Abstract
Annona leptopetala (R.E.Fr.) H. Rainer, Annonaceae, is used in folk medicine like antitumor and anti-inflammatory. The aim of this study was to determine chemical composition, toxicity and antitumor activity of A. leptopetala leaves volatile oil. Fresh leaves were hydrodistilled and then the volatile oil chemical composition was assessed by gas chromatography and mass spectrometry. Toxicity was assessed using haemolysis, micronucleus and acute toxicity protocols. Antitumor effects were determined in vitro and in vivo, using sulforhodamine B assay and sarcoma 180 murine tumor model, respectively. Spathulenol was the major component identified (12.56%). The volatile oil showed in vitro antitumor activity mainly in leukemia cell line (K-562), with Total growth inhibit (TGI) (concentration producing TGI) of 0.64 µg/ml. In other hand, the volatile oil <250 µg/ml did not inhibit HaCat non-tumor cell line growth. The concentration that produced 50% haemolysis was 372.8 µg/ml. The 50% lethal dose in mice was approximately 447.2 mg/kg intraperitoneally. Sarcoma 180 tumor growth inhibition rates were 59.29% and 58.77% at 100 and 150 mg/kg intraperitoneally, respectively. The volatile oil presented moderate gastrointestinal toxicity and no genotoxicity was observed at 350 mg/kg. Thus, the volatile oil shows antitumor activity with moderate toxicity.
Keywords
Antitumor activity; Cytotoxicity; Genotoxicity; Sarcoma 180; Sphatulenol
Introduction
Annona leptopetala (R.E.Fr) H. Rainer, Annonaceae, commonly known as “pinha-brava” is a tree or shrub endemic in Brazil used in folk medicine like antitumor and anti-inflammatory (Agra et al., 2007Agra, M.F., Freitas, P.F., Barbosa-Filho, J.M., 2007. Synopsis of the plants known as medicinal and poisonous in Northeast of Brazil. Rev. Bras. Farmacogn. 17, 114-140.; David et al., 2007David, J.P., Meira, M., David, J.M., Brandão, H.N., Branco, A., Agra, M.F., Barbosa, M.R.V., de Queiroz, L.P., Giulietti, A.M., 2007. Radical scavenging, antioxidant and cytotoxic activity of Brazilian Caatinga plants. Fitoterapia 78, 215-218.). Antioxidant and in vivo antitumor activities for extracts from A. leptopetala have been reported (David et al., 2007David, J.P., Meira, M., David, J.M., Brandão, H.N., Branco, A., Agra, M.F., Barbosa, M.R.V., de Queiroz, L.P., Giulietti, A.M., 2007. Radical scavenging, antioxidant and cytotoxic activity of Brazilian Caatinga plants. Fitoterapia 78, 215-218.; Costa et al., 2012Costa, V.C.O., Tavares, J.F., Queiroga, C.S., Castello-Branco, M.V.S., Diniz, M.F.F.M., Lima, C.U.G.B., Santos, B.V.O.S., Pita, J.C.L.R., Silva, M.S., 2012. Constituintes químicos das folhas de Rollinia leptopetala RE Fries. Quim. Nova 22, 1-5.), in addition to antispasmodic effect in guinea pig ileum (Monteiro et al., 2008Monteiro, F.S., Costa, V.C.O., Queiroga, C.S., Tavares, J.F., Santos, B.V.O., Silva, M.S., Silva, B.A., 2008. Comparação do efeito antiespasmódico de Rollinia leptopetala R.E. In: Fries e Rollinia exsucca A. DC. em íleo isolado de cobaia. Anais do XX Simpósio de Plantas Medicinais do Brasil. X Congresso Internacional de Etnofarmacologia, São Paulo.).
