Abstract
In this study, mango seed kernels extract contained a considerable amount of phenolics and flavonoids (17,400 and 3325 mg/100 g seed, respectively). The HPLC profiling revealed that hesperidin was the major phenolic compound of the mango seed kernels extract. This is the first report find hesperidin in mango extracts. The phenolic compounds of mango seed kernels extract were effective in scavenging free radicals of DPPH and ABTS with IC50 values of 47.3 and 7.9 µg/ml, respectively. The total antioxidant activity of mango seed kernels extract based on the reduction of molybdenum was also measured. The phenolic compounds of mango seed kernels extract potentially inhibited the protease, fibrinogenase, phospholipase A2, l-amino acid oxidase, hyaluronidase, and hemolytic activities of the most dangerous Cerastes cerastes and Echis coloratus viper venoms. The phenolic compounds of mango seed kernels extract could completely neutralize the hemorrhage and lethality of both venoms in experimental animals. It could be concluded that the mango seed kernels extract phenolic compounds with potential antioxidant activity are considered as a new avenue in the viper bite treatment.
Keywords
Antioxidant; Anti-venom; Egyptian mango; Oxidative stress; Phenolic compounds
Introduction
Mango (Mangifera indica L., Anacardiaceae) is one of the most important tropical fruits in the world. Mango fruit occupies the second position as a tropical crop with a global production exceeding 35 million tons (Jahurul et al., 2015Jahurul, M.H.A., Zaidul, I.S.M., Kashif, G., Fahad, Y., Al-Juhaimi, Kar-Lin Nyam, N.A.N., Sahena, F., Mohd Omar, A.K., 2015. Mango (Mangifera indica L.) by-products and their valuable components: a review. Food Chem. 183, 173-180.). In Egypt, over 4 million tons of mango fruits are produced (Abdalla et al., 2007Abdalla, A.E.M., Darwish, S.M., Ayad, E.H.E., El-Hamahmy, R.M., 2007. Egyptian mango by-product 1: compositional quality of mango seed kernel. Food Chem. 103, 1134-1140.). Mango fruits are processed in various products as puree, canned slices, syrup, nectar, leather, pickles, chutney and jam, resulting in a significant amount of wastes. Mango seed kernel represents about 20% of the whole mango fruit and includes rich levels of health-enhancing compounds and natural antioxidants (Sogi et al., 2013Sogi, D.S., Siddiq, M., Greiby, I., Dolan, K.D., 2013. Total phenolics, antioxidant activity, and functional properties of ‘Tommy Atkins’ mango peel and kernel as affected by drying methods. Food Chem. 141, 2649-2655.; Jahurul et al., 2015Jahurul, M.H.A., Zaidul, I.S.M., Kashif, G., Fahad, Y., Al-Juhaimi, Kar-Lin Nyam, N.A.N., Sahena, F., Mohd Omar, A.K., 2015. Mango (Mangifera indica L.) by-products and their valuable components: a review. Food Chem. 183, 173-180.). Further, kernels have anti-tyrosinase, anti-inflammatory, anti-obesity, and hepato-protective effects (Kobayashi et al., 2013Kobayashi, M., Matsui-Yuasa, I., Fukuda-Shimizu, M., Mandai, Y., Tabuchi, M., Munakata, H., Kojima-Yuasa, A., 2013. Effect of mango seed kernel extract on the adipogenesis in 3T3-L1 adipocytes and in rats fed a high fat diet. Health (N.Y.) 5, 9-15.).
Vipers are the main cause (over 80%) of snake bite worldwide. Venoms of the vipers are plentiful sources of active molecules that affect a large number of physiological functions. These molecules include diverse hydrolytic enzymes that causing local harmful effects like hemorrhage, necrosis and edema end with tissue loss. They also cause systemic effects resulting in coagulopathy, cardiopathy, and neuropathy (Oussedik-Oumehdi and Laraba-Djebari, 2008Oussedik-Oumehdi, H., Laraba-Djebari, F., 2008. Irradiated Cerastes cerastes venom as a novel tool for immunotherapy. Immunopharm. Immunot. 30, 37-43.). Furthermore, viper venom causes various complications as thrombocytopenia, renal abnormalities, hypopituitarism and permanent tissue damage. Oxidative stress plays a main role in viper bite pathophysiology and the constancy of the viper bite complications (Zengin et al., 2012Zengin, S., Al, B., Yarbil, P., Guzel, R., Orkmez, M., Yildirim, C., Taysi, S., 2012. An assessment of oxidant/antioxidant status in patients with snake envenomation. Emerg. Med. J. 31, 48-52.; Sunitha et al., 2015Sunitha, K., Hemshekhar, M., Thushara, R.M., Santhosh, M.S., Sundaram, M.S., Kemparaju, M.K., Girish, K.S., 2015. Inflammation and oxidative stress in viper bite: an insight within and beyond. Toxicon 98, 89-97.). Insufficiency of anti-venom to reverse these complications leads to viper bite treatment still a challenge till now (Girish and Kemparaju, 2011Girish, K.S., Kemparaju, K., 2011. Overlooked issues of snakebite management: time for strategic approach. Curr. Top. Med. Chem. 11, 2494-2508.). Cerastes cerastes (Linnaeus, 1758) (horn viper) and Echis coloratus Günther, 1878 (red carpet viper) are the most dangerous and medically important vipers in Egypt and are responsible for the greatest incidence of envenomation (Wahby et al., 2012Wahby, A.F., Abdel-Aty, A.M., El-Kady, E.M., 2012. Purification of hemorrhagic SVMPs from venoms of three vipers of Egypt. Toxicon 59, 329-337.).
The recovery and utilization of valuable compounds from mango by-products are an important challenge, hence, this study aims to determine the total phenolic, flavonoid contents and antioxidant capacity of Egyptian mango seed kernel extract (MSKE) and to assess its anti-venom properties against Egyptian Cerastes cerastes and Echis coloratus viper venoms.
Materials and methods
Plant
Egyptian mango Hindi cultivar (Mangifera indica L., Anacardiaceae) was obtained from Horticulture Institute Research, Agriculture Research Centre, Cairo, Egypt.
Animal
Cerastes cerastes (Linnaeus, 1758) and Echis coloratus Günther, 1878 adult vipers were obtained from laboratory animal unit of Helwan Farm-VACSERA, Egypt. The C. cerastes and E. coloratus venoms were milked, lyophilized and stored at -20 ºC.
Male Swiss-albino mice weighing (20 ± 2.4 g) were used for this study. All animals were housed at the animal house of the National Research Centre (NRC), under standardized conditions and diet. All the experimental protocols described in this study were performed in accordance with the recommendations of the ethical committee, NRC, Egypt (Protocol permit #15-097).
Plant extraction
Mango seed kernel Hindi cultivar (8 g) were chopped, grinded, soaked in 60 ml of 80% methanol and shaken overnight at 120 rpm and room temperature. The extract was centrifuged at 7200 × g for 10 min. The supernatant was evaporated under vacuum and dissolved in least volume of 0.1% DMSO and designated as a mango seed kernel extract (MSKE).
