ABSTRACT
Celtis iguanaea (Jacq.) Sarg., Cannabaceae, is popularly used in the treatment of diabetes mellitus. However, chemical and pharmacological investigations are lacking. In this study, we investigated the effects of the hydroalcoholic extract from C. iguanaea on markers of cardiovascular diseases and the glucose metabolism in cholesterol-fed rats. Therefore, hypercholesterolemic rats (1% cholesterol) were orally treated with C. iguanaea extract (C-150, CI-300, or CI-600 mg/kg) or simvastatin (4 mg/kg) (n = 6) once a day (30 days) with a hypercholesterolemic diet. A control group (C) was given saline. C. iguanaea extract showed significant decreases in serum levels of total cholesterol, LDL-cholesterol, HMG-CoA-reductase, interleukin-1 and 6, TNF-α and IFN-γ when compared to group C (p < 0.001). Hypoglycemic effects were observed along with a decrease of the activity of sucrase (CI-600), maltase (CI-150, CI-300), and an increase in muscle glycogen levels (CI-300). Antioxidant effects were observed in plasma by the decrease of TBARS and increase of nonprotein thiols levels (CI-600). The histopathological analysis showed a significant decrease in the liver fat area for C. iguanaea extract compared to group C (p < 0.001). Our results suggest that the biological effects of C. iguanaea extract could be related to the flavonoids that possibly exert antioxidant, enzymatic inhibitory, and insulin-mimetic effects.
Keywords:
Medicinal plants; Flavonoids; Hypoglycemic; Hypolipidemic; Antiatherogenic
Introduction
Cardiovascular diseases (CVD), a group of disorders of the heart and blood vessels, are considered the first cause of death globally (WHO, 2016aWHO, 2016a. Cardiovascular Diseases (CVDs). World Health Organization, Geneva.). The risk for developing CVD such as atherosclerosis increases significantly with elevated total cholesterol and LDL-cholesterol and decreased HDL-cholesterol values (American Heart Association, 2017American Heart Association, 2017. Prevention and Treatment of High Cholesterol (Hyperlipidemia). American Heart Association, Dallas.). In addition to hypercholesterolemia, oxidative stress plays an important role in the progress of atherosclerosis. Excessive production of reactive species contributes to converts LDL-cholesterol in oxidized LDL (oxLDL-C) that is recognized by macrophages. Macrophages activated by oxLDL-C induce further oxidative stress, which in turn, contributes to inflammatory response by secreting pro-inflammatory cytokines (Chávez-Sánchez et al., 2014Chávez-Sánchez, L., Espinosa-Luna, J.E., Chávez-Rueda, K., Legorreta-Haquet, M.V., Montoya-Díaz, E., Blanco-Favela, F., 2014. Innate immune system cells in atherosclerosis. Arch. Med. Res. 45, 1-14.).
Other risk factor for the development of CVD is diabetes, a chronic disease that occurs when the body is unable to produce or effectively use the hormones responsible for regulating blood glucose levels (WHO, 2016bWHO, 2016b. Global Report on Diabetes. World Health Organization, Geneva.). Diabetes can contribute to the occurrence of CVD such as hyperlipidemias, characterized by an excess of lipids, mainly cholesterol, triacylglycerides (TG), and low-density lipoprotein (LDL) (Aronow, 2013Aronow, W.A., 2013. Treatment of hypercholesterolemia. J. Clin. Exp. Cardiol. S1, 1-8.; Nirosha et al., 2014Nirosha, K., Divya, M., Vamsi, S., Sadiq, M., 2014. A review on hyperlipidemia. IJNTPS 4, 81-92.; WHO, 2016aWHO, 2016a. Cardiovascular Diseases (CVDs). World Health Organization, Geneva.).
For the treatment and prevention of hyperlipidemia, statins are the drugs of choice. However, these molecules can cause several side effects (Stroes et al., 2015Stroes, E.S., Thompson, P.D., Corsini, A., Vladutiu, G.D., Raal, F.J., Ray, K.K., Roden, M., Stein, E., Tokgõzoglu, L., Nordestgaard, B.G., Bruckert, E., Krauss, R.M., Laufs, U., Santos, R.D., Mãrz, W., Newman, C.B., Chapman, M.J., Ginsberg, H.N., Chapman, M., Ginsberg, H.N., Backer, G., Catapano, A.L., Hegele, R.A., Hovingh, G.K., Jacobson, T.A., Leiter, L., Mach, F., Wiklund, O., 2015. Statin-associated muscle symptoms: impact on statin therapy European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management. Eur. Heart J. 18, 2-13.). Various plants, which contain substances such as saponins, polyphenols, and flavonoids, have shown good results in reducing plasma lipid levels (Shimoda et al., 2006Shimoda, H., Seki, E., Aitani, M., 2006. Inhibitory effect of green coffee bean extract on fat accumulation and body weight gain in mice. BMC Complement. Altern. Med. 6, 9-13.), and recently, advances have been made in the search for natural products able to reduce hyperlipidemias with fewer side effects (Koriem, 2014Koriem, K.M.M., 2014. Antihyperlipidemic activity of the medicinal plants among Kadazan and Dusun communities in Sabah, Malaysia: a review. Asian Pac. J Trop. Biomed. 4, 768-779.; Mohamed et al., 2014Mohamed, G.A., Ibrahim, S.R.M., Elkhayat, E.S., El Dine, R.S., 2014. Natural anti-obesity agents. Bull. Fac. Pharm. Cairo Univ. 52, 269-284.; Waltenberger et al., 2016Waltenberger, B., Mocan, A., Šmejkal, K., Heiss, E.H., Atanasov, A.G., 2016. Natural products to counteract the epidemic of cardiovascular and metabolic disorders. Molecules 21, 1-33.).
Celtis iguanaea (Jacq) Sarg., Cannabaceae, is popularly known as “esporão-de-galo”, “taleira”, “sarã” and “gurrupiá”, according to the region where it is found in Brazil, and may develop in temperate or tropical regions (Silva and Proença, 2008Silva, C.S.P., Proença, C.E.B., 2008. Uso e disponibilidade de recursos medicinais no município de Ouro Verde de Goiás, GO, Brasil. Acta Bot. Bras. 22, 481-492.; Martins and Pirani, 2009Martins, E.G.A., Pirani, J.R., 2009. Flora da Serra do Cipó, Minas Gerais: Cannabaceae. Bol. Bot. Univ. São Paulo 27, 247-251.; Paula et al., 2010Paula, M.A., Couto, R.O., Bara, M.T.F., Rezende, M.H., Paula, J.R., Costa, E.A., 2010. Caracterização farmacognóstica da Celtis iguanaea (Jacq.) Sargent. Lat. Am. J. Pharm. 29, 526-533.). Previous studies showed the traditional use of leaves of C. iguanaea in the treatment of body pain, rheumatism, asthma, cramping, dyspepsia, urinary infections, and for the control of diabetes mellitus (Hernandez-Galicia et al., 2002Hernandez-Galicia, E., Aguilar-Contreras, A., Aguilar-Santamaria, L., Roman-Ramos, R., Chavez-Miranda, A.A., Garcia-Veja, L.M., Flores-Saenz, J.L., Alarcon-Aguilar, F.J., 2002. Studies on hypoglycemic activity of Mexican medicinal plants. Proc. West. Pharmacol. Soc. 45, 118-124.; Tene et al., 2007Tene, V., Malagóri, O., Finzi, P.V., Vidari, G., Armijos, C., Zaragoza, T., 2007. Anethnobotanical survey of medicinal plants used in Loja and Zamora-Chinchipe, Ecuador. J. Ethnopharmacol. 111, 63-81.; Silva and Proença, 2008Silva, C.S.P., Proença, C.E.B., 2008. Uso e disponibilidade de recursos medicinais no município de Ouro Verde de Goiás, GO, Brasil. Acta Bot. Bras. 22, 481-492.; Paula et al., 2010Paula, M.A., Couto, R.O., Bara, M.T.F., Rezende, M.H., Paula, J.R., Costa, E.A., 2010. Caracterização farmacognóstica da Celtis iguanaea (Jacq.) Sargent. Lat. Am. J. Pharm. 29, 526-533.; Martins et al., 2015Martins, J.L., Rodrigues, O.R., Sousa, F.B., Fajemiroye, J.O., Galdino, P.M., Florentino, I.F., Costa, E.A., 2015. Medicinal species with gastroprotective activity found in the Brazilian Cerrado. Fundam. Clin. Pharmacol. 29, 238-251.).
Recently, a gastro-protective effect of the hexane fraction of the ethanolic extract was observed using different models of gastric ulcers (Sousa et al., 2013Sousa, F.B., Martins, J.L.R., Florentino, I.F., Couto, R.O., Nascimento, M.V.M., Galdino, P.M., Ghedini, P.C., Paula, J.R., Costa, E.A., 2013. Preliminary studies of gastroprotective effect of Celtis iguanaea (Jacq.) Sargent leaves (Ulmaceae). Nat. Prod. Res. 27, 1102-1107.; Martins et al., 2014aMartins, J.L.R., Rodrigues, O.R.P., Silva, D.M., Galdino, P.M., Paula, J.R., Romão, W., Costa, H.B., Vaz, B.G., Ghedini, P.C., Costa, E.A., 2014. Mechanisms involved in the gastroprotective activity of Celtis iguanaea (Jacq.) Sargent on gastric lesions in mice. J. Ethnopharmacol. 155, 1616-1624.,bMartins, J.L.R., Sousa, F.B., Fajemiroye, J.O., Ghedini, P.C., Ferreira, P.M., Costa, E.A., 2014. Anti-ulcerogenic and antisecretory effects of Celtis iguanaea (Jacq.) Sargent hexane leaf extract. Rev. Bras. Plantas Med. 16, 250-255.). The extracts administered to mice and Artemia salina demonstrated no cytotoxic or genotoxic effects (Trevisan et al., 2012Trevisan, R.R., Lima, C.P., Miyazaki, C.M.S., Pesci, F.A., Silva, C.B., Hirota, B.C.K., Lordello, A.L.L., Miguel, O.G., Miguel, M.D., Zanin, S.M.W., 2012. Evaluation of the phytotoxic activity focused on the allelopathic effect of the extract from the bark of Celtis iguanaea (Jacq.) Sargent Ulmaceae and purification of two terpenes. Rev. Bras. Plantas Med. 14, 494-499.; Borges et al., 2013Borges, F.F.V., Machado, T.C., Cunha, K.S., Pereira, K.C., Costa, E.A., Paula, J.R., Chen-Chen, L., 2013. Assessment of thecytotoxic, genotoxic, and antigenotoxic activities of Celtis iguanaea (Jacq.) in mice. An. Acad. Bras. Ciênc. 85, 955-963.). Among the chemical constituents, the presence of pentacyclic triterpenes of type friedelano, friedelin, and epifriedelinol is reported (Trevisan et al., 2012Trevisan, R.R., Lima, C.P., Miyazaki, C.M.S., Pesci, F.A., Silva, C.B., Hirota, B.C.K., Lordello, A.L.L., Miguel, O.G., Miguel, M.D., Zanin, S.M.W., 2012. Evaluation of the phytotoxic activity focused on the allelopathic effect of the extract from the bark of Celtis iguanaea (Jacq.) Sargent Ulmaceae and purification of two terpenes. Rev. Bras. Plantas Med. 14, 494-499.). Preliminary phytochemical analyses of leaves and stems of C. iguanaea also revealed the presence of coumarins, mucilage, and flavonoids (Paula et al., 2010Paula, M.A., Couto, R.O., Bara, M.T.F., Rezende, M.H., Paula, J.R., Costa, E.A., 2010. Caracterização farmacognóstica da Celtis iguanaea (Jacq.) Sargent. Lat. Am. J. Pharm. 29, 526-533.).