Different compounds has been obtained from Annona genus, such as flavonols (Júnior et al., 2016Júnior, J.G.A.S., Coutinho, H.D.M., Boris, T.C.C., Cristo, J.S., Pereira, N.L.F., Figueiredo, F.G., Cunha, F.A.B., Aquino, P.E.A., Nascimento, P.A.C., Mesquita, F.J.C., Moreira, P.H.F., Coutinho, S.T.B., Souza, I.T., Teixeira, G.C., Ferreira, N.M.N., Farina, E.O., Torres, C.M.G., Holanda, V.N., Pereira, V.S., Guedes, M.I.F., 2016. Chemical characterization and cytoprotective effect of the hydroethanol extract from Annona coriacea Mart (Araticum). Pharmacognosy Res. 8, 253-325.; Novaes et al., 2018Novaes, P., Ferreira, M.J.P., Santos, D.Y.A.C., 2018. Flavonols from Annona coriacea Mart, (Annonaceae). Biochem. Syst. Ecol. 78, 77-80.), terpenes (Santana et al., 2017Santana, K.L., Galvão, M.S., Jesus, M.S., Nogueira, J.P., Narain, N., 2017. HS-SPME optimization and extraction of volatile compounds from soursop (Annona muricata L.) pulp with emphasis on their characteristic impact compounds. Food Sci. Technol. 37, 250-260.), tannins, saponins, cardiac glycosides, monosaccharides, aromatic and phenolic amino acids, steroids (Agu and Okolie, 2017Agu, K.C., Okolie, P.N., 2017. Proximate composition, phytochemical analysis, and in vitro antioxidant potentials of extracts of Annona muricata (Soursop). Food Sci. Nutr. 5, 1029-1036.), isoquinolonic and indolic alkaloids (Kuo et al., 2001Kuo, R.Y., Chang, F.R., Chen, C.Y., Teng, C.M., Yen, H.F., Wu, Y.C., 2001. Antiplatelet activity of N-methoxycarbonyl aporphines from Rollinia mucosa. Phytochemistry 57, 421-425.), lignoids (Fevier et al., 1999Fevier, A., Ferreira, M.E., Fournet, A., Valuff, G., Inchausti, A., De Arias, A.R., Hocquemiller, R., Waechter, A., 1999. Acetogenins and other compounds from Rollinia emarginata and their antiprotozoal activities. Plant. Med. 65, 47-49.) and acetogenins (Mangal et al., 2016Mangal, M., Imran, K.M., Mohan, A.S., 2016. Acetogenins as potential anticancer agents. Anticancer Agents Med. Chem. 16, 138-159.). Regarding volatile oils from Annona species, monoterpenes and sesquiterpenes were isolated, including β-elemene (Kossouoh et al., 2007Kossouoh, C., Moudachirou, M., Adjakidje, V., Chalchat, J., Figuérédo, G., 2007. Essential oil chemical composition of Annona muricata L. leaves from Benin. J. Essent. Oil Res. 19, 307-309.), bicyclogermacrene (Siqueira et al., 2011Siqueira, C.A.T., Oliani, J., Sartoratto, A., Queiroga, C.L., Moreno, P.R.H., Reimão, J.Q., Tempone, A.G., Fischer, D.C.H., 2011. Chemical constituents of the volatile oil from leaves of Annona coriacea and in vitro antiprotozoal activity. Rev. Bras. Farmacogn. 21, 33-40.), α-copaene (Costa et al., 2013Costa, E.V., Dutra, L.M., Salvador, M.J., Ribeiro, L.H.G., Gadelha, F.R., Carvalho, J.E., 2013. Chemical composition of the essential oils of Annona pickelii and Annona salzmannii (Annonaceae), and their antitumour and trypanocidal activities. Nat. Prod. Res. 27, 997-1001.) and α-phellandrene (Meira et al., 2014Meira, C.S., Guimarães, E.T., Macedo, T.S., Silva, T.B., Menezes, L.R.A., Costa, E.V., Soares, M.B.P., 2014. Chemical composition of essential oils from Annona vepretorum Mart. and Annona squamosa L, (Annonaceae) leaves and their antimalarial and trypanocidal activities. J. Essent. Oil Res. 27, 160-168.). In addition, literature data showed antitumor