Total phenols measurement
The total phenolic content of MSKE was measured according to Velioglu et al. (1998)Velioglu, Y.S., Mazza, G., Gao, L., Oomah, B.D., 1998. Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. J. Agric. Food Chem. 46, 4113-4117.. The reaction mixture includes: 0.1 ml methanol extract, 0.1 ml Folin-Ciocalteu reagent and 0.8 ml distilled water were incubated for 5 min at room temperature. Then 0.5 ml sodium carbonate (20%) was added and incubated at room temperature for 30 min. The absorbance was measured at 750 nm. The results were expressed as mg gallic acid equivalent (GAE)/100 g seed.
Total flavonoids measurement
The total flavonoid content of MSKE was measured according to Zhishen et al. (1999)Zhishen, J., Mengcheng, T., Jianming, W., 1999. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 64, 555-559.. Incubation of 0.25 ml methanol extract, 1.25 ml distilled water and 0.075 ml of 5% NaNO2 for 6 min, then add 0.15 ml of 10% AlCl3. After 5 min, 0.5 ml of 1.0 M NaOH and 0.275 ml distilled water were added. The absorbance was measured at 510 nm. The results were expressed as mg catechin equivalent (CE)/100 g seed.
HPLC analysis of phenolic compounds
The high performance liquid chromatography (HPLC) analysis was carried out for MSKE according to Kim et al. (2006)Kim, K.H., Tsao, R., Yang, R., Cui, S.W., 2006. Phenolic acid profiles and antioxidant activities of wheat bran extracts and the effect of hydrolysis conditions. Food Chem. 95, 466-473.. The separation and determination were performed on XDB-C18 column (150 × 4.6 µm). The column was eluted by acetonitrile (solvent A) and 2% acetic acid (solvent B) at a flow rate of 1 ml/min. The obtained peaks were monitored simultaneously at 280, 320 and 360 nm. Commercial phenolic compounds were used as standards.
Antioxidant assays
DPPH assay
1,1-Diphenyl-2-picrylhydrazyl (DPPH) method was used for determination of the antioxidant activity of MSKE (Ao et al., 2008Ao, C., Li, A., Elzaawely, A., Xuan, T.D., Tawata, S., 2008. Evaluation of antioxidant and antibacterial activities of Ficus microcarpa L. extract. Food Control 19, 940-948.). The reaction mixture includes: 0.1 ml methanol extract and 0.9 ml of 0.1 mM DPPH dissolved in methanol were incubated for 30 min at room temperature. The absorbance was measured at 517 nm. DPPH scavenging percent = [(O.D. control - O.D. sample)/O.D. control] × 100.
ABTS assay
ABTS (2,2′-azino-bis (3-ethylbenzo-thiazoline-6-sulfonic acid) reagent was prepared and used for determination of the antioxidant activity of MSKE (Re et al., 1999Re, R., Pellegrini, N., Proteggente, A., Pannala, A., Yang, M., Rice-Evans, C., 1999. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 26, 1231-1237.). The reaction mixture includes: 1 ml of ABTS reagent and 0.1 ml of the extract were incubated for 1 min at room temperature and the reduction of absorbance was measured at 734 nm. ABTS scavenging percent = [(O.D. control - O.D. sample)/O.D. control] × 100.
Phosphomolybdenum complex assay
The antioxidant activity of MSKE was also evaluated by formation of a phosphomolybdenum complex according to Prieto et al. (1999)Prieto, P., Pineda, M., Aguilar, M., 1999. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal Biochem. 269, 337-341.. The reaction mixture includes: 4 mM ammonium molybdate, 28 mM sodium phosphate, 600 mM sulfuric acid, and 50 µl of the extract were incubated at 95 ºC for 90 min. After cooling, the absorbance was read at 695 nm. EC50 is defined as a concentration of the MSKE gives absorbance of 0.5.
Protease inhibition
Azocasein assay
The protease activity of crude venom was measured according to Lemos et al. (1991)Lemos, F.J.A., Campos, F.A.P., Silva, C.P., Xavier-Filho, J., 1991. Proteinases and amylases of larval midgut of Zabrotes subfasciatus reared on cowpea (Vigna unguiculata) seeds. Entomol. Exp. Appl. 56, 219-228. using azocasein as a substrate. One ml reaction mixture includes: 0.2% azocasein, 10 µg of crude venom and 0.02 M Tris–HCl buffer, pH 7.0 were incubated at 37 ºC. The reaction was stopped after 1 h by adding 100 µl of 20% TCA and precipitation removed by centrifugation at 5000 × g for 5 min. The change of absorbance (0.01 O.D.) was measured at 366 nm which considered as a one unit/h. Inhibition studies, the crude venom (10 µg) was pre-incubated with various concentrations of MSKE for 15 min at 37 ºC and measurement of residual activity.
Gelatin zymography
Gelatin zymography of crude venom (30 µg) was performed in 12% native polyacrylamide gel co-polymerized with 0.2% gelatin according to Bee et al. (2001)Bee, A., Theakston, R.D.G., Harrison, R.A., Cartera, S.D., 2001. Novel in vitro assays for assessing the hemorrhagic activity of snake venoms and for demonstration of venom metalloproteinase inhibitors. Toxicon 39, 1429-1434.. After electrophoresis, the gel was incubated overnight in 0.02 M Tris–HCl buffer, pH 7.0 at 37 ºC. After gel staining by Coomassie Brilliant Blue R-250, clear zones were appeared. Inhibition experiments, 30 µg of crude venom was pre-incubated with different concentrations of MSKE for 15 min at 37 ºC and performed according to the method previously described.
Fibrinogen degradation inhibition
Fibrinogenase activity of crude venom was determined according to Ouyang and Teng (1976)Ouyang, C., Teng, C.M., 1976. Fibrinogenolytic enzymes of Trimeresurus mucrosquamatus venom. Biochim. Biophys. Acta 420, 298-308.. One ml reaction mixture includes: Human plasma fibrinogen (2 mg), venom sample (2 µg) and 5 mM Tris–HCl buffer, pH 7.5 containing 5 mM CaCl2 were incubated at 37 ºC. The reaction was stopped after 2 h by adding 100 µl of stopping solution (4% SDS, 4% 2-mercaptoethanol and 10 M urea). The samples were electrophoresis on 10% SDS-PAGE according to Laemmli (1970)Laemmli, U.K., 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680-685.. Inhibition experiments were performed by pre-incubated venom sample with different concentrations of MSKE for 15 min at 37 ºC and performed according to the method previously described.