Flavonoids have demonstrated multiple pharmacological effects, including anti-inflammatory, antioxidant, anti-cancer, hypoglycemic, and hypolipidemic activities (Lago et al., 2014Lago, J.H.G., Arruda, A.C.T., Mernak, M., Barrosa, K.H., Martins, M.A., Tibério, I.F.L.C., Prado, C.M., 2014. Structure-activity association of flavonoids in lung diseases. Molecules 19, 3570-3595.; Zhang et al., 2016Zhang, Y., Chen, S., Wei, C., Gong, H., Li, L., Ye, X., 2016. Chemical and cellular assays combined with in vitro digestion to determine the antioxidant activity of flavonoids from Chinese bayberry (Myrica rubra Sieb. et Zucc.) Leaves. PLOS ONE, http://dx.doi.org/10.1371/journal.pone.0167484.
http://dx.doi.org/10.1371/journal.pone.0...
; Li et al., 2016Li, J., Gong, F., Li, F., 2016. Hypoglycemic and hypolipidemic effects of flavonoids from tartary buckwheat in type 2 diabetic rats. Biomed. Res. 27, 132-137.). However, to C. iguanaea have not been performed studies evaluating protection against the risk factors for CVD and the hypoglycemic effects. Therefore, the present study aimed to investigate the chemical composition and to evaluate the effects of C. iguanaea on markers of lipids and glucose metabolism in cholesterol-fed rats.
Materials and methods
Solvents and chemicals
The solvents MeOH and acetic acid used for HPLC analysis were purchased from Merck® (São Paulo; SP, Brazil). Ethanol for the production of extracts was purchased from Vetec® (Rio de Janeiro; RJ, Brazil). Nylon membrane filters (0.45 mm) were purchased from of Flow Supply®, and the water was purified using a Milli-Q plus system from Millipore®.
Plant material
The leaves of Celtis iguanaea (Jacq) Sarg., Cannabaceae, were collected in Chapecó (SC), Brazil (27° 01′ 55.14′ S – 52° 47′ 29.42′ O) in September 2015, and authenticated by Professor Adriano Dias de Oliveira of the Community University of the Region of Chapecó (Unochapecó), where a voucher specimen is deposited (#3463).
Production of hydroalcoholic extract of Celtis iguanaea
The leaves of C. iguanaea were dried at room temperature (25 ± 5 °C), pounded in a knife mill (Ciemlab®, CE430), selected in a sieve (425 µm; 35 Tyler/Mesch), identified, and stored with protection from light. The extracts were produced via maceration (5 days) at room temperature using dry-milled leaves of the plant (100 g) and ethanol 70% (1:20, w/v). After filtration through Büchner funnel, the hydroalcoholic extract of C. iguanaea was concentrated via evaporation under reduced pressure, lyophilized, weighed, and stored at −20 °C.
Chemical analysis of Celtis iguanaea
To increase the phytochemical study of C. iguanaea in addition to the extracts, a dichloromethane extract of the leaves (1:20 w/v) was prepared via maceration (5 days). Both the extracts were analyzed via liquid chromatography tandem mass spectrometry. The samples (5 mg) were dissolved in methanol (3 ml) and filtered in a Sepak RP-18® cartridge, and then through a nylon membrane (Flow Supply®) with a 22.25 mm diameter and a 0.22 µm pore size.
HPLC-ESI-IT-MSn analyses
An aliquot of CI and dichloromethane extract were analyzed separately via in-line HPLC-ESI-IT-MSn, using a SURVEYOR MS micro system coupled in-line to an LCQ Fleet ion-trap mass spectrometer (Thermo Scientific). HPLC separation was conducted on a chromatographic column (250 × 4.6 mm i.d. 5 micron) using a gradient mobile phase with a flow rate of 0.8 ml/min of water and MeOH plus 0.1% acetic acid. Initial conditions were 5% MeOH increasing to reach 100% MeOH and hold at 100% MeOH at 80 min and held at 100% MeOH for 10 min. Both extracts were analyzed by ESI-MSn in negative ion mode with a LCQ Fleet ion-trap instrument from Thermo Scientific. The capillary voltage was set at −20 kV, the spray voltage at −5 kV and the tube lens offset at 100 V, sheath gas (nitrogen) flow rate at 80 (arbitrary units) and auxiliary gas flow rate at 5 (arbitrary units). Data were acquired in MS1 and MSn scanning modes. The capillary temperature was 275 °C. Xcalibur 2.1 software (Thermo Scientific) was used for data analysis.
ESI-MSn analysis
For analysis via mass spectrometry, CI and dichloromethane extract (5 mg) were dissolved in methanol (3 ml) and filtered in a Sepak RP-18 cartridge, and then through a nylon membrane (Flow Supply®) with a 22.25 mm diameter and a 0.22 µm pore size. The samples were analyzed online via the LCQ Fleet, Thermo Scientific® mass spectrometer, equipped with a direct sample insertion device for streaming injection analysis (FIA). The samples were ionized via electrospray ionization (ESI) and fragmentations into multiple stages (MSn) were held in an Ion-Trap (IT) interface. The negative mode was chosen for the generation and analysis of all the spectra, and the experimental conditions were as follows: capillary voltage −35 V, spray voltage −5000 V, capillary temperature 350 °C, drag gas (N2) and flow rate 60 (arbitrary units). The acquisition track was m/z 100–2000, with two or more scan events held simultaneously in the spectrum. The experiment was performed by Laboratory of Bioprospecting of Natural Products (LBPN), UNESP, Coastal Campus IB-CLP.
Animals
The International Guidelines for Care and Use of Laboratory Animals were followed for all experiments, and the experimental protocol was approved by the Ethics Committee on Animal Use (CEUA: 013/2015) of Unochapecó, Brazil. Male adult Wistar rats (n = 36), weighing 200 ± 15 g, were purchased from the animal facility of Unochapecó. Each animal was housed in wire-bottomed 17 × 33.5 × 40.5 cm cage in a controlled environment at 22 ± 2 °C with a 12 h light-dark cycle (lights on at 7 am and off at 7 pm) and minimal noise.
Hyperlipidemic diet
The high cholesterol diet was prepared by mixing a normal basal diet [composition (w/w): 49.8% carbohydrate, 23.5% crude protein, 5.9% crude fat, 5.9% crude ash, and 3.9% crude fiber] with cholic acid and cholesterol (989:1:10 w/w). The mixture was pelleted prior to use (Kin et al., 2008Kin, H.Y., Jeong, D.M., Jung, H.J., Jung, Y.J., Yokozawa, T., Choi, J.S., 2008. Hypolipidemic effects of Sophora flavescens and its constituents in polaxamer 407-induced hyperlipidemic and cholesterol-fed rats. Biol. Pharm. Bull. 31, 73-78.).
Experimental design
After 7 days of acclimatization the animals were divided randomly in two groups: a normal group (N) (n = 6) fed on pelleted food (Biobase®) and an induced group (I) (n = 30) fed with hyperlipidemic diet (pelleted food Biobase® + 1% cholesterol + 0.1% cholic acid Sigma–Aldrich®, St. Louis, MO, USA). The rats of both groups had access to water ad libitum. After 15 days, group I was divided randomly into 5 groups (n = 6). The groups were treated intragastrically once a day for 30 days and were divided as follows: Control (saline 0.9%) (C); hydroalcoholic extracts of CI (150, 300 or 600 mg/kg, respectively) (CI: 150, 300 or 600) and simvastatin (4 mg/kg) (SIMV) (Pankaj et al., 2010Pankaj, G.J., Savita, D.P., Nitin, G.H., Manoj, V.G., Sanjay, J.S., 2010. Hypolipidemic activity of Moringa oleifera Lam., Moringaceae, on high fat diet induced hyperlipidemia in albino rats. Rev. Bras. Farmacogn. 20, 969-973.). All treatments (at defined doses) were administered in a volume of 0.5 ml/200 g body weight diluted in saline (0.9% saline) and subjected to an ultrasonic bath (20 °C) to facilitate solubility. Dietary intake was measured daily and body weights of animals were recorded before and every three days following the initiation of treatment. All animals were fasted for a 12 h period before euthanasia, but with free access to water. The rats were anesthetized with a mixture of lidocaine and sodium thiopental (10 and 40 mg/kg, respectively), administered intraperitoneally. Blood aliquots were collected via cardiac puncture and the animals were euthanized using an overdose of sodium thiopental (120 mg/kg). The right soleus muscle, liver samples and intestine were collected for biochemical and histopathological analyses.
Biochemical analysis of blood samples
Upon collection, the blood samples were immediately centrifuged (3000 × g) for 15 min. Serum total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), triacylglycerides (TG), urea, interleukin-1 (IL-1) IL-6 tumor necrosis factor alpha (TNF-α), interferon gamma (IFN-γ), IL-10, glucose and alanine aminotransferase (ALT) levels were determined by enzymatic colorimetric methods (UV/vis) using commercial kits and according to the manufacturer's instructions (Li et al., 2012Li, W., Zhang, M., Gu, J., Meng, Z., Zhao, L.C., Zheng, Y., Chen, L., Yang, G.L., 2012. Hypoglycemic effect of protopanaxadiol-type ginsenosides and compound K on type 2 diabetes mice induced by high-fat diet combining with streptozotocin via suppression of hepatic gluconeogenesis. Fitoterapia 83, 192-198.) Serum LDL-C was calculated using the equation by Friedewald et al. (1972)Friedewald, W.T., Levy, R.I., Fredrickson, D.S., 1972. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 18, 499-502., very low density lipoprotein (VLDL) was calculated according to Sposito et al. (2007)Sposito, A.C., Caramelli, B., Fonseca, F.A.H., Bertolami, M.C., 2007. IV Diretriz Brasileira sobre Dislipidemias e Prevenção da Aterosclerose: Departamento de Aterosclerose da Sociedade Brasileira de Cardiologia. Arq. Bras. Cardiol. 88, 2-19. and the atherogenic index (AI) calculated by Xia et al. (2011)Xia, W., Sun, C., Zhao, Y., Wu, L., 2011. Hypolipidemic e antioxidant activities of Sanchi (Radix Notoginseng) in rats with a high fat diet. Phytomedicine 18, 516-520..