activity for other components of Annona volatile oil, such as α-terpineol (Hassan et al., 2010Hassan, S.B., Gali-Muhtasib, H., Goransson, H., Larsson, R., 2010. Alpha terpineol: a potential anticancer agent which acts through suppressing NF-kappa B signalling. Anticancer Res. 30, 1911-1919.), spathulenol (Bomfim et al., 2016Bomfim, L.M., Menezes, L.R.A., Rodrigues, A.C.B.C., Dias, R.B., Rocha, C.A.G., Soares, M.B.P., Neto, A.F.S., Nascimento, M.P., Campos, A.F., Silva, L.C.R.C., Costa, E.V., Bezerra, D.P., 2016. Antitumour activity of the microencapsulation of Annona vepretorum essential oil. Basic Clin. Pharmacol. Toxicol. 118, 208-213.), trans-caryophyllene (Hadri et al., 2010Hadri, A., Rio, M.A.G., Sanz, J., Coloma, A.G., Idaomar, M., Ozonas, B.R., Gonzales, J.B., Reus, M.I.S., 2010. Cytotoxic activity of α-humulene and trans-caryophyllene from Salvia officinallis in animal and human tumor cells. Ann. R. Acad. Nac. Farm. 76, 343-356.) and germacrene-D (Salvador et al., 2011Salvador, M.J., Carvalho, J.E.D., Wisniewski-Jr, A., Kassuya, C.A., Santos, É.P., Riva, D., Stefanello, M.E.A., 2011. Chemical composition and cytotoxic activity of the essential oil from the leaves of Casearia lasiophylla. Rev. Bras. Farmacogn. 21, 864-868.),
Considering that the theory of synergistic action associated with the antitumor activity of volatile oils appears to be rather than its components separately (Bhalla et al., 2013Bhalla, Y., Gupta, V.K., Jaitak, V., 2013. Anticancer activity of essential oils: a review. J. Sci. Food Agric. 93, 3643-3653.), this study determined the chemical composition, antitumor activity and toxicity of the volatile oil from A. leptopetala leaves (ALO).
Materials and methods
Drugs and reagents
RPMI 1640 culture medium, glutamine, penicillin, streptomycin, 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) and fetal bovine serum (FBS) were obtained from GIBCO (Carlsbad, CA). 5-Fluorouracil (5-FU), Triton X-100, Tween-80, cyclophosphamide, Trizma base and sulforhodamine (SRB) were purchased from Sigma–Aldrich (St. Louis, MO). Dimethylsulfoxide (DMSO) was purchased from Mallinckrodt Chemicals (Phillipsburg, NJ). Kits for biochemical analysis were purchased from LABTEST (Lagoa Santa, MG, Brazil). Sodium thiopental (Thiopentax®) was purchased from Cristália (Itapira, SP, Brazil) and heparin (Parinex®) from Hipolabor (Sabara, MG, Brazil). Doxorubicin (DOX) was from Tecnofarma International (Uruguay) and trichloroacetic acid (TCA) from Merck (Darnstadt, Germany).
Plant material
Annona leptopetala (R.E.Fr.) H. Rainer, Annonaceae, leaves were collected in August 2016 in Serra Branca, Paraíba State, Brazil. The plant material was identified by Dr. Maria de Fátima Agra. Voucher specimen number AGRA 3567 was deposited at the Herbarium Lauro Pires Xavier of Federal University of Paraíba (UFPB), Brazil.
Hydrodistillation of the volatile oil
Fresh leaves (1000 g) were collected over ice and hydro-distilled using Clevenger type apparatus for 4 h, at a temperature of 40 ºC, yielding 400 mg of the volatile oil (yield of 0.04% relative to the weight of fresh material used). The resulting oil was dried with anhydrous sodium sulfate, stored in amber bottle and kept at 4 ºC lower temperature. Thereafter, ALO was submitted for Gas Chromatography with Mass Spectrometry (GC-MS) analysis.