Phospholipase A2 (PLA2) inhibition
PLA2 activity of crude venom was determined using egg yolk as a substrate according to Marinetti (1965)Marinetti, G.V., 1965. The action of phospholipase A on lipoproteins. Biochem. Biophys. Acta 60, 554-565.. One ml of egg yolk solution (1:5 w/v saline), crude venom alone (200 µg) or pre-incubated with different phenolic concentrations of MSKE at 37 ºC for 15 min were mixed and reached to a final volume of 5 ml with saline. The absorbance was recorded each 5 min for 15 min at 900 nm. PLA2 activity was also determined according to Gutierrez et al. (1988)Gutierrez, J.M., Avila, C., Rojas, E., Cerdas, L., 1988. An alternative in vitro method for testing the potency of the polyvalent anti-venom produced in Costa Rica. Toxicon 26, 411-413.. Crude venom (200 µg) alone or preincubated with various concentrations of MSKE for 15 min at 37 ºC was loaded into wells (3 mm diameter) of 1% agarose plates containing 4% washed human erythrocytes, 4% egg yolk suspension and 10 mM CaCl2. The fortified plate was incubated for 20 h at 37 ºC. The decreasing of the clear zone diameters around the wells was measured and equivalent to the PLA2 inhibition. The venom PLA2 activity in the absence of MSKE was taken as a 100%.
l-Amino acid oxidase inhibition
l-Amino acid oxidase (LAAO) activity of crude venom was measured according to Kishimoto and Takahashi (2001)Kishimoto, M., Takahashi, T., 2001. A spectrophotometric microplate assay for L-amino-acid oxidase. Anal. Biochem. 298, 136-139.. The reaction mixture includes: 0.005 M l-leucine, as a substrate, horseradish peroxidase (5 U/ml) and 0.002 M O-phenylenediamine, as a substrate for peroxidase, 2 µg of venom sample and 0.05 M of Tris–HCl buffer, pH 8.0 were incubated at 37 ºC. The reaction was stopped after 1 h by adding 50 µl of 2 M H2SO4. The absorbance was measured at 490 nm using ELISA reader. One unit of LAAO enzyme is the amount of enzyme which produces 1 µmol of H2O2. Inhibition experiments were performed by pre-incubated venom sample with different concentrations of MSKE at 37 ºC for 15 min and measurement of remaining activity. Crude venom alone (a positive control) was taken as a 100%.
Hyaluronidase inhibition
Hyaluronidase activity of crude venom was measured according to Pukrittayakamee et al. (1988)Pukrittayakamee, S., Warrel, D.A., Deasakorn, V., McMichael, A.J., White, N.J., Bunnag, G.D., 1988. The hyaluronidase activities of some Southeast Asian snake venoms. Toxicon 34, 1119-1125.. The reaction mixture includes: 50 µg hyaluronic acid, 10 µg of crude venom (in a final volume of 500 µl) and 200 mM sodium acetate buffer, pH 5.5, including 0.15 M NaCl were incubated at 37 ºC. The reaction was stopped after 15 min by adding 100 µl of (2.5% acetyltrimethylammonium bromide in 2% NaOH). The absorbance was recorded at 400 nm. One unit is defined as a concentration of enzyme that causes 50% turbidity reduction. Inhibition experiments, 10 µg of crude venom was pre-incubated with different concentrations of MSKE at 37 ºC for 15 min and measurement of the residual activity. Crude venom alone (a positive control) was taken as a 100%.
Hemolytic inhibition
The hemolytic activity of crude venom was determined according to Al-Abdulla et al. (1991)Al-Abdulla, I.H., Sidki, A.M., Landon, J., 1991. An indirect hemolytic assay for assessing anti-venoms. Toxicon 29, 1043-1046.. The reaction mixture includes: 100 µl of egg yolk solution (diluted 1:5 in saline), 1 ml of 2.5% washed human erythrocytes (v/v), 3.8 ml of 0.001 M Tris–HCl buffer, pH 7.5 containing 0.1 M NaCl, 0.1 M KCl and 0.01 M CaCl2 and 10 µg of crude venom alone or pre-incubated with various concentrations of MSKE at 37 ºC for 15 min. The reaction mixture was centrifuged at 2500 rpm for 10 min and the concentration of hemoglobin that released was estimated at 540 nm.
Hemorrhagic inhibition
Hemorrhagic activity of crude venom was evaluated by Kondo et al. (1960)Kondo, H., Kondo, S., Ikezawa, H., Murata, R., 1960. Studies on the quantitative method for the determination of hemorrhagic activity of Habu snake venom. Jpn. J. Med. Sci. Biol. 13, 43-52.. Mice groups (n = 5) were injected with venom samples dorsally and intradermally in 0.1 ml of saline. Groups (a1) and (a2) were injected with two minimum hemorrhagic doses (2MHD) of C. cerastes and E. coloratus venoms, respectively. Groups (b1–f1) and (b2–d2) were injected with 2MHD of C. cerastes and E. coloratus venoms pre-incubated for 15 min at 37 ºC with various concentrations of the MSKE, respectively. Groups (g2) and (K2) were injected with 0.1 ml of MSKE and saline, as controls, respectively. After 2 h, the mice skins were removed and the diameters of the hemorrhagic spots were recorded.
Neutralization of lethal potency of crude venoms
The median lethal dose (LD50) of C. cerastes and E. coloratus venoms was measured according to Meier and Theakston (1986)Meier, J., Theakston, R.D., 1986. Approximate LD50 determinations of snake venoms using eight to ten experimental animals. Toxicon 24, 395-401.. Mice groups (n = 5) were injected intraperitonially with different concentrations of venom (2–16 µg protein/mouse) in 0.5 ml of saline. In neutralization studies, groups 1 and 5 were injected with 2LD50 of C. cerastes and E. coloratus venoms, respectively. Groups 2–4 and groups 6–7 were injected with 2LD50 of C. cerastes and E. coloratus venoms, respectively, and after 10 min following venom injection different phenolic concentrations of the MSKE were injected individually. Groups 8 and 9 were received saline and 500 µg phenolic of the MSKE, respectively. The survival time of mice in each group was recorded during 24 h.
Statistical analysis
The data were statistically analyzed by a one-way ANOVA, followed by Dennett's post hoc test. Differences were significant at p < 0.01. The results were expressed as mean ± S.E.
Results and discussion
The total phenolic content of MSKE was found to be 17,400 ± 348 mg GAE/100 g seed (Table 1). Different of total phenolic contents ranged from 112 to 44,760 mg/100 g seed were reported in other mango cultivars (Abdalla et al., 2007;Abdalla, A.E.M., Darwish, S.M., Ayad, E.H.E., El-Hamahmy, R.M., 2007. Egyptian mango by-product 1: compositional quality of mango seed kernel. Food Chem. 103, 1134-1140. Ribeiro et al., 2008Ribeiro, S.M.R., Barbosa, L.C.A., Queiroz, J.H., Knödler, M., Schieber, A., 2008. Phenolic compounds and antioxidant capacity of Brazilian mango (Mangifera indica L.) varieties. Food Chem. 110, 620-626.; Sogi et al., 2013Sogi, D.S., Siddiq, M., Greiby, I., Dolan, K.D., 2013. Total phenolics, antioxidant activity, and functional properties of ‘Tommy Atkins’ mango peel and kernel as affected by drying methods. Food Chem. 141, 2649-2655.; Dorta et al., 2014Dorta, E., Gonzez, M., Lobo, M.G., Snchez-Moreno, C., Ancos, B., 2014. Screening of phenolic compounds in by-product extracts from mangoes (Mangifera indica L.) by HPLC–ESI–QTOF–MS and multivariate analysis for use as a food ingredient. Food Res. Int. 57, 51-60.). These differences in phenolic contents might be due to mango cultivars, geographical location, extraction conditions and used different standard equivalents.
Total phenolic, flavonoid contents and antioxidant capacity of the mango seed kernels extract.