Estimation of HMG-CoA reductase activity (HMG-CoA/mevalonate ratio)
HMG-CoA reductase activity was measured in liver homogenates using the ratio of HMG-CoA to mevalonate, an index of enzyme activity which catalyzes the conversion of HMG-CoA to mevalonate. Therefore, the liver tissue was removed as quickly as possible and a 10% w/v homogenate was prepared in saline arsenate solution. The homogenate was deproteinized using an equal volume of dilute perchloric acid and allow to stand for 5 min, followed by centrifugation. To 1 ml of the filtrate, 0.5 ml of freshly prepared (alkaline hydroxylamine reagent in the case of HMG-CoA) was added. It was mixed and 1.5 ml of ferric chloride reagent was added after 5 min. The absorbance was read after 10 min at 540 nm versus a similarly treated saline arsenate blank. The ratio of HMG-CoA/mevalonate was calculated (Venugopala Rao and Ramakrishnan, 1975Venugopala Rao, A., Ramakrishnan, S., 1975. Indirect assessment of hydroxymethylglutaryl-CoA reductase (NADPH) activity in liver tissue. Clin. Chem. 21, 1523-1525.).
Fecal cholesterol
Fecal material was collected in the last 3 days of the experiment and stored at -20 °C. The stools were thawed e dried at 60 °C (24 h). To the powdered samples (0.5 g), isopropanol (5 ml) was added and the solutions were agitated and stored at -20 °C for 24 h with subsequent centrifugation (12,000 × g) for 10 min. The supernatant was analyzed for levels of fecal cholesterol at 500 nm via enzymatic colorimetric methods (UV/Vis) using commercial Labtest® kits according to the manufacturer's instructions (Kalek et al., 1984Kalek, H.D., Stellaard, F., Kruis, W., Paumgartner, G., 1984. Detection of increase bile acid excretion by determination of bile acid contend in simple stool samples. Clin. Chim. Acta 140, 85-90.).
Glycogen measurements
The harvested liver and soleus muscle were assessed for glycogen content. Glycogen was isolated from these tissues. The tissue was weighed, homogenized in 33% KOH, and boiled at 100 °C for 30 min, with occasional stirring. After cooling, 96% ethanol was added to the samples, which were then heated to boiling 100 °C temperature and cooled in an ice bath to aid glycogen precipitation. The homogenate was centrifuged (1300 × g) for 15 min, the supernatant was discarded, and the resulting pellet was washed and resolubilized in water. Glycogen content was determined via treatment with an iodine reagent, and the absorbance was measured at 460 nm. The results were expressed as milligrams of glycogen per gram of tissue (Krisman, 1962Krisman, C.R., 1962. A method for the colorimetric estimation of glycogen with iodine. Anal. Biochem. 4, 14-23.).
Disaccharidase extraction and assays
The extracted small intestine segment was washed in 0.9% NaCl solution, dried on filter paper, weighed, trimmed, and homogenized (300 × g) with 0.9% NaCl (0.4 g of duodenum per 1 ml of 0.9% NaCl) for 1 min at 4 °C. The resulting extract was centrifuged at (1300 × g) for 8 min. The supernatant was assessed to measure in vivo maltase, sucrase, and lactase activity as well as protein determination. The activity of maltase (EC 3.2.1.20), lactase (EC 3.2.1.23), and sucrase (EC 3.2.1.48) was determined using a glucose diagnosis kit based on the reagent glucose oxidase. To determine dissaccharidase activity, duodenum homogenates (10 µl) were incubated at 37 °C for 60 min with 10 µl of the substrate equivalent to 0.056 µM of maltose, sucrose, or lactose dissolved in sodium maleate buffer (pH 6.0). Then, the reagent solution containing glucose oxidase and peroxidase was added and incubated at 37 °C for 10 min. The absorbance was read at 500 nm, and the activity calculation was based on a glucose standard (Dahlqvist, 1984Dahlqvist, A., 1984. Assay of intestinal disaccharidases. Scand. J. Clin. Lab. Invest. 44, 169-172.; Pereira et al., 2011Pereira, D.F., Cazarolli, L.H., Lavado, C., Mengatto, V., Figueiredo, M.S.R.B., Guedes, A., Pizzolatti, M.G., Silva, F.R.M.B., 2011. Effects of flavonoids on α-glucosidase activity: potential targets for glucose homeostasis. Nutrition 27, 1161-1167.). One enzyme unit (U) was defined as the amount of enzyme that catalyzed the release of 1 µmol of glucose per minute under the assay conditions. The specific activity was defined as enzyme activity (U) per milligram of protein. The protein concentration was determined using bovine serum albumin as a standard. The assays were performed in duplicate along with the appropriate controls (Lowry et al., 1951Lowry, O.H., Rosebrough, N.J., Farr, A.L., Randall, R.J., 1951. Protein measurement with the folin phenol reagent. J. Biol. Chem. 193, 265-275.).
Lipid peroxidation
To evaluate lipid peroxidation and antioxidant enzyme activity, the liver samples were homogenized in three volumes of Tris–HCl (150 mM; pH 7.4) and centrifuged (3000 × g at 4 °C) for 10 min yielding a low-speed supernatant, which was used for further analysis. Lipid peroxidation was evaluated by measuring the TBARS level (Ohkawa et al., 1979Ohkawa, H., Ohishi, N., Yagi, K., 1979. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 95, 351-358.) after the addition of 7.2 mmol/l of butylated hydroxytoluene to the homogenates or plasma to prevent further oxidation. Liver homogenates and plasma samples were deproteinized with 10% trichloroacetic acid (TCA) and preincubated with 0.67% thiobarbituric acid (TBA) at 100 °C for 1 h. The colored product of the reaction was then extracted with n-butanol and measured at 535 nm using a standard curve of 1,1,3,3-tetraethoxypropane.
Antioxidant activity
Epinephrine (5 µl, 60 mM) was added to a medium containing glycine buffer (50 mM, pH 10.2) and an aliquot of sample was added in a final volume of 200 µl. The inhibition of epinephrine auto-oxidation to adrenochrome at alkaline pH was spectrophotometrically determined at 480 nm. Liver SOD activity was expressed as U/g of tissue (1 U) is the amount of enzyme that inhibits the oxidation of epinephrine by 50% whereas plasma SOD was expressed as U/ml of plasma (Misra and Fridovich, 1972Misra, H.P., Fridovich, I., 1972. The role of superoxide anion in the auto-oxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 247, 3170-3175.). Liver catalase (CAT) activity was determined by adding hydrogen peroxide (0.5 M H2O2) in a medium containing 30 µl of sample and 50 mM potassium phosphate buffer, pH 7.0 (final volume of 2 ml). The rate of decrease in H2O2 absorbance was monitored at 240 nm. The pseudo-first order reaction constant (k) of the decrease in H2O2 absorption at 25 °C was determined and specific activity was expressed as k/g wet tissue (Aebi, 1984Aebi, H., 1984. Catalase in vitro. Methods Enzymol. 105, 121-126.). The nonprotein thiols (NPSH) content was accessed in the plasma according to Ellman (1959)Ellman, G.L., 1959. Tissue sulphydryl groups. Arch. Biochem. Biophys. 82, 70-77. with minor modifications. Briefly, the reaction was conducted with 5,5-dithio-bis-(2-nitrobenzoic acid) and the samples were read at 412 nm. The content of NPSH in plasma samples were expressed as µmol/ml.
Histopathological analysis
Hepatic tissue was fixed in 10% formalin and trimmed to obtain 2-mm-thick cross sections. The tissue was dehydrated in an ascending graded series of ethanol, cleared in xylene, and embedded in paraffin. Sections of 3 µm were obtained with a standard microtome and were mounted on glass slides. The sections were later stained with hematoxylin and eosin for histological analyses and morphometry of fat area in the liver. The fat area was measured from ten pictures (400× magnification) per sample using the Image J 1.45s program. All white area was considered to contain fat. To identify and visualize the glycogen, each liver sample was sectioned and stained to the Periodic Acid Schiff (PAS) (Prophet et al., 1992Prophet, E.B., Mills, B., Arrington, J.B., Sobin, L.H., 1992. Laboratory Methods in Histotechnology. Armed Forces Institute of Pathology, Washington, DC.). Each PAS glass slide was used to capture ten pictures (400× magnification). The Image J 1.45s program was used to measure the glycogen area considering all PAS positive areas as glycogen area. All pictures were obtained using a binocular microscope Leica DM 500 coupled to an ICC 50 HD camera. The software Leica Application Suite EZ (LAS-EZ) version 3.0 was used to capture the images and to measure thickness of tunica.
Statistical analysis
The data were expressed as means ± standard deviation (SD). Statistical analysis between the treated and the control groups were performed using one-way ANOVA followed by Tukey post hoc test. A difference in the mean values of p < 0.05 was considered statistically significant.
Results
Chemical constituents of Celtis iguanaea
The chemical profile of C. iguanaea obtained via mass spectrometry in TIC mode was showed in Table 1. The compounds obtained were registered as their respective ions [M−H]−. For these ions, partitions in multiple stages were obtained, which allowed the structures of some molecules to be suggested based on their fragmentation mechanisms. Several free and glycosylated flavonoids were identified among these compounds. It should be noted that orientin was present in both the hydroalcoholic and dichloromethane extract. This molecule was one of the major components of hydroalcoholic extract of the plant.
ESI-MS and ESI-MSn product ions of compounds occurring in the extracts of the Celtis iguanaea.
Effects of hydroalcoholic extract of Celtis iguanaea on serum lipid profiles
At the end the four-week treatment, as expected, the C group (treated with saline 0.9%) showed higher levels of serum TC and LDL-C (p < 0.001) than rats fed with a normal diet (group N). This effect was reversed in hypercholesterolemic rats treated with C. iguanaea extract (CI-300 or CI-600 mg/kg) or simvastatin (SIMV, 4 mg/kg) that exhibited significant decrease in TC (40.7, 37.0 and 41.9%, respectively) compared with group C (p < 0.001) (Fig. 1A). All hypercholesterolemic rats treated with CI-150, 300 or 600 mg/kg or SIMV revealed significant decrease in LDL-C (37.0, 57.5, 52.2 and 46.0%, respectively) compared to group C (p < 0.001) (Fig. 1B). The analyses also demonstrated an accentuated reduction of the atherogenic index after treatment with CI-300 and 600 mg/kg 51.56% and 46.44%, respectively, compared to group C (p < 0.05) (Fig. 1C). There were no differences in the serum levels of alanine aminotransferase (ALT) enzyme activity and urea compared to control group, which suggests the absence of hepatic and renal toxicity. There were also no differences in TG and HDL-C values between the treated groups and group N and C (data not shown).