GC-MS analysis
Analysis of the oil was carried out on a Shimadzu GC-MS instrument under the following conditions: DB-5 ms (30 m × 0.25 mm internal diameter, film thickness 0.25 µm), fused-silica capillary column, programmed temperature of 60–240 ºC (3 ºC/min), injector temperature at 220 ºC, helium carrier gas adjusted to a linear velocity of 32 cm/s (measured at 100 ºC), splitless injection (2 µl of hexane solution 1:1000), split flow adjusted to yield a 20:1 ratio, septum sweep constant at 10 ml/min, Electron Ionization Mass Spectrometry (EIMS) electron energy of 70 eV, ion source and connections at 200 ºC. The quantitative data for the volatile constituents were obtained by peak-area normalization using a Focus Gas Chromatography with Flame Ionization Detector (GC/FID), operated under GC-MS similar conditions except for the carrier gas, which was nitrogen. The retention index was calculated for all the volatile constituents by volatile oil co-injection using an n-alkane (C8-C20, Sigma–Aldrich) homologous series applying the equation of Van den Dool and Kratz (1963)Van den Dool, H., Kratz, P.D., 1963. A generalization of the retention index system including linear temperature programmed gas liquid partition chromatography. J. Chromatogr. A 11, 463-471.. Individual components were identified by comparison of both mass spectrum and Gas Chromatography (GC) retention data with previously analyzed authentic compounds stored in our private library, as well as with the aid of commercial libraries containing mass spectra, and retention indices of volatile compounds commonly found in volatile oils (Adams, 2001Adams, R.P., 2001. Identification of Essential Oil Components by Gas Chromatography/Quadrupole Mass Spectroscopy. Allured Publishing Corporation, Carol Stream (IL).).
Cell lines
The tumor cell lines used were: U251 – glioma, MCF-7 – breast, NCI/ADR-RES - multidrug-resistant ovarian, 786-0 – kidney, NCI-H460 – non-small cell lung cancer, PC-3 – prostate, OVCAR – ovarian, HT29 – colon and K562 – leukemia, and HaCaT human keratinocytes served as the normal cell line. The cells lines were cultivated in RPMI-1640 supplemented with FBS 10%, glutamine 2 mM, penicillin 100 U/ml, streptomycin 100 µg/ml and HEPES 2 mM, at 37 ºC with CO2 5%, in the Chemical, Biological and Agricultural Pluridisciplinary Research Center, State University of Campinas, Campinas, Brazil. Sarcoma 180 tumor cells were maintained in the peritoneal cavity of Swiss mice.
Animals
Swiss albino mice (Mus musculus), females (36–42 g), were obtained from the Dr. Thomas George Bioterium of Research Institute in Drugs and Medicines of Federal University of Paraíba, Brazil. The animals were randomly housed in cages containing six animals with free access to food and water. All animals were kept on a 12 h/12 h off light-dark cycle (lights on at 6 am). All procedures were previously approved by the Animal Studies Committee (CEUA) of UFPB, nº. 0912/10.
Haemolysis assay
The haemolytic activity of ALO was tested using mouse erythrocytes according to Kang et al. (2009)Kang, C., Munawir, A., Cha, M., Sohn, E.-T., Lee, H., Kim, J.S., Yoon, W.D., Lim, D., Kim, E., 2009. Cytotoxicity and hemolytic activity of jellyfish Nemopilema nomurai (Scyphozoa: Rhizostomeae) venom. Comp. Biochem. Physiol. 150, 85-90.. Erythrocytes from fresh blood samples were suspended in phosphate buffered saline (PBS) to make a 1% (v/v) solution. Red blood cell suspension was incubated with various concentrations (0–750 µg/ml) of ALO dissolved in DMSO (5%, v/v, in PBS) in plates on a shaker for 60 min and then centrifuged. The absorbance of the supernatants was read at 540 nm using a UV–vis spectrophotometer (UV-1650PC Shimadzu) to measure the extent of red blood cell (RBC) lysis, and the concentration producing 50% haemolysis (HC50) was determined. Positive controls (100% haemolysis) and negative controls (0% haemolysis) were also determined by incubating erythrocytes using Triton X-100 1% in PBS and DMSO 5% in PBS, respectively. The haemolysis assay was performed in quadruplicate and repeated three times.