The total flavonoid content of MSKE was found to be 3325 ± 120 mg CE/100 g seed (Table 1). The obtained results were much higher than that reported for peel and kernel of other mango cultivars (10–1170 mg/100 g) (Ribeiro et al., 2008Ribeiro, S.M.R., Barbosa, L.C.A., Queiroz, J.H., Knödler, M., Schieber, A., 2008. Phenolic compounds and antioxidant capacity of Brazilian mango (Mangifera indica L.) varieties. Food Chem. 110, 620-626.; Dorta et al., 2012Dorta, E., Lobo, M.G., González, M., 2012. Using drying treatments to stabilize mango peel and seed: effect on antioxidant activity. LWT Food Sci. Technol. 45, 261-268.; Ajila and Rao, 2013Ajila, C.M., Rao, U.J.S.P., 2013. Mango peel dietary fiber: composition and associated bound phenolics. J. Funct. Foods 202, 11-17.; Dorta et al., 2014Dorta, E., Gonzez, M., Lobo, M.G., Snchez-Moreno, C., Ancos, B., 2014. Screening of phenolic compounds in by-product extracts from mangoes (Mangifera indica L.) by HPLC–ESI–QTOF–MS and multivariate analysis for use as a food ingredient. Food Res. Int. 57, 51-60.).
Different phenolic compounds were identified and quantified by HPLC technique (Table 2). Hesperidin was the major compound in MSKE which represented 3000 ± 112 mg/100 g seed and 55.6% of total polyphenols, followed by cinnamic and tannic acids which represented 1200 ± 52 and 987 ± 44 mg/100 g seed with 22.2 and 18.3% of total polyphenols, respectively. Whereas MSKE contained minor amount of gallic, protocatechuic, p-coumaric, catachine, and cholorgenic. This is the first report find hesperidin in mango extracts. However, hesperidin was extracted in large concentrations from the discarded rinds of the ordinary orange (Kanes et al., 1993Kanes, K., Tisserat, B., Berhow, M., Vandercook, C., 1993. Phenolic composition of various tissues of Rutaceae species. Phytochemistry 324, 967-974.).
The MSKE showed a concentration dependent scavenging of DPPH and ABTS radicals with IC50 values 47.3 ± 0.85 and 7.9 ± 0.14 µg/ml, respectively (Table 1). The scavenging activity of MSKE was attributed to its phenolic compounds, like hesperdin, cinnamic, and tannin (Kim et al., 2004Kim, J.Y., Jung, K.J., Choi, J.S., Chung, H.Y., 2004. Hesperetin: a potent antioxidant against peroxynitrite. Free Radic. Res. 38, 761-769.; Abdalla et al., 2007Abdalla, A.E.M., Darwish, S.M., Ayad, E.H.E., El-Hamahmy, R.M., 2007. Egyptian mango by-product 1: compositional quality of mango seed kernel. Food Chem. 103, 1134-1140.). Total antioxidant capacity of the MSKE based on the reduction of molybdenum (VI) to molybdenum (V) was also measured with EC50 value of 4.0 ± 0.11 µg/ml (Table 1). Many studies reported that mango seed kernel is a rich source of natural antioxidants (Abdalla et al., 2007Abdalla, A.E.M., Darwish, S.M., Ayad, E.H.E., El-Hamahmy, R.M., 2007. Egyptian mango by-product 1: compositional quality of mango seed kernel. Food Chem. 103, 1134-1140.; Jahurul et al., 2015Jahurul, M.H.A., Zaidul, I.S.M., Kashif, G., Fahad, Y., Al-Juhaimi, Kar-Lin Nyam, N.A.N., Sahena, F., Mohd Omar, A.K., 2015. Mango (Mangifera indica L.) by-products and their valuable components: a review. Food Chem. 183, 173-180.).
Viper venom contains a set of multifunctional proteases like snake venom metalloproteases (SVMP), serine and thrombin like proteases. These proteases cause numerous pathologies including hemorrhage, necrosis, hemostatic disturbances, inflammation and alter the prey redox state (Markland and Swenson, 2013Markland, F.S., Swenson, S., 2013. Snake venom metalloproteinases. Toxicon 62, 3-18.; Sunitha et al., 2015Sunitha, K., Hemshekhar, M., Thushara, R.M., Santhosh, M.S., Sundaram, M.S., Kemparaju, M.K., Girish, K.S., 2015. Inflammation and oxidative stress in viper bite: an insight within and beyond. Toxicon 98, 89-97.). The MSKE exhibited 50% inhibition (IC50) of the C. cerastes and E. coloratus protease activity at 18 and 9 µg phenolics, respectively and full inhibition for both venoms was given at 30 µg phenolics (Fig. 1A). However, full inhibition for C. cerastes and E. coloratus protease zones was achieved at 80 and 40 µg phenolic of the MSKE in gelatin zymography (Fig. 1B and 1C), respectively.
Inhibition of Cerastes cerastes (Cc) and Echis coloratus (Eco) protease activity by the mango seed kernels extract (MSKE). (A) Ten µg of crude venoms were pre-incubated individually with different phenolic concentrations of the MSKE for 15 min at 37 ºC and the residual activity % was measured using the standard assay. The values represent mean ± S.E. (n = 3) and the results p > 0.01 (a) were considered as significant. (B) Gelatin zymography of C. cerastes venom protease inhibition by the MSKE. Lane 1, 30 µg of C. cerastes venom sample alone, and lanes (2–5) 30 µg of C. cerastes venom pre-incubated with 10, 20, 40 and 80 µg phenolic of the MSKE, respectively. (C) Gelatin zymography of E. coloratus venom protease inhibition by the MSKE. Lane 1, 30 µg of E. coloratus venom alone, and lanes (2–5) 30 µg of E. coloratus venom pre-incubated with 10, 20, 30 and 40 µg phenolic of the MSKE, respectively.