Effect of hydroalcoholic extract from Celtis iguanaea (CI-150, 300, and 600 mg/kg), and simvastatin (SIMV 4 mg/kg) in the values of total cholesterol (A), LDL-C (B) and atherogenic index (C) in cholesterol-fed rats. Rats were either given a normal diet (N) or the following treatment with saline (C). Values are expressed as means ± SD (n = 6). ANOVA one way *p < 0.001 compared with group N. # p < 0.001 compared with group C. & p < 0.05 compared with group C.
Effects of Celtis iguanaea extract on body and liver weights, HMG-CoA/mevalonate ratio, and fecal excretion of cholesterol
All animals gained weight from the beginning to the end of the experiment. However, no differences in weight gain were observed between the groups. The average liver weight was significantly higher in rats that received the high-cholesterol diet as compared to the group fed with the normal diet. To propose a possible mechanism of the hypolipidemic effects of C. iguanaea extract, the activity of hepatic 3-hydroxy-3-methyl-glutaryl-CoA (HMG-CoA) reductase, which is the regulatory enzyme in cholesterol biosynthesis, was measured indirectly. Analysis of the enzymatic activity revealed an accentuated increase in HMG-CoA/mevalonate ratio in the groups treated with C. iguanaea extract or SIMV compared to untreated rats fed with the high-fat diet and water (group C) (p < 0.001). The fecal cholesterol levels differed between the experimental group C (p < 0.01) and the N group. In cholesterol-fed rats, treated with CI-300 and 600 mg/kg, and SIMV exhibited significantly decreased cholesterol excretion values compared to rat belonging to group C (p < 0.01) (Table 2).
The effects of hydroalcoholic extract of Celtis iguanaea on animal body and liver weights, HMG-CoA/mevalonate ratio, and in the fecal excretion of cholesterol (n = 6) (mean ± SD).
Effects of Celtis iguanaea extract on inflammatory markers
The inflammatory reaction and the associated immune response are the main events that lead to the process of atherogenesis. The analysis of pro-inflammatory interleukins revealed a significant decrease in interleukin-1 (IL-1), IL-6, tumor necrosis factor-α (TNF-α), and interferon gamma (IFN-γ) values (p < 0.001) in all hypercholesterolemic rats treated with CI and SIMV compared with group C (Fig. 2A–D). Conversely, the groups N, CI-150, CI-300, CI-600, and SIMV exhibited higher levels of anti-inflammatory IL-10 (p < 0.001) compared to group C (p < 0.001) (Fig. 2E).
Effect of hydroalcoholic extract from Celtis iguanaea (CI-150, 300, and 600 mg/kg), and simvastatin (SIMV 4 mg/kg) on the values of IL-1 (A), IL-6 (B), TNF-α (C), IFN-γ (D), IL-10 (E) (mean ± SD; n = 6) in rats fed a high-fat diet. Rats were either given a normal diet (N) or the following treatment with saline (C). ANOVA one way *p < 0.001 compared with group N. # p < 0.001 compared with the group C.
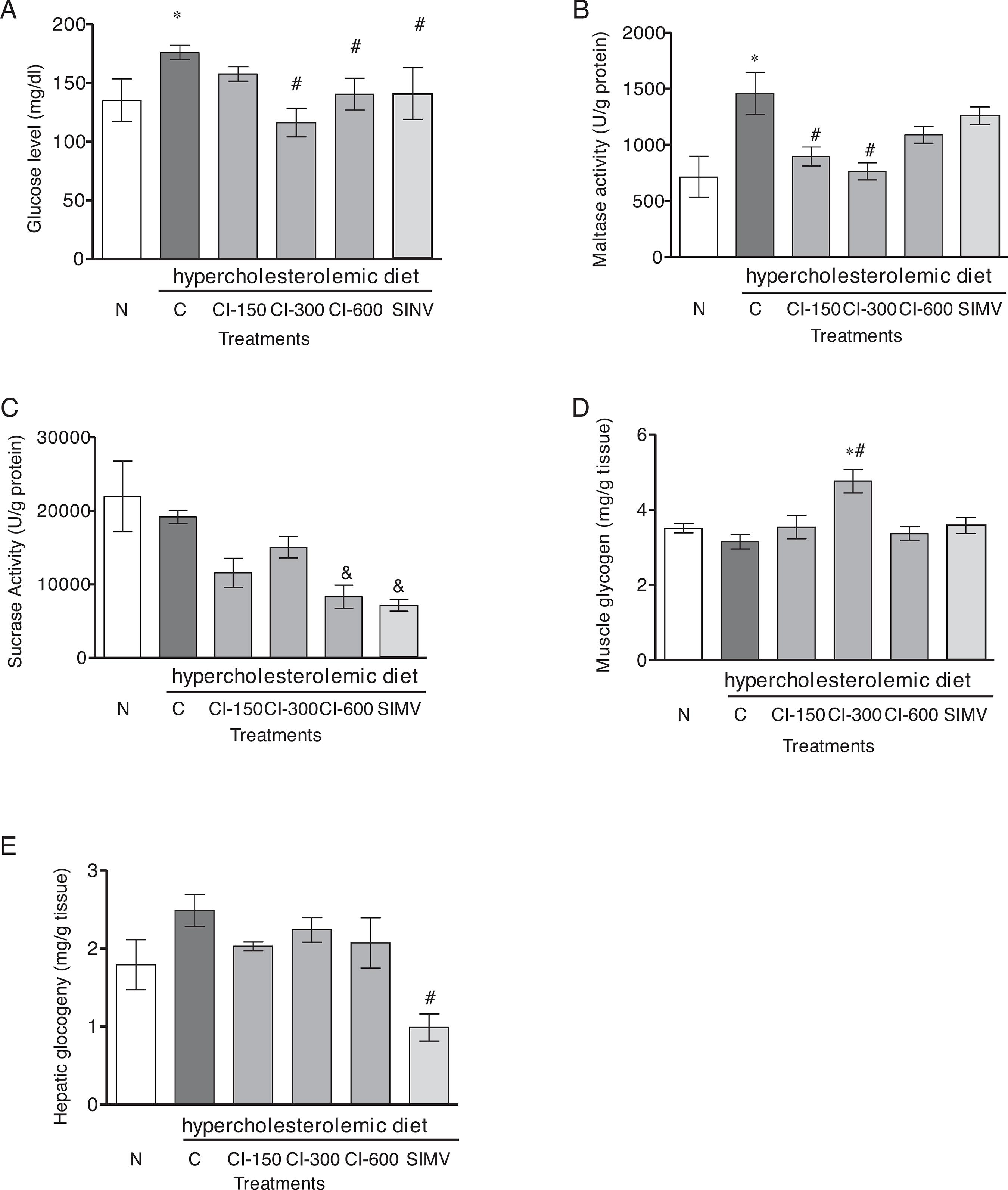
Effects of Celtis iguanaea extract on glycemia, disaccharidase activity, and concentration of hepatic and muscular glycogen
The serum glucose levels of CI-300, CI-600 and SIMV rats display a significant decrease (20.11, 19.85 and 19.84%, respectively), when compared to the C group (p < 0.001) (Fig. 3A). The disaccharidase activity was significantly decrease by C. iguanaea extract; maltase activity was also inhibited at concentrations of 150 and 300 mg/kg when compared to group C (p < 0.01) (Fig. 3B). The sucrase activity was reduced by 600 mg/kg and SIMV (p < 0.05) compared to group C (Fig. 3C). Lactase activity was not detected in any of the evaluated groups (data not shown). Moreover, analysis showed that CI-300 mg/kg increased significantly muscular glycogen (p < 0.001) (Fig. 3D), and that none of the doses of CI affected hepatic glycogen content (Fig. 3E).
Effect of hydroalcoholic extract from Celtis iguanaea (CI-150, 300, and 600 mg/kg), and simvastatin (SIMV 4 mg/kg) on serum glucose levels (A), disaccharidases (maltase and sucrase activity) (B and C), muscular and hepatic glycogen (D and E) in rats fed a high-fat diet (mean ± SD; n = 6). Rats were either given a normal diet (N) or the following treatment with saline (C). ANOVA one way *p < 0.001 compared with group N. # p < 0.001 compared with the group C. & p < 0.05 compared with the group C.
