F., 2013. Phenylethanoid glycosides with anti-inflammatory activities from the stems of Cistanche deserticola cultured in Tarim desert. Fitoterapia 89, 167-174.
- Palazzo, E., Marabese, I., de Novellis, V., Rossi, F., Maione, S., 2014. Supraspinal metabotropic glutamate receptors: a target for pain relief and beyond. Eur. J. Neurosci. 39, 444-454.
- Sakurada, T., Katsumata, K., Tanno, K., Sakurada, S., Kisara, K., 1992. The capsaicin test in mice for evaluating tachykinin antagonists in the spinal-cord. Neuropharmacology 31, 1279-1285.
- Sarsenbayev, K.N., Isabaev, S.O., Kolosov, N.G., 2011. Proceedings of the International Scientific Conference “Modern Ecological State of the Aral Sea Region, the Prospects for Solving Problems” , Kyzylorda, pp. 195–200.
- Schapoval, E.E.S., Vargas, M.R.W., Chaves, C.G., Bridi, R., Zuanazzi, J.A., Henriques, A.T., 1998. Antiinflammatory and antinociceptive activities of extracts and isolated compounds from Stachytarpheta cayennensis J. Ethnopharmacol. 60, 53-59.
- Shimoda, H., Tanaka, J., Takahara, Y., Takemoto, K., Shan, S.J., Su, M.H., 2009. The hypocholesterolemic effects of Cistanche tubulosa extract, a Chinese traditional crude medicine, in mice. Am. J. Chin. Med. 37, 1125-1138.
- Sui, Z.F., Gu, T.M., Liu, B., Peng, S.W., Zhao, Z.L., Le, L., Shi, D.F., Yang, R.Y., 2011. Water-soluble carbohydrate compound from the bodies of Herba Cistanche: isolation and its scavenging effect on free radical in skin. Carbohydr. Polym. 85, 75-79.
- Szteyn, K., Rowen, M.P., Gomez, R., Du, J., Carlton, S.M., Jeske, N.A., 2015. A-kinase anchoring protein 79/150 coordinates metabotropic glutamate receptor sensitization of peripheral sensory neurons. Pain 156, 2364-2372.
- Wang, X.Y., Qi, Y., Cai, R.L., Li, X.H., Yang, M.H., Shi, Y., 2009. The effect of Cistanche deserticola polysaccharides (CDPS) on marcrophages activation. Chin. Pharmacol. Bull. 25, 787-789.
- Xie, J.N., Zhao, M.B., Wu, F.W., Tu, P.F., 2005. Chromatographic fingerprint of Cistanche deserticola by HPLC. Chin. Trad. Herb. Drugs 36, 268-271.
- Yang, F.R., Wen, D.S., Fang, B.W., Lou, J.S., Meng, L., 2013. Prevention of Cistanche salsa extract on hepatic fibrosis induced by carbon tetrachloride in rats. Chin. Herb. Med. 5, 199-204.
- Yong, J., Tu, P.F., 2009. Analysis of chemical constituents in Cistanche species. J. Chromatogr. A 1216, 1970-1979.
- Zhang, X., Li, X., Rena, K., Du, N.S., 2003. RP-HPLC determination of echinacoside and acteoside in Herba Cistanches cultivated on different parasitic species and habitats. Chin. J. Pharm. Anal. 23, 254-256.
- Zhou, J., Zhang, Q., Bing Sun, J., Li Sun, X., Zeng, P., 2014. Two-phase hollow fiber liquid phase microextraction based on magnetofluid for simultaneous determination of echinacoside, tubuloside B, acteoside and isoacteoside in rat plasma after oral administration of Cistanche salsa extract by high performance liquid chromatography. J. Pharm. Biomed. Anal. 94, 30-35.
Publication Dates
-
Publication in this collection
Sep-Oct 2017
History
-
Received
19 Apr 2017 -
Accepted
17 May 2017