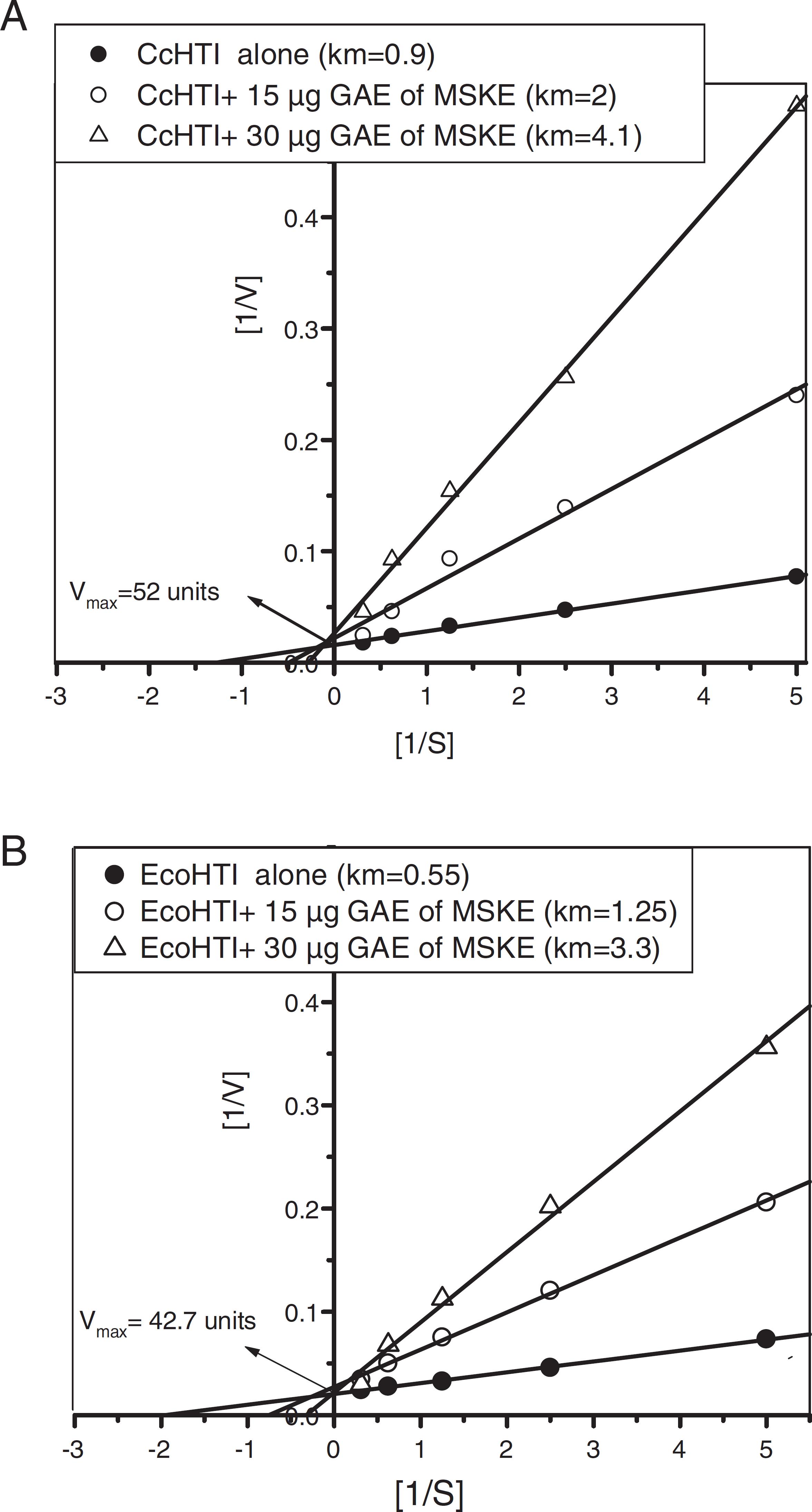
For studying the mode of inhibition by MSKE, the kinetic of the CcHTI and EcoHTI metalloproteases, previously purified from Egyptian C. cerastes and E. coloratus venoms (Wahby et al., 2012Wahby, A.F., Abdel-Aty, A.M., El-Kady, E.M., 2012. Purification of hemorrhagic SVMPs from venoms of three vipers of Egypt. Toxicon 59, 329-337.), was evaluated in presence of the MSKE. The k m values of the CcHTI and EcoHTI enzymes increased from 0.9 and 0.55 to 2.0, 1.25, and 4.1, 3.3 mg at 15 and 30 µg phenolic of the MSKE, respectively. The V max values of the CcHTI and EcoHTI were unaffected (Fig. 2A and B). This increase in the K m values reflects the decline of the affinity of both enzymes toward the azocasein, as a substrate, and at the same time reflects the competitive inhibitory effect of the phenolic compounds of the MSKE on the active sites of both enzymes.
Lineweaver–Burk plot for inhibition of CcHTI (A) and EcoHTI (B) metlalloproteases toward azocasein as a substrate by 15 and 30 µg phenolic of the mango seed kernels extract (MSKE).
The action of venom proteases on fibrinogen lead to unstable fibrin, causing change in blood coagulation and platelet function (Mc-Cleary and Kini, 2013Mc-Cleary, R.J., Kini, R.M., 2013. Snake bites and hemostasis/thrombosis. Thrombosis Res. 132, 642-646.). When the C. cerastes and E. coloratus venoms (2 µg) were individually incubated with fibrinogen (2 mg/ml) for 2 h, α and β chains of the fibrinogen were completely digested. Whereas the ð-chain of the fibrinogen partially digested, demonstrating that both venoms have a potential fibrinogenase activity. At 1–4 µg of the MSKE, the degradation inhibition of β and ð chains was preferred over α chain in both venoms. At 6 and 10 µg of the MSKE, full inhibition of α, β and ð chains degradation was achieved in E. coloratus and C. cerastes venoms, respectively (Fig. 3A and B) suggesting that the MSKE inhibits proteases induced hemostatic disturbances. Thai mango ethanolic extract inhibited the fibriongenase activity of the Malayan and Thai cobra venoms (Pithayanukul et al., 2009Pithayanukul, P., Leanpolchareanchai, J., Saparpakorn, P., 2009. Molecular docking studies and anti-snake venom metalloproteinase activity of Thai mango seed kernel extract. Molecules 14, 3198-3213.).
Inhibition of Cerastes cerastes and Echis coloratus fibrinogenase activity by the mango seed kernels extract (MSKE). (A) Lane (1) 2 mg fibrinogen/ml alone, lane (2) fibrinogen + 2 µg of C. cerastes venom, and lanes (3–8) fibrinogen + 2 µg of C. cerastes venom pre-incubated with 1, 2, 3, 4, 8 and 10 µg phenolic of the MSKE, respectively. (B) Lane (1) 2 mg fibrinogen/ml alone, lane (2) fibrinogen + 2 µg of E. coloratus venom, and lanes (3–8) fibrinogen + 2 µg of E. coloratus venom pre-incubated with 1, 2, 3, 4, 5 and 6 µg phenolic of the MSKE, respectively.