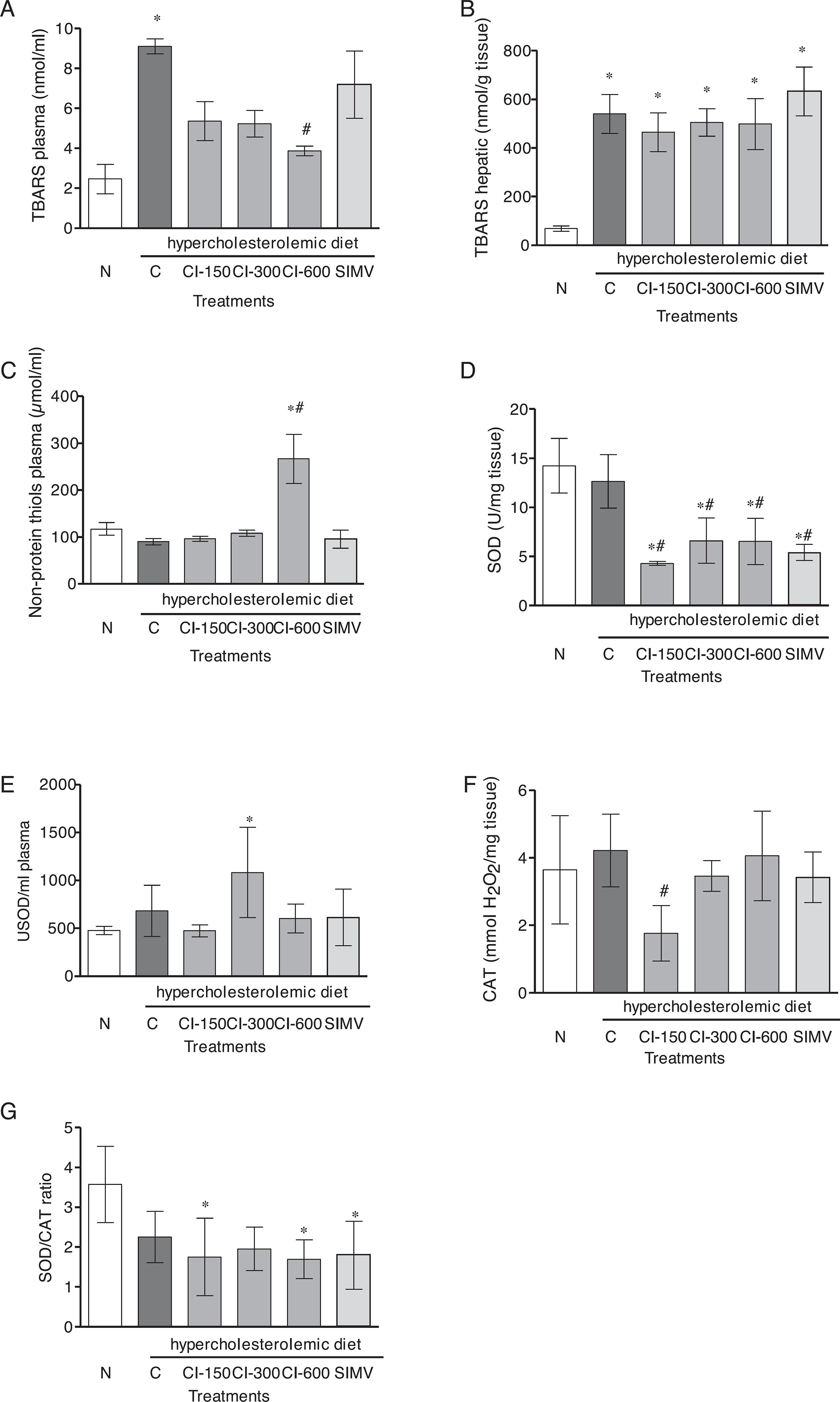
Effects of Celtis iguanaea extract on the serum and liver markers of oxidative damage and antioxidant enzyme activity
The results for plasma thiobarbituric acid reactive substances (TBARS) revealed a significant increase in lipid peroxidation in group C compared to group N (p < 0.001), while CI-600 mg/kg induced a lipid peroxidation decrease compared to group C levels (p < 0.01) (Fig. 4A). The analyses of hepatic TBARS demonstrated a significant increase in lipid peroxidation after high cholesterol diet (C group) (p < 0.01) (Fig. 4B), and this effect was not protected by C. iguanaea extract or simvastatin treatments. A significant increase in the values of plasma nonprotein thiols (NPSH) was noted after administration of CI-600 mg/kg as compared to that of group C (p < 0.001) (Fig. 4C). Regarding antioxidant enzymes activity, all treatments with C. iguanaea extract and SIMV caused a decrease of hepatic superoxide dismutase (SOD) activity in comparison to both groups N and C (p < 0.001) (Fig. 4D). However, plasma SOD was increased in the CI-300 group compared to group N (p < 0.01) (Fig. 4E). The results for hepatic catalase (CAT) activity had also showed the effect in the dose of 150 mg/kg compared with group C (p < 0.05) (Fig. 4F). The hepatic SOD/CAT ratio decreased after CI-150 and CI-600 and SIMV treatment when compared to the ratio obtained for group N (p < 0.05) (Fig. 4G).
Effect of hydroalcoholic extract from Celtis iguanaea (CI-150, 300, and 600 mg/kg), and simvastatin (SIMV 4 mg/kg) on TBARS plasma (A), TBARS hepatic (B), NPSH (C), plasma and hepatic SOD activity (D and E), CAT activity (F) and hepatic SOD/CAT ratio (G) in rats fed a high-fat diet (mean ± SD; n = 6). Rats were either given a normal diet (N) or the following treatment with saline (C). ANOVA one way *p < 0.001 compared with group N. # p < 0.05 compared with the group C. & p < 0.001 compared with the group C.
Histological results
Histopathological analyses of liver tissue did not show any increase in liver fat area in group N compared to group C (data not shown). However, all animals treated with CI-150, 300 and 600 mg/kg and SIMV revealed a significant decrease on the fat liver area (24.26, 22.91, 20.63 and 24.60%, respectively) compared to group C (p < 0.001). CI-150 and 300 mg/kg as well as SIMV decreased the liver fat area where compared with the N group as well group C (p < 0.05) (Fig. 5A). The evaluation of liver glycogen content in histological sections did not show differences between group N and any of the treated groups.
Effect of hydroalcoholic extract from Celtis iguanaea (CI-150, 300, and 600 mg/kg), and simvastatin (SIMV 4 mg/kg) on fat liver area in rats fed a high-fat diet (mean ± SD; n = 6). Rats were either given a normal diet (N) or the following treatment with saline (C). ANOVA one way *p < 0.05 compared with group N. # p < 0.001 compared with the group C.
Discussion
The relevance of the search for new drugs for its treatment and plants compounds with hypolipidemic potential for the treatment and prevention of atherosclerosis and other injuries related to the cardiovascular system is well-recognized by the scientific community (Koriem, 2014Koriem, K.M.M., 2014. Antihyperlipidemic activity of the medicinal plants among Kadazan and Dusun communities in Sabah, Malaysia: a review. Asian Pac. J Trop. Biomed. 4, 768-779.). This is the first report on the hypolipidemic and hypoglycemic effects of a hydroalcoholic extract of the C. iguanaea in cholesterol-fed rats. The results of the present study show that a cholesterol-enriched diet causes an increase in serum TC and LDL-C levels, which can be mitigated by C. iguanaea extract at doses of 150, 300 and 600 mg/kg (for LDL-C) and 300 and 600 mg/kg (for TC). The atherogenic index (AI), decreased in all groups treated with C. iguanaea extract when compared to group C. This index has been associated with the risk for developing atherosclerosis. Increased AI values are associated with an increased risk of organ damage, due to the oxidative stress, which is in turn related to the increase in blood lipid levels (Balzan et al., 2013Balzan, S., Hernandes, A., Reichert, C.L., Donaduzzi, C., Pires, V.A., Gasparotto Junior, A., Cardozo Junior, E.L., 2013. Lipid-lowering effects of standardized extracts of Ilex paraguariensis in high-fat-diet rats. Fitoterapia 86, 115-122.). Several flavonoids and polyphenolic compounds are present in C. iguanaea extracts of different polarities and orientin is one of the major components of the hydroalcoholic extract. The hypolipidemic potential of C. iguanaea extract must be related to the presence of flavonoids in their composition, since these substances, as found in similar studies, are able to reduce TG, CT and LDL-C levels and further increase HDL-C levels (Li et al., 2016Li, J., Gong, F., Li, F., 2016. Hypoglycemic and hypolipidemic effects of flavonoids from tartary buckwheat in type 2 diabetic rats. Biomed. Res. 27, 132-137.; Mocelin et al., 2016Mocelin, R., Marcon, M., Santo, G.D., Zanatta, L., Sachett, A., Schönell, A.P., Bevilaqua, F., Giachini, M., Chitolina, R., Wildner, S.M., Duarte, M.M.M.F., Conterato, G.M.M., Piato, A.L., Gomes, D.B., Roman Junior, W.A., 2016. Hypolipidemic and antiatherogenic effects of Cynara scolymus in cholesterol-fed rats. Rev. Bras. Farmacogn. 26, 233-239.; El-Newary et al., 2016El-Newary, S.A., Sulieman, A.M., El-Attar, S.R., Sitohy, M.Z., 2016. Hypolipidemic and antioxidant activity of the aqueous extract from the uneaten pulp of the fruit from Cordia dichotoma in healthy and hyperlipidemic Wistar albino rats. J. Nat. Med. 70, 539-553.). The reduction of lipid levels in the blood stream in cholesterol fed-rats, is related to decreased enzyme activities of HMG-CoA reductase and acyl-CoA acetyltransferase, which cause decreased levels of cholesterol esters available to form very low density lipoproteins (VLDL), resulting in reduced VLDL secretion by the liver (Mocelin et al., 2016Mocelin, R., Marcon, M., Santo, G.D., Zanatta, L., Sachett, A., Schönell, A.P., Bevilaqua, F., Giachini, M., Chitolina, R., Wildner, S.M., Duarte, M.M.M.F., Conterato, G.M.M., Piato, A.L., Gomes, D.B., Roman Junior, W.A., 2016. Hypolipidemic and antiatherogenic effects of Cynara scolymus in cholesterol-fed rats. Rev. Bras. Farmacogn. 26, 233-239.). The results of this study, showed a significant reduction of the activity of the HMG-CoA reductase enzyme, consistent with the observed reduction of the fecal cholesterol levels of the rats. These effects may be related to flavonoids that exhibited potent anti-adipogenesis activity and inhibition of intracellular triacylglyceride accumulation, by inhibiting the expression of CCAAT-enhancer-binding proteins α (C/EBPα) and peroxisome proliferator-activated receptors γ (PPARγ) (Kim et al., 2010Kim, J., Lee, I., Seo, J., Jung, M., Kim, Y., Yim, N., Bae, K., 2010. Vitexin, orientin and other flavonoids from Spirodela polyrhiza inhibit adipogenesis in 3T3-L1 cells. Phytother. Res. 24, 1543-1548.). A previous study showed that dietary polyphenols can bind the HMG-CoA reductase enzyme that catalyzes the reaction converting HMG-CoA to mevalonate, which leads to the production of cholesterol and blocks the binding of nicotinamide adenine dinucleotide phosphate (NADP+) (Islam et al., 2015Islam, B., Sharma, C., Adem, A., Aburawi, E., Ojha, S., 2015. Insight into the mechanism of polyphenols on the activity of HMGR by molecular docking. Drug. Des. Dev. Ther. 28, 4943-4951.).