In vitro antitumor activity
The sulforhodamine B assay (SRB) was performed as described by Monks et al. (1991)Monks, A., Scudiero, D., Skehan, P., Shoemaker, R., Paull, K., Vistica, D., Hose, C., Langley, J., Cronise, P., Vaigro-Wolff, A., Gray-Goodrich, M., Campbell, H., Mayo, J., Boyd, M., 1991. Feasibility of a high-flux anticancer drug screen using a diverse panel of cultured human tumor cell lines. J. Natl. Cancer. Inst. 83, 757-766.. This assay relies on the ability of SRB to bind to protein components of cells that have been fixed to tissue-culture plates by TCA. SRB is a bright-pink aminoxanthene dye with two sulphonic groups that bind to basic amino acid residues under acidic conditions and dissociate under basic conditions.
Cells in 96-well plates (100 µl cells/well) were exposed to different concentrations of ALO (0.25, 2.5, 25 and 250 µg/ml) in DMSO/RPMI/FBS 5% at 37 ºC and CO2 5%, for 48 h. Final DMSO concentration did not affect cell viability. Cells were then fixed with TCA solution (50%, v/v), and cell proliferation was determined by spectrophotometric quantification (540 nm) of cellular protein content. DOX (0.025–25 µg/ml) was used as the positive control. Three measurements were obtained: at the beginning of incubation (T0) and 48 h post-incubation for compound-free (C) and exposed (T) cells. Cell proliferation was determined according to the equation: cell proliferation = 100 × [(T - T0)/C - T0]. The cytostatic effect was observed when T0 ≤ T < C, while cytocidal effect occurred when T < T0. The experiments were done in triplicate to calculate the total growth inhibition (TGI) (concentration that produces TGI).
Acute preclinical toxicity
The evaluation of acute preclinical toxicity of ALO was performed according to the Guide for the conduct of non-clinical toxicology studies and safety pharmacology necessary for the development of drugs, published by the Brazilian Health Surveillance Agency (Anvisa, 2013Anvisa, 2013. Guia para a condução de estudos não clínicos de toxicologia e segurança farmacológica necessários ao desenvolvimento de medicamentos. Agência Nacional de Vigilância Sanitária. Ministério da Saúde, Brasília.), with some modifications. Mice (six females) were given doses of 250, 375 and 500 mg/kg ALO intraperitoneally (i.p.), and the control group received vehicle alone (Tween-80 5% in saline). For detection of toxic signs suggestive of activity on the central nervous system (CNS) or autonomic nervous system (ANS) of a general nature, the animals were closely observed at the following times: 0, 15, 30 and 60 min, 4 h, and daily for 14 d (Almeida et al., 1999Almeida, R.N., Falcão, A.C.G.M., Diniz, R.S.T., Quintans-Júnior, L.J., Polari, R.M., Barbosa-Filho, J.M., Agra, M.F., Duarte, J.C., Ferreira, C.D., Antoniolli, A.R., Araújo, C.C., 1999. Metodologia para avaliação de plantas com atividade no Sistema Nervoso Central e alguns dados experimentais. Rev. Bras. Farm. 80, 72-76.). For 50% lethal dose (LD50) determination, the number of death animals was observed during 14 days after the treatments.
Genotoxicity
For the micronucleus assay, groups of six mice were treated intraperitoneally with different doses of ALO at 110, 230 and 350 mg/kg (25, 50 and 80% of the LD50 value, respectively) (Ribeiro, 2003Ribeiro, L.R., 2003. Teste do micronúcleo em medula óssea de roedores in vivo. In: Ribeiro, L.R., Salvadori, D.M.F., Marques, E.K. (Eds.), Mutagênese ambiental. ULBRA, Canoas, pp. 173–198.). A group treated with a standard drug (cyclophosphamide, 50 mg/kg, i.p.), and a control group (Tween-80 5% in saline) were included. After 24 h, the animals were anesthetized with sodium thiopental (40 mg/kg), and peripheral blood samples were collected from the retro-orbital plexus for blood smears. Three blood smears were prepared for each animal, and a minimum of 2000 erythrocytes were counted to determine the number of micronucleated erythrocytes (OECD, 1997OECD, 1997. Guideline for Testing of Chemicals n. 474: Mammalian erythrocyte micronucleus test, Organization for