PLA2 plays a vital role in systemic oxidative stress and inflammation. It implicated in many necrotic actions on red blood cells (RBC) and skeletal muscle cells resulting in production of inflammatory mediators like prostaglandins and leukotrienes. These mediators are responsible for tissue damage (Barone et al., 2014Barone, J.M., Frezzatti, R., Silveira, P.F., 2014. Effects of N-acetyl-l-cysteine on redox status and markers of renal function in mice inoculated with Bothrops jararaca and Crotalus durissusterrificus venoms. Toxicon 79, 1-10.; Sunitha et al., 2015Sunitha, K., Hemshekhar, M., Thushara, R.M., Santhosh, M.S., Sundaram, M.S., Kemparaju, M.K., Girish, K.S., 2015. Inflammation and oxidative stress in viper bite: an insight within and beyond. Toxicon 98, 89-97.). For determination of PLA2 activity, 200 µg of both viper venoms was used because they showed a low PLA2 activity. This weak activity is probably related to a sub-group (2b) of sPLA2 which contains a Lys at position 49 instead of Asp. These sPLA2 have a low activity but retain high levels of toxicity and are found exclusively in viper venoms (Harris and Scott-Davey, 2013Harris, J.B., Scott-Davey, T., 2013. Secreted phospholipases A2 of snake venoms: effects on the peripheral neuromuscular system with comments on the role of phospholipases A2 in disorders of the CNS and their uses in industry. Toxins 5, 2533-2571.). The MSKE efficiently inhibited the PLA2 activity of the C. cerastes and E. coloratus venoms with IC50 values of 30 and 35 µg phenolics, respectively. Full inhibition was achieved at 50 µg phenolic of the MSKE for both venoms (Fig. 4A). The PLA2 inhibition was also confirmed by reduction the activity zone diameters on gel plate (Fig. 4B and C). At 80 µg of MSKE, 88 and 100% inhibition of PLA2 activity were observed in C. cerastes and E. coloratus venoms, respectively. Similarly, the hesperidin is a potent anti-inflammatory agent and inhibited snake PLA2 (Garg et al., 2001Garg, A., Garg, S., Zaneveld, L.J.D., Singla, A.K., 2001. Chemistry and pharmacology of the Citrus bioflavonoid hesperidin. Phytother. Res. 15, 655-669.; Soares et al., 2005Soares, A.M., Ticli, F.K., Marcussi, S., Lourenco, M.V., Januarioc, A., Sampaioa, S.V., Gigliob, J.R., Lomonted, B., Pereirac, P.S., 2005. Medicinal plants with inhibitory properties against snake venoms. Curr. Med. Chem. 12, 2625-2641.). Further, tannic acid had anti-snake effect which could be precipitated proteins, formed insoluble complexes with many metal ions and inhibited snake PLA2 (Haslam, 1996Haslam, E., 1996. Natural polyphenols (vegetable tannins) as drugs and medicine: possible modes of action. J. Nat. Prod. 59, 205-215.). Moreover, cinnamic acid was also tested as an anti-snake agent (Mors et al., 2000Mors, W.B., Nascimento, M.C., Pereira, B.M.R., Pereira, N.A., 2000. Plant natural products active against snake bite-the molecular approach. Phytochemistry 55, 627-642.).
Inhibition of Cerastes cerastes (Cc) and Echis coloratus (Eco) PLA2 activity by the mango seed kernels extract (MSKE). (A) Two hundred µg of each venom sample was pre-incubated with different phenolic concentrations of the MSKE for 15 min at 37 ºC and the residual activity % was measured using the standard assay. The values represent mean ± S.E. (n = 3) and the results p >0.01 (a) were considered as significant. (B) Inhibition of C. cerastes PLA2 activity in fortified agarose gel (1). Eighty µg phenolic of MSKE alone, (2) C. cerastes venom alone and (3–7) C. cerastes venom pre-incubated with 5, 10, 20, 40 and 80 µg phenolic of the MSKE, respectively. C. Inhibition of E. coloratus PLA2 activity in fortified agarose gel. (1) E. coloratus venom alone and (2–6) E. coloratus venom pre-incubated with 5, 10, 20, 40 and 80 µg phenolic of the MSKE, respectively.
LAAO are flavoenzymes which catalyze the oxidative deamination of l-amino acids to α-keto acids and liberate ammonia and H2O2. The excess production of H2O2 affect victim's endogenous antioxidant defense followed by apoptotic, cytotoxic, platelet aggregation, edema and hemorrhage (Sunitha et al., 2015Sunitha, K., Hemshekhar, M., Thushara, R.M., Santhosh, M.S., Sundaram, M.S., Kemparaju, M.K., Girish, K.S., 2015. Inflammation and oxidative stress in viper bite: an insight within and beyond. Toxicon 98, 89-97.). The MSKE inhibited the LAAO activity of C. cerastes and E. coloratus venoms with IC50 values of 16 and 18 µg phenolics, respectively. Full inhibition of both venoms LAAO activity was achieved at 40 µg phenolic of the MSKE (Fig. 5A). This inhibition may be due to the antioxidant potency of MSKE components. Kalpana et al. (2009)Kalpana, K.B., Srinivasan, M., Menon, V.P., 2009. Evaluation of antioxidant activity of hesperidin and its protective effect on H2O2 induced oxidative damage on pBR322 DNA and RBC cellular membrane. Mol. Cell Biochem. 323, 21-29. found that the hesperidin prevented H2O2-induced oxidative damage on the RBC.
Inhibition of l-amino acid oxidase activity (A) hyaluronidase activity (B) and hemolytic activity (C) of Cerastes cerastes (Cc) and Echis coloratus (Eco) venoms by the mango seed kernels extract (MSKE). Venom samples were pre-incubated individually with different phenolic concentrations of the MSKE for 15 min at 37 ºC and the residual activities % were measured using the standard assays. The values represent mean ± S.E. (n = 3) and the results p > 0.01 (a) were considered as significant.
Snake venom hyaluronidases are known as spreading factors. They facilitate inflow of the toxins from the bite site to the circulation (Kemparaju and Girish, 2006Kemparaju, K., Girish, K.S., 2006. Snake venom hyaluronidase: a therapeutic target. Cell. Biochem. Funct. 24, 7-12.). The IC50 and full inhibition of C. cerastes and E. coloratus hyaluronidase activity were recorded at 9, 30 and 14, 40 µg of MSKE, respectively (Fig. 5B). The hesperidin is considered as a potent hyaluronidase inhibitor (Garg et al., 2001Garg, A., Garg, S., Zaneveld, L.J.D., Singla, A.K., 2001. Chemistry and pharmacology of the Citrus bioflavonoid hesperidin. Phytother. Res. 15, 655-669.).
During viper bite, hemolysis was occurred by the action of both SVMP and PLAs2 on the RBC causing accumulation of Hb and heme and mediated pro-oxidant and pro-inflammatory effects (Sunitha et al., 2015Sunitha, K., Hemshekhar, M., Thushara, R.M., Santhosh, M.S., Sundaram, M.S., Kemparaju, M.K., Girish, K.S., 2015. Inflammation and oxidative stress in viper bite: an insight within and beyond. Toxicon 98, 89-97.). The IC50 and 100% inhibition of C. cerastes and E. coloratus hemolytic activity were observed at 21, 19 and 40 µg of MSKE (Fig. 5C), respectively. The MSKE alone didn’t induce any RBC hemolysis. This proves that MSKE is non-toxic. Hemolytic inhibition could be explained by the ability of phenolic compounds of MSKE to inhibit the proteases and PLA2s in both venoms.
Hemorrhagic SVMP are the major components of viper venoms and considered as the basic causative of viper lethality (Sells et al., 1997Sells, P.G., Richards, A.M., Laing, G.D., Theakston, R.D.G., 1997. The use of hens’eggs as an alternative to the conventional in vivo rodent assay for antidotes to hemorrhagic venoms. Toxicon 35, 1413-1421.). Inhibition of viper hemorrhagic activity is considered as a core test to evaluate the extract anti-snake property (Shirwaikar et al., 2004Shirwaikar, A., Rajendran, K., Bodla, R., Kumar, C.D., 2004. Neutralization potential of viper Russelli Russelli (Russell's viper) venom by ethanol leaf extract of Acalypha indica. J. Ethnopharmacol. 94, 267-273.). The results showed that the hemorrhagic spots in mice skin injected with 1 and 3 µg of C. cerastes and E. coloratus venoms in absence of MSKE were about 20 ± 0.2 and 21 ± 0.3 mm, respectively. The MSKE was impressively able to relieve the hemorrhagic effect of both venoms in mice dose dependently. The hemorrhagic zones of C. cerastes and E. coloratus venoms were totally disappeared at 25 and 15 µg phenolic of the MSKE, respectively (Fig. 6A and B). Also, the MSKE alone didn’t induce any hemorrhage.