It is well established that high levels of LDL-C precede inflammatory processes that cause damage to the blood vessel wall and atherosclerosis (Lewis, 2011Lewis, S.J., 2011. Lipid-lowering therapy: who can benefit?. Vasc. Health Risk Manag. 7, 525-534.). Many inflammatory markers associated with atherosclerosis have been described over the years (Casiglia and Tikhonoff, 2007Casiglia, E., Tikhonoff, V., 2007. Inflammatory and coagulative markers of atherosclerosis. Eur. Heart J. 28, 271-273.) including the pro-inflammatory cytokines involved in the atherosclerotic process such as IL-1, IL-6, TNF-α, C-reactive protein (CRP), and INF-γ (Vaddi et al., 1994Vaddi, K., Nicolini, F.A., Mehta, P., Metha, J.L., 1994. Increased secretion of tumor necrosis factor-alpha and interferon-gamma by mononuclear leukocytes inpatients with ischemic heart disease. Relevance in superoxide anion generation. Circulation 90, 694-699.; Ridker et al., 2002Ridker, P.M., Rifai, N., Rose, L., Buring, J.E., Cook, N.R., 2002. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N. Engl. J. Med. 347, 1557-1565.; Harvey and Ramji, 2005Harvey, E.J., Ramji, D.P., 2005. Interferon-gamma and atherosclerosis: pro- or anti-atherogenic?. Cardiovasc. Res. 67, 11-20.; Larsson et al., 2005Larsson, P.T., Hallerstam, S., Rosfors, S., Wallen, N.H., 2005. Circulating markers of inflammation are related to carotid artery atherosclerosis. Int. Angiol. 24, 43-51.; Tzoulaki et al., 2005Tzoulaki, I., Murray, G.D., Lee, A.J., Rumley, A., Lowe, G.D., Fowkes, F.G., 2005. C-reactive protein, interleukin-6, and soluble adhesion molecules as predictors of progressive peripheral atherosclerosis in the general population. Edinburgh Artery Study. Circulation 112, 976-983.). In this study, the group of animals that was fed a hypercholesterolemic diet showed higher concentrations of pro-inflammatory interleukins; however, in the treatment groups (all concentrations of C. iguanaea extract), these levels decreased significantly. The decrease in pro-inflammatory interleukins can be related to inhibition of transcription factors, signal transducer and activator of transcription 1 (STAT1) and nuclear factor-κB (NF-kB), major polyphenol targets in the vascular system (Quiñones et al., 2013Quiñones, M., Miguel, M., Aleixandre, A., 2013. Beneficial effects of polyphenols on cardiovascular disease. Pharmacol. Res. 68, 125-131.). Orientin, one of the main components of C. iguanaea extract, has already demonstrated to inhibit the high mobility group box-1 (HMGB1) protein levels in lipopolysaccharide (LPS)-induced human umbilical vein endothelial cells (HUVEC) and HMGB1-mediated cytoskeletal rearrangements (Yoo et al., 2014Yoo, H.Y., Kum, S.K., Lee, T.H., Bae, J.S., 2014. Orientin inhibits HMGB1-induced inflammatory responses in HUVECs and in murine polymicrobial sepsis. Inflammation 37, 1705-1717.). Furthermore, in vivo assessments showed that orientin inhibited HMGB1-mediated and LPS-induced hyperpermeability, cecal ligation and puncture (CLP)-induced release of HMGB1 level, leukocyte migration, LPS-induced TNF-α level, IL-6 level, NF-kB level, extracellular regulated kinases (ERK) 1/2 level, and lethality of mice (Lee et al., 2014Lee, W.H., Ku, S.K., Bae, J.S., 2014. Vascular barrier protective effects of orientin and isoorientin in LPS-induced inflammation in vitro and in vivo. Vascul. Pharmacol. 62, 3-14.). In addition, the pre-treatment with orientin or isoorientin may contribute to decreased vascular inflammatory effects of high glucose levels in HUVECs and in mice, through the inhibition of NF-κB (Ku et al., 2014Ku, S.K., Kwak, S., Bae, J.S., 2014. Orientin inhibits high glucose-induced vascular inflammation in vitro and in vivo. Inflammation 37, 2164-2173.).
However, the inflammatory process related to atherosclerosis appears to be regulated by anti-inflammatory cytokines such as IL-10. This cytokine has a powerful impact on macrophage cholesterol metabolism, stimulating both the absorption of cholesterol from modified lipoproteins, as well as cholesterol efflux from the cell, typical for atherosclerotic processes (Han et al., 2009Han, X., Kitamoto, S., Lian, Q., Boisvert, W.A., 2009. Interleukin-10 facilitates both cholesterol uptake and efflux in macrophages. J. Biol. Chem. 284, 32950-32958.). The major mechanisms for these anti-atherogenic effects is the activation of the PPARγ/LXR pathway up-regulates the expression of genes such as ATP-binding cassette transporter 1 (ABCA1) and ATP-binding cassette sub-family G member 1 (ABCG1) (Han et al., 2010Han, X., Kitamoto, S., Wang, H., Boisvert, W.A., 2010. Interleukin-10 overexpression in macrophages suppresses atherosclerosis in hyperlipidemic mice. FASEB J. 24, 2869-2880.) in macrophages and the ability of IL-10 to inhibit the expression of inflammatory mediators, probably by regulating the expression of extracellular matrix (ECM)-degrading enzymes (Glass and Witztum, 2001Glass, C.K., Witztum, J.L., 2001. Atherosclerosis the road ahead. Cell 104, 503-516.) and by inhibiting NF-kB activity (Moore et al., 2001Moore, K.W., de Waal Malefyt, R., Coffman, R.L., O’Garra, A., 2001. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 19, 683-765.; Driessler et al., 2004Driessler, F., Venstrom, K., Sabat, R., Asadullah, A., Schottelius, A.J., 2004. Molecular mechanisms of interleukin-10-mediated inhibition of NF-κB activity: a role for p50. Am. J. Clin. Exp. Immunol. 135, 164-173.). Thus, the increase of IL-10 at doses of 300 and 600 mg/kg of C. iguanaea extract might be involved in both cholesterol-lowering and anti-inflammatory effects observed in cholesterol-fed rats. Further studies are needed to elucidate the mechanisms responsible for these effects induced by C. iguanaea extract.
In addition to a hypolipidemic potential, C. iguanaea extract groups showed to reduce blood glucose levels and promote changes in carbohydrate digestion and metabolism. Carbohydrates are stored in the form of glycogen in humans in the skeletal muscles and liver. This mechanism is regulated by insulin, leading to difficulties in reducing blood glucose levels for individuals with diabetes and insulin resistance (Jensen et al., 2011Jensen, J., Rustad, P.I., Kolnes, A.J., Lai, Y.C., 2011. The role of skeletal muscle glycogen breakdown for regulation of insulin sensitivity by exercise. Front. Physiol. 2, 1-11.). This study demonstrated that C. iguanaea extract at a concentration of 300 mg/kg increases significantly the levels of glycogen in the soleus muscle without changing the hepatic glycogen, inhibits the activity of maltase, and decreases the activity of sucrase (at 600 mg/kg), what probably caused the reduction of blood glucose. Flavonoids such as orientin, can decrease the disaccharidase activity (Pereira et al., 2011Pereira, D.F., Cazarolli, L.H., Lavado, C., Mengatto, V., Figueiredo, M.S.R.B., Guedes, A., Pizzolatti, M.G., Silva, F.R.M.B., 2011. Effects of flavonoids on α-glucosidase activity: potential targets for glucose homeostasis. Nutrition 27, 1161-1167.; Toma et al., 2014Toma, A., Makonnen, E., Mekonnen, Y., Debella, A., Addisakwattana, S., 2014. Intestinal α-glucosidase and some pancreatic enzymes inhibitory effect of hydroalcholic extract of Moringa stenopetala leaves. BMC Complement. Altern. Med. 14, 1-5.), promote alterations in the carbohydrate metabolism and glucose absorption in the intestine, stimulate the insulin secretion from the pancreatic β-cells, modulate glucose release from the liver, activate the insulin receptors and glucose uptake in the insulin-sensitive tissues, and modulate the intracellular signaling pathways and gene expression (Hanhineva et al., 2010Hanhineva, K., Törrönen, R., Bondia-Pons, I., Pekkinen, J., Kolehmainen, M., Mykkänen, H., Poutanen, K., 2010. Impact of dietary polyphenols on carbohydrate metabolism. Int. J. Mol. Sci. 11, 1365-1402.).
Another important condition related to the atherogenic process is the presence of oxidative stress. Our results demonstrated an increase in the lipid peroxidation in the hypercaloric diet group, which could be due to an elevated production of reactive oxygen species (ROS), an important condition for the development of metabolic syndrome and some disorders such obesity, systemic arterial hypertension (SAH), atherosclerosis, and diabetes (Youn et al., 2014Youn, J.Y., Siu, K.L., Lob, H.E., Itani, H., Harrison, D.G., Cai, H., 2014. Role of vascular oxidative stress in obesity and metabolic syndrome. Diabetes 63, 2344-2355.; Demir et al., 2014Demir, B., Demir, E., Acıksarı, G., Uygun, T., Utku, I.K., Gedikbasi, A., Caglar, I.M.1., Pirhan, O., Tureli, H.O., Oflar, E., Ungan, İ., Ciftci, S., Karakaya, O., 2014. The association between the epicardial adipose tissue thickness and oxidative stress parameters in isolated metabolic syndrome patients: a multimarker approach. Int. J. Endocrinol. 1, 1-9.). However, an increase in the plasma TBARS was prevented by the C. iguanaea extract at dose of 600 mg/kg. The concomitant enhancement of plasma NPSH levels observed in CI-600 group may have contributed to alleviate the lipid peroxidation. NPSH are mostly composed of glutathione (GSH), a tripeptide involved in hydrogen peroxide (H2O2) removal via reaction catalyzed by glutathione peroxidase. The H2O2 decomposition contributes to prevent the formation of the highly reactive hydroxyl radical (•OH), which is involved in the initiation and propagation of lipid peroxidation (Halliwell and Gutteridge, 1984Halliwell, B., Gutteridge, J.M., 1984. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem. J. 219, 1-14.). The higher antioxidant activity of C. iguanaea extract at dose of 600 mg/kg was also shown by the increase in plasma SOD activity, which suggests higher capacity in superoxide (O2•−) removal in plasma. On the other hand, the antioxidant properties of C. iguanaea extract appears to be not effective in liver, since the results showed a decrease in both liver SOD and CAT activity, while no dose of extract was able to protect against lipid peroxidation induced by high cholesterol diet. However, the decrease in antioxidant enzymes could not have contributed to maintain the increased liver TBARS levels, because liver SOD/CAT ratio either decrease (at doses of 150 and 600 mg/kg) or did not change (at dose of 150 of C. iguanaea extract). SOD enzyme catalyze the dismutation of superoxide anions into hydrogen peroxide (H2O2) that is easily diffuse across cell membranes and cytosol, to soften this action, CAT and other enzymes are able to neutralize this reactive species, producing water (H2O) and molecular oxygen (Kotani and Taniguchi, 2011Kotani, K., Taniguchi, N., 2011. The association between reactive oxygen metabolites and metabolic syndrome in asymptomatic Japanese men. J. Clin. Med. Res. 3, 247-251.; Avelar et al., 2015Avelar, T.M.T., Storch, A.S., Castro, L.A., Azevedo, S.V.M.M., Ferraz, L., Lopes, P.F., 2015. Oxidative stress in the pathophysiology of metabolic syndrome: which mechanisms are involved?. J. Bras. Patol. Med. Lab. 51, 231-239.). Then, a lower SOD/CAT ratio indicates that there was no loss of capacity in H2O2 decomposition in liver. Therefore is unlikely that H2O2 originated from catalase reaction has been decomposed in •OH radical via Fenton reaction and contributed to the hepatic lipid peroxidation. Thus, the increase of these antioxidants may have contributed to protect against lipid peroxidation in plasma. In addition, flavonoids such as orientin, isoorientin, vitexin and also statins exhibit radical scavenging activity, which may explain the protective effect against lipid peroxidation at all doses of CI and simvastatin (Anantachoke et al., 2015Anantachoke, N., Kitphati, W., Mangmool, S., Bunyapraphatsara, N., 2015. Polyphenolic compounds and antioxidant activities of the leaves of Glochidion hypoleucum. Nat. Prod. Commun. 10, 479-482.; Puttananjaiah et al., 2011Puttananjaiah, M.K., Dhale, M.A., Gaonkar, V., Keni, S., 2011. Reductase inhibitors demonstrate anti-atherosclerotic character due to their antioxidant capacity. Appl. Biochem. Biotechnol. 163, 215-222.). On the other hand, the lack of protection against the increase of liver TBARS levels induced by high-fat diet may indicate that ROS production overcome the antioxidant capacity of liver.
In addition to the protective oxidative damage by C. iguanaea extract, the histological evaluation showed a reduction of the fat liver in all groups treated with the extract, which might be related to the decrease of hepatic HMG-CoA activity with reduced cholesterol synthesis. Over the years, research has shown that drugs with hypolipidemic potential, such as statins (pleiotropic effects), may increase endothelial function, modulate the inflammatory response related to atherogenesis and reduce platelet aggregation (Mitsios et al., 2010Mitsios, J.V., Papathanasiou, A.I., Goudevenos, J.A., Tselepis, A.D., 2010. The antiplatelet and antithrombotic actions of statins. Curr. Pharm. Des. 16, 3808-3814.).
This study revealed that the administration of C. iguanaea extract in cholesterol-fed rats can significantly reduce the LDL and TC levels related to the decreased HMG-CoA activity, inhibition of pro-inflammatory cytokines, and reduction of the glucose levels and lipid peroxidation. In addition, the ALT levels do not differ between treatments groups, indicating the absence of C. iguanaea extract toxicity at the doses tested. Thus, these results indicate that C. iguanaea extract may be beneficial for may be beneficial for lipids and glucose metabolism, and most likely, for the prevention of atherosclerosis.
Conclusions
The hydroalcoholic extract of C. iguanaea may be effective in the prevention of hypercholesterolemia and the protection against atherosclerosis and hyperglycemia. These biological activities are possibly related to its antioxidant effect, enzymatic inhibitory activity, and insulin mimetic potential.
Ethical disclosures
-
Protection of human and animal subjects. The authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).Confidentiality of data. The authors declare that no patient data appear in this article.Right to privacy and informed consent. The authors declare that no patient data appear in this article.
Acknowledgment
This work was supported by CAPES and Unochapecó [modality Art. 170 and 171 – FUMDES].
References
- Aebi, H., 1984. Catalase in vitro Methods Enzymol. 105, 121-126.
- American Heart Association, 2017. Prevention and Treatment of High Cholesterol (Hyperlipidemia). American Heart Association, Dallas.
- Anantachoke, N., Kitphati, W., Mangmool, S., Bunyapraphatsara, N., 2015. Polyphenolic compounds and antioxidant activities of the leaves of Glochidion hypoleucum Nat. Prod. Commun. 10, 479-482.
- Aronow, W.A., 2013. Treatment of hypercholesterolemia. J. Clin. Exp. Cardiol. S1, 1-8.
- Avelar, T.M.T., Storch, A.S., Castro, L.A., Azevedo, S.V.M.M., Ferraz, L., Lopes, P.F., 2015. Oxidative stress in the pathophysiology of metabolic syndrome: which mechanisms are involved?. J. Bras. Patol. Med. Lab. 51, 231-239.
- Balzan, S., Hernandes, A., Reichert, C.L., Donaduzzi, C., Pires, V.A., Gasparotto Junior, A., Cardozo Junior, E.L., 2013. Lipid-lowering effects of standardized extracts of Ilex paraguariensis in high-fat-diet rats. Fitoterapia 86, 115-122.
- Borges, F.F.V., Machado, T.C., Cunha, K.S., Pereira, K.C., Costa, E.A., Paula, J.R., Chen-Chen, L., 2013. Assessment of thecytotoxic, genotoxic, and antigenotoxic activities of Celtis iguanaea (Jacq.) in mice. An. Acad. Bras. Ciênc. 85, 955-963.
- Casiglia, E., Tikhonoff, V., 2007. Inflammatory and coagulative markers of atherosclerosis. Eur. Heart J. 28, 271-273.
- Chávez-Sánchez, L., Espinosa-Luna, J.E., Chávez-Rueda, K., Legorreta-Haquet, M.V., Montoya-Díaz, E., Blanco-Favela, F., 2014. Innate immune system cells in atherosclerosis. Arch. Med. Res. 45, 1-14.
- Dahlqvist, A., 1984. Assay of intestinal disaccharidases. Scand. J. Clin. Lab. Invest. 44, 169-172.
- Demir, B., Demir, E., Acıksarı, G., Uygun, T., Utku, I.K., Gedikbasi, A., Caglar, I.M.1., Pirhan, O., Tureli, H.O., Oflar, E., Ungan, İ., Ciftci, S., Karakaya, O., 2014. The association between the epicardial adipose tissue thickness and oxidative stress parameters in isolated metabolic syndrome patients: a multimarker approach. Int. J. Endocrinol. 1, 1-9.
- Driessler, F., Venstrom, K., Sabat, R., Asadullah, A., Schottelius, A.J., 2004. Molecular mechanisms of interleukin-10-mediated inhibition of NF-κB activity: a role for p50. Am. J. Clin. Exp. Immunol. 135, 164-173.
- Ellman, G.L., 1959. Tissue sulphydryl groups. Arch. Biochem. Biophys. 82, 70-77.
- El-Newary, S.A., Sulieman, A.M., El-Attar, S.R., Sitohy, M.Z., 2016. Hypolipidemic and antioxidant activity of the aqueous extract from the uneaten pulp of the fruit from Cordia dichotoma in healthy and hyperlipidemic Wistar albino rats. J. Nat. Med. 70, 539-553.
- Friedewald, W.T., Levy, R.I., Fredrickson, D.S., 1972. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 18, 499-502.
- Glass, C.K., Witztum, J.L., 2001. Atherosclerosis the road ahead. Cell 104, 503-516.
- Halliwell, B., Gutteridge, J.M., 1984. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem. J. 219, 1-14.
- Han, X., Kitamoto, S., Lian, Q., Boisvert, W.A., 2009. Interleukin-10 facilitates both cholesterol uptake and efflux in macrophages. J. Biol. Chem. 284, 32950-32958.
- Han, X., Kitamoto, S., Wang, H., Boisvert, W.A., 2010. Interleukin-10 overexpression in macrophages suppresses atherosclerosis in hyperlipidemic mice. FASEB J. 24, 2869-2880.
- Hanhineva, K., Törrönen, R., Bondia-Pons, I., Pekkinen, J., Kolehmainen, M., Mykkänen, H., Poutanen, K., 2010. Impact of dietary polyphenols on carbohydrate metabolism. Int. J. Mol. Sci. 11, 1365-1402.
- Harvey, E.J., Ramji, D.P., 2005. Interferon-gamma and atherosclerosis: pro- or anti-atherogenic?. Cardiovasc. Res. 67, 11-20.
- Hernandez-Galicia, E., Aguilar-Contreras, A., Aguilar-Santamaria, L., Roman-Ramos, R., Chavez-Miranda, A.A., Garcia-Veja, L.M., Flores-Saenz, J.L., Alarcon-Aguilar, F.J., 2002. Studies on hypoglycemic activity of Mexican medicinal plants. Proc. West. Pharmacol. Soc. 45, 118-124.
- Islam, B., Sharma, C., Adem, A., Aburawi, E., Ojha, S., 2015. Insight into the mechanism of polyphenols on the activity of HMGR by molecular docking. Drug. Des. Dev. Ther. 28, 4943-4951.
- Jensen, J., Rustad, P.I., Kolnes, A.J., Lai, Y.C., 2011. The role of skeletal muscle glycogen breakdown for regulation of insulin sensitivity by exercise. Front. Physiol. 2, 1-11.
- Kalek, H.D., Stellaard, F., Kruis, W., Paumgartner, G., 1984. Detection of increase bile acid excretion by determination of bile acid contend in simple stool samples. Clin. Chim. Acta 140, 85-90.
- Kim, J., Lee, I., Seo, J., Jung, M., Kim, Y., Yim, N., Bae, K., 2010. Vitexin, orientin and other flavonoids from Spirodela polyrhiza inhibit adipogenesis in 3T3-L1 cells. Phytother. Res. 24, 1543-1548.
- Kin, H.Y., Jeong, D.M., Jung, H.J., Jung, Y.J., Yokozawa, T., Choi, J.S., 2008. Hypolipidemic effects of Sophora flavescens and its constituents in polaxamer 407-induced hyperlipidemic and cholesterol-fed rats. Biol. Pharm. Bull. 31, 73-78.
- Koriem, K.M.M., 2014. Antihyperlipidemic activity of the medicinal plants among Kadazan and Dusun communities in Sabah, Malaysia: a review. Asian Pac. J Trop. Biomed. 4, 768-779.
- Kotani, K., Taniguchi, N., 2011. The association between reactive oxygen metabolites and metabolic syndrome in asymptomatic Japanese men. J. Clin. Med. Res. 3, 247-251.
- Krisman, C.R., 1962. A method for the colorimetric estimation of glycogen with iodine. Anal. Biochem. 4, 14-23.
- Ku, S.K., Kwak, S., Bae, J.S., 2014. Orientin inhibits high glucose-induced vascular inflammation in vitro and in vivo Inflammation 37, 2164-2173.
- Lago, J.H.G., Arruda, A.C.T., Mernak, M., Barrosa, K.H., Martins, M.A., Tibério, I.F.L.C., Prado, C.M., 2014. Structure-activity association of flavonoids in lung diseases. Molecules 19, 3570-3595.
- Larsson, P.T., Hallerstam, S., Rosfors, S., Wallen, N.H., 2005. Circulating markers of inflammation are related to carotid artery atherosclerosis. Int. Angiol. 24, 43-51.
- Lee, W.H., Ku, S.K., Bae, J.S., 2014. Vascular barrier protective effects of orientin and isoorientin in LPS-induced inflammation in vitro and in vivo Vascul. Pharmacol. 62, 3-14.