Inhibition of Cerastes cerastes and Echis coloratus hemorrhagic activity by the mango seed kernels extract (MSKE). (A) a1 contains 2 MHD of C. cerastes alone, b1, c1, d1, e1 and f1 contain 2 MHD of C. cerastes venom pre-incubated with 5, 10, 15, 20 and 25 µg phenolic of the MSKE, respectively. (B) a2 contains 2MHD of E. coloratus alone, b2, c2, and d2 contain 2 MHD of E. coloratus venom pre-incubated with 5, 10, and 15 µg phenolic of the MSKE, respectively, (g2) 100 µl of MSKE alone, and (k2) 100 µl of saline as controls. The values represent mean ± S.E. (n = 5).
The LD50 of C. cerastes and E. coloratus venoms were established at 8 and 4 µg/20 g mouse, respectively (data not shown). Mice injected with 2LD50 of C. cerastes and E. coloratus survived for 0.43 and 0.49 h, respectively. The mice could survive up to 16 and 19 h when MSKE administrated by 20 µg phenolics after 10 min of 2 LD50 C. cerastes and E. coloratus venoms injection, respectively, and about fifty percent survival in mice groups. Further, the 2LD50 of C. cerastes and E. coloratus venoms totally neutralized at 80 and 40 µg of MSKE, respectively and all mice still alive even after 24 h of injection. In addition, intraperitonial administration of the MSKE did not induce any toxic effect (Table 3). The full inhibition of both venoms lethality may be due to interact of phenolic compounds of the extract with venom harmful enzymatic components (Houghton, 1998Houghton, P.J., 1998. Plant extracts active towards snake venom enzymes. In: Bailey, G.S. (Ed.), Enzymes from Snake Venom. Colorado, Alaken, pp. 689–703.). Many recent studies reported that oxidative stress and the complications associated with viper bite could be improved by the antioxidants treatment strategy (Zengin et al., 2012Zengin, S., Al, B., Yarbil, P., Guzel, R., Orkmez, M., Yildirim, C., Taysi, S., 2012. An assessment of oxidant/antioxidant status in patients with snake envenomation. Emerg. Med. J. 31, 48-52.; Katkar et al., 2014Katkar, G.D., Sundaram, M.S., Hemshekhar, M., Sharma, R.D., Santhosh, M.S., Sunitha, K., Rangappa, K.S., Girish, K.S., Kemparaju, K., Habiby, S.A., 2014. Melatonin alleviates Echis carinatus venom-induced toxicities by modulating inflammatory mediators and oxidative stress. J. Pineal Res. 56, 295-312.). Therefore, the venom neutralization action of the MSKE may be attributed to its phenolic compounds and their antioxidant activity.
Neutralization of Cerastes cerastes and Echis coloratus lethality by the mango seed kernels extract (MSKE) administered intraperitoneal. The values represent mean ± S.E. (n = 5). Values with superscript (a) were remarkable significant (p < 0.01).
Conclusion
In the present study, the MSKE contained a considerable amount of phenolic and flavonoid contents and antioxidant activity. Hesperidin was the major phenolic compounds of MSKE. This is the first report find hesperidin in mango extracts. The phenolic compounds of the MSKE potentially inhibited the protease, fibrinogenase, PLA2, LAAO, hyaluronidase, hemolytic, hemorrhagic activities and lethality of Cerastes cerastes and Eicus coloratus venoms. Therefore, the cost effective phenolic compounds from mango waste products could be used as a therapeutic way for viper bites management.
Ethical disclosures
-
Protection of human and animal subjects. The authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
-
Confidentiality of data. The authors declare that no patient data appear in this article.
-
Right to privacy and informed consent. The authors declare that no patient data appear in this article.
References
- Abdalla, A.E.M., Darwish, S.M., Ayad, E.H.E., El-Hamahmy, R.M., 2007. Egyptian mango by-product 1: compositional quality of mango seed kernel. Food Chem. 103, 1134-1140.
- Ajila, C.M., Rao, U.J.S.P., 2013. Mango peel dietary fiber: composition and associated bound phenolics. J. Funct. Foods 202, 11-17.
- Al-Abdulla, I.H., Sidki, A.M., Landon, J., 1991. An indirect hemolytic assay for assessing anti-venoms. Toxicon 29, 1043-1046.
- Ao, C., Li, A., Elzaawely, A., Xuan, T.D., Tawata, S., 2008. Evaluation of antioxidant and antibacterial activities of Ficus microcarpa L. extract. Food Control 19, 940-948.
- Barone, J.M., Frezzatti, R., Silveira, P.F., 2014. Effects of N-acetyl-l-cysteine on redox status and markers of renal function in mice inoculated with Bothrops jararaca and Crotalus durissusterrificus venoms. Toxicon 79, 1-10.
- Bee, A., Theakston, R.D.G., Harrison, R.A., Cartera, S.D., 2001. Novel in vitro assays for assessing the hemorrhagic activity of snake venoms and for demonstration of venom metalloproteinase inhibitors. Toxicon 39, 1429-1434.
- Dorta, E., Gonzez, M., Lobo, M.G., Snchez-Moreno, C., Ancos, B., 2014. Screening of phenolic compounds in by-product extracts from mangoes (Mangifera indica L.) by HPLC–ESI–QTOF–MS and multivariate analysis for use as a food ingredient. Food Res. Int. 57, 51-60.
- Dorta, E., Lobo, M.G., González, M., 2012. Using drying treatments to stabilize mango peel and seed: effect on antioxidant activity. LWT Food Sci. Technol. 45, 261-268.
- Garg, A., Garg, S., Zaneveld, L.J.D., Singla, A.K., 2001. Chemistry and pharmacology of the Citrus bioflavonoid hesperidin. Phytother. Res. 15, 655-669.
- Girish, K.S., Kemparaju, K., 2011. Overlooked issues of snakebite management: time for strategic approach. Curr. Top. Med. Chem. 11, 2494-2508.
- Gutierrez, J.M., Avila, C., Rojas, E., Cerdas, L., 1988. An alternative in vitro method for testing the potency of the polyvalent anti-venom produced in Costa Rica. Toxicon 26, 411-413.
- Harris, J.B., Scott-Davey, T., 2013. Secreted phospholipases A2 of snake venoms: effects on the peripheral neuromuscular system with comments on the role of phospholipases A2 in disorders of the CNS and their uses in industry. Toxins 5, 2533-2571.
- Haslam, E., 1996. Natural polyphenols (vegetable tannins) as drugs and medicine: possible modes of action. J. Nat. Prod. 59, 205-215.
- Houghton, P.J., 1998. Plant extracts active towards snake venom enzymes. In: Bailey, G.S. (Ed.), Enzymes from Snake Venom. Colorado, Alaken, pp. 689–703.