- Lewis, S.J., 2011. Lipid-lowering therapy: who can benefit?. Vasc. Health Risk Manag. 7, 525-534.
- Li, J., Gong, F., Li, F., 2016. Hypoglycemic and hypolipidemic effects of flavonoids from tartary buckwheat in type 2 diabetic rats. Biomed. Res. 27, 132-137.
- Li, W., Zhang, M., Gu, J., Meng, Z., Zhao, L.C., Zheng, Y., Chen, L., Yang, G.L., 2012. Hypoglycemic effect of protopanaxadiol-type ginsenosides and compound K on type 2 diabetes mice induced by high-fat diet combining with streptozotocin via suppression of hepatic gluconeogenesis. Fitoterapia 83, 192-198.
- Lowry, O.H., Rosebrough, N.J., Farr, A.L., Randall, R.J., 1951. Protein measurement with the folin phenol reagent. J. Biol. Chem. 193, 265-275.
- Martins, E.G.A., Pirani, J.R., 2009. Flora da Serra do Cipó, Minas Gerais: Cannabaceae. Bol. Bot. Univ. São Paulo 27, 247-251.
- Martins, J.L., Rodrigues, O.R., Sousa, F.B., Fajemiroye, J.O., Galdino, P.M., Florentino, I.F., Costa, E.A., 2015. Medicinal species with gastroprotective activity found in the Brazilian Cerrado. Fundam. Clin. Pharmacol. 29, 238-251.
- Martins, J.L.R., Rodrigues, O.R.P., Silva, D.M., Galdino, P.M., Paula, J.R., Romão, W., Costa, H.B., Vaz, B.G., Ghedini, P.C., Costa, E.A., 2014. Mechanisms involved in the gastroprotective activity of Celtis iguanaea (Jacq.) Sargent on gastric lesions in mice. J. Ethnopharmacol. 155, 1616-1624.
- Martins, J.L.R., Sousa, F.B., Fajemiroye, J.O., Ghedini, P.C., Ferreira, P.M., Costa, E.A., 2014. Anti-ulcerogenic and antisecretory effects of Celtis iguanaea (Jacq.) Sargent hexane leaf extract. Rev. Bras. Plantas Med. 16, 250-255.
- Misra, H.P., Fridovich, I., 1972. The role of superoxide anion in the auto-oxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 247, 3170-3175.
- Mitsios, J.V., Papathanasiou, A.I., Goudevenos, J.A., Tselepis, A.D., 2010. The antiplatelet and antithrombotic actions of statins. Curr. Pharm. Des. 16, 3808-3814.
- Mocelin, R., Marcon, M., Santo, G.D., Zanatta, L., Sachett, A., Schönell, A.P., Bevilaqua, F., Giachini, M., Chitolina, R., Wildner, S.M., Duarte, M.M.M.F., Conterato, G.M.M., Piato, A.L., Gomes, D.B., Roman Junior, W.A., 2016. Hypolipidemic and antiatherogenic effects of Cynara scolymus in cholesterol-fed rats. Rev. Bras. Farmacogn. 26, 233-239.
- Mohamed, G.A., Ibrahim, S.R.M., Elkhayat, E.S., El Dine, R.S., 2014. Natural anti-obesity agents. Bull. Fac. Pharm. Cairo Univ. 52, 269-284.
- Moore, K.W., de Waal Malefyt, R., Coffman, R.L., O’Garra, A., 2001. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 19, 683-765.
- Nirosha, K., Divya, M., Vamsi, S., Sadiq, M., 2014. A review on hyperlipidemia. IJNTPS 4, 81-92.
- Ohkawa, H., Ohishi, N., Yagi, K., 1979. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 95, 351-358.
- Pankaj, G.J., Savita, D.P., Nitin, G.H., Manoj, V.G., Sanjay, J.S., 2010. Hypolipidemic activity of Moringa oleifera Lam., Moringaceae, on high fat diet induced hyperlipidemia in albino rats. Rev. Bras. Farmacogn. 20, 969-973.
- Paula, M.A., Couto, R.O., Bara, M.T.F., Rezende, M.H., Paula, J.R., Costa, E.A., 2010. Caracterização farmacognóstica da Celtis iguanaea (Jacq.) Sargent. Lat. Am. J. Pharm. 29, 526-533.
- Pereira, D.F., Cazarolli, L.H., Lavado, C., Mengatto, V., Figueiredo, M.S.R.B., Guedes, A., Pizzolatti, M.G., Silva, F.R.M.B., 2011. Effects of flavonoids on α-glucosidase activity: potential targets for glucose homeostasis. Nutrition 27, 1161-1167.
- Prophet, E.B., Mills, B., Arrington, J.B., Sobin, L.H., 1992. Laboratory Methods in Histotechnology. Armed Forces Institute of Pathology, Washington, DC.
- Puttananjaiah, M.K., Dhale, M.A., Gaonkar, V., Keni, S., 2011. Reductase inhibitors demonstrate anti-atherosclerotic character due to their antioxidant capacity. Appl. Biochem. Biotechnol. 163, 215-222.
- Quiñones, M., Miguel, M., Aleixandre, A., 2013. Beneficial effects of polyphenols on cardiovascular disease. Pharmacol. Res. 68, 125-131.
- Ridker, P.M., Rifai, N., Rose, L., Buring, J.E., Cook, N.R., 2002. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N. Engl. J. Med. 347, 1557-1565.
- Shimoda, H., Seki, E., Aitani, M., 2006. Inhibitory effect of green coffee bean extract on fat accumulation and body weight gain in mice. BMC Complement. Altern. Med. 6, 9-13.
- Silva, C.S.P., Proença, C.E.B., 2008. Uso e disponibilidade de recursos medicinais no município de Ouro Verde de Goiás, GO, Brasil. Acta Bot. Bras. 22, 481-492.
- Sousa, F.B., Martins, J.L.R., Florentino, I.F., Couto, R.O., Nascimento, M.V.M., Galdino, P.M., Ghedini, P.C., Paula, J.R., Costa, E.A., 2013. Preliminary studies of gastroprotective effect of Celtis iguanaea (Jacq.) Sargent leaves (Ulmaceae). Nat. Prod. Res. 27, 1102-1107.
- Sposito, A.C., Caramelli, B., Fonseca, F.A.H., Bertolami, M.C., 2007. IV Diretriz Brasileira sobre Dislipidemias e Prevenção da Aterosclerose: Departamento de Aterosclerose da Sociedade Brasileira de Cardiologia. Arq. Bras. Cardiol. 88, 2-19.
- Stroes, E.S., Thompson, P.D., Corsini, A., Vladutiu, G.D., Raal, F.J., Ray, K.K., Roden, M., Stein, E., Tokgõzoglu, L., Nordestgaard, B.G., Bruckert, E., Krauss, R.M., Laufs, U., Santos, R.D., Mãrz, W., Newman, C.B., Chapman, M.J., Ginsberg, H.N., Chapman, M., Ginsberg, H.N., Backer, G., Catapano, A.L., Hegele, R.A., Hovingh, G.K., Jacobson, T.A., Leiter, L., Mach, F., Wiklund, O., 2015. Statin-associated muscle symptoms: impact on statin therapy European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management. Eur. Heart J. 18, 2-13.
- Tene, V., Malagóri, O., Finzi, P.V., Vidari, G., Armijos, C., Zaragoza, T., 2007. Anethnobotanical survey of medicinal plants used in Loja and Zamora-Chinchipe, Ecuador. J. Ethnopharmacol. 111, 63-81.
- Toma, A., Makonnen, E., Mekonnen, Y., Debella, A., Addisakwattana, S., 2014. Intestinal α-glucosidase and some pancreatic enzymes inhibitory effect of hydroalcholic extract of Moringa stenopetala leaves. BMC Complement. Altern. Med. 14, 1-5.
- Trevisan, R.R., Lima, C.P., Miyazaki, C.M.S., Pesci, F.A., Silva, C.B., Hirota, B.C.K., Lordello, A.L.L., Miguel, O.G., Miguel, M.D., Zanin, S.M.W., 2012. Evaluation of the phytotoxic activity focused on the allelopathic effect of the extract from the bark of Celtis iguanaea (Jacq.) Sargent Ulmaceae and purification of two terpenes. Rev. Bras. Plantas Med. 14, 494-499.
- Tzoulaki, I., Murray, G.D., Lee, A.J., Rumley, A., Lowe, G.D., Fowkes, F.G., 2005. C-reactive protein, interleukin-6, and soluble adhesion molecules as predictors of progressive peripheral atherosclerosis in the general population. Edinburgh Artery Study. Circulation 112, 976-983.
- Vaddi, K., Nicolini, F.A., Mehta, P., Metha, J.L., 1994. Increased secretion of tumor necrosis factor-alpha and interferon-gamma by mononuclear leukocytes inpatients with ischemic heart disease. Relevance in superoxide anion generation. Circulation 90, 694-699.
- Venugopala Rao, A., Ramakrishnan, S., 1975. Indirect assessment of hydroxymethylglutaryl-CoA reductase (NADPH) activity in liver tissue. Clin. Chem. 21, 1523-1525.
- Waltenberger, B., Mocan, A., Šmejkal, K., Heiss, E.H., Atanasov, A.G., 2016. Natural products to counteract the epidemic of cardiovascular and metabolic disorders. Molecules 21, 1-33.
- WHO, 2016a. Cardiovascular Diseases (CVDs). World Health Organization, Geneva.
- WHO, 2016b. Global Report on Diabetes. World Health Organization, Geneva.
- Xia, W., Sun, C., Zhao, Y., Wu, L., 2011. Hypolipidemic e antioxidant activities of Sanchi (Radix Notoginseng) in rats with a high fat diet. Phytomedicine 18, 516-520.
- Yoo, H.Y., Kum, S.K., Lee, T.H., Bae, J.S., 2014. Orientin inhibits HMGB1-induced inflammatory responses in HUVECs and in murine polymicrobial sepsis. Inflammation 37, 1705-1717.
- Youn, J.Y., Siu, K.L., Lob, H.E., Itani, H., Harrison, D.G., Cai, H., 2014. Role of vascular oxidative stress in obesity and metabolic syndrome. Diabetes 63, 2344-2355.
- Zhang, Y., Chen, S., Wei, C., Gong, H., Li, L., Ye, X., 2016. Chemical and cellular assays combined with in vitro digestion to determine the antioxidant activity of flavonoids from Chinese bayberry (Myrica rubra Sieb. et Zucc.) Leaves. PLOS ONE, http://dx.doi.org/10.1371/journal.pone.0167484
» http://dx.doi.org/10.1371/journal.pone.0167484
Publication Dates
-
Publication in this collection
Jan-Feb 2018
History
-
Received
17 Sept 2017 -
Accepted
5 Dec 2017