- Jahurul, M.H.A., Zaidul, I.S.M., Kashif, G., Fahad, Y., Al-Juhaimi, Kar-Lin Nyam, N.A.N., Sahena, F., Mohd Omar, A.K., 2015. Mango (Mangifera indica L.) by-products and their valuable components: a review. Food Chem. 183, 173-180.
- Kalpana, K.B., Srinivasan, M., Menon, V.P., 2009. Evaluation of antioxidant activity of hesperidin and its protective effect on H2O2 induced oxidative damage on pBR322 DNA and RBC cellular membrane. Mol. Cell Biochem. 323, 21-29.
- Kanes, K., Tisserat, B., Berhow, M., Vandercook, C., 1993. Phenolic composition of various tissues of Rutaceae species. Phytochemistry 324, 967-974.
- Katkar, G.D., Sundaram, M.S., Hemshekhar, M., Sharma, R.D., Santhosh, M.S., Sunitha, K., Rangappa, K.S., Girish, K.S., Kemparaju, K., Habiby, S.A., 2014. Melatonin alleviates Echis carinatus venom-induced toxicities by modulating inflammatory mediators and oxidative stress. J. Pineal Res. 56, 295-312.
- Kemparaju, K., Girish, K.S., 2006. Snake venom hyaluronidase: a therapeutic target. Cell. Biochem. Funct. 24, 7-12.
- Kim, J.Y., Jung, K.J., Choi, J.S., Chung, H.Y., 2004. Hesperetin: a potent antioxidant against peroxynitrite. Free Radic. Res. 38, 761-769.
- Kim, K.H., Tsao, R., Yang, R., Cui, S.W., 2006. Phenolic acid profiles and antioxidant activities of wheat bran extracts and the effect of hydrolysis conditions. Food Chem. 95, 466-473.
- Kishimoto, M., Takahashi, T., 2001. A spectrophotometric microplate assay for L-amino-acid oxidase. Anal. Biochem. 298, 136-139.
- Kobayashi, M., Matsui-Yuasa, I., Fukuda-Shimizu, M., Mandai, Y., Tabuchi, M., Munakata, H., Kojima-Yuasa, A., 2013. Effect of mango seed kernel extract on the adipogenesis in 3T3-L1 adipocytes and in rats fed a high fat diet. Health (N.Y.) 5, 9-15.
- Kondo, H., Kondo, S., Ikezawa, H., Murata, R., 1960. Studies on the quantitative method for the determination of hemorrhagic activity of Habu snake venom. Jpn. J. Med. Sci. Biol. 13, 43-52.
- Laemmli, U.K., 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680-685.
- Lemos, F.J.A., Campos, F.A.P., Silva, C.P., Xavier-Filho, J., 1991. Proteinases and amylases of larval midgut of Zabrotes subfasciatus reared on cowpea (Vigna unguiculata) seeds. Entomol. Exp. Appl. 56, 219-228.
- Marinetti, G.V., 1965. The action of phospholipase A on lipoproteins. Biochem. Biophys. Acta 60, 554-565.
- Markland, F.S., Swenson, S., 2013. Snake venom metalloproteinases. Toxicon 62, 3-18.
- Mc-Cleary, R.J., Kini, R.M., 2013. Snake bites and hemostasis/thrombosis. Thrombosis Res. 132, 642-646.
- Meier, J., Theakston, R.D., 1986. Approximate LD50 determinations of snake venoms using eight to ten experimental animals. Toxicon 24, 395-401.
- Mors, W.B., Nascimento, M.C., Pereira, B.M.R., Pereira, N.A., 2000. Plant natural products active against snake bite-the molecular approach. Phytochemistry 55, 627-642.
- Oussedik-Oumehdi, H., Laraba-Djebari, F., 2008. Irradiated Cerastes cerastes venom as a novel tool for immunotherapy. Immunopharm. Immunot. 30, 37-43.
- Ouyang, C., Teng, C.M., 1976. Fibrinogenolytic enzymes of Trimeresurus mucrosquamatus venom. Biochim. Biophys. Acta 420, 298-308.
- Pithayanukul, P., Leanpolchareanchai, J., Saparpakorn, P., 2009. Molecular docking studies and anti-snake venom metalloproteinase activity of Thai mango seed kernel extract. Molecules 14, 3198-3213.
- Prieto, P., Pineda, M., Aguilar, M., 1999. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal Biochem. 269, 337-341.
- Pukrittayakamee, S., Warrel, D.A., Deasakorn, V., McMichael, A.J., White, N.J., Bunnag, G.D., 1988. The hyaluronidase activities of some Southeast Asian snake venoms. Toxicon 34, 1119-1125.
- Re, R., Pellegrini, N., Proteggente, A., Pannala, A., Yang, M., Rice-Evans, C., 1999. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 26, 1231-1237.
- Ribeiro, S.M.R., Barbosa, L.C.A., Queiroz, J.H., Knödler, M., Schieber, A., 2008. Phenolic compounds and antioxidant capacity of Brazilian mango (Mangifera indica L.) varieties. Food Chem. 110, 620-626.
- Sells, P.G., Richards, A.M., Laing, G.D., Theakston, R.D.G., 1997. The use of hens’eggs as an alternative to the conventional in vivo rodent assay for antidotes to hemorrhagic venoms. Toxicon 35, 1413-1421.
- Shirwaikar, A., Rajendran, K., Bodla, R., Kumar, C.D., 2004. Neutralization potential of viper Russelli Russelli (Russell's viper) venom by ethanol leaf extract of Acalypha indica J. Ethnopharmacol. 94, 267-273.
- Soares, A.M., Ticli, F.K., Marcussi, S., Lourenco, M.V., Januarioc, A., Sampaioa, S.V., Gigliob, J.R., Lomonted, B., Pereirac, P.S., 2005. Medicinal plants with inhibitory properties against snake venoms. Curr. Med. Chem. 12, 2625-2641.
- Sogi, D.S., Siddiq, M., Greiby, I., Dolan, K.D., 2013. Total phenolics, antioxidant activity, and functional properties of ‘Tommy Atkins’ mango peel and kernel as affected by drying methods. Food Chem. 141, 2649-2655.
- Sunitha, K., Hemshekhar, M., Thushara, R.M., Santhosh, M.S., Sundaram, M.S., Kemparaju, M.K., Girish, K.S., 2015. Inflammation and oxidative stress in viper bite: an insight within and beyond. Toxicon 98, 89-97.
- Velioglu, Y.S., Mazza, G., Gao, L., Oomah, B.D., 1998. Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. J. Agric. Food Chem. 46, 4113-4117.
- Wahby, A.F., Abdel-Aty, A.M., El-Kady, E.M., 2012. Purification of hemorrhagic SVMPs from venoms of three vipers of Egypt. Toxicon 59, 329-337.
- Zengin, S., Al, B., Yarbil, P., Guzel, R., Orkmez, M., Yildirim, C., Taysi, S., 2012. An assessment of oxidant/antioxidant status in patients with snake envenomation. Emerg. Med. J. 31, 48-52.
- Zhishen, J., Mengcheng, T., Jianming, W., 1999. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 64, 555-559.
Publication Dates
-
Publication in this collection
Sep-Oct 2018
History
-
Received
21 Mar 2018 -
Accepted
13 June 2018 -
Published
21 July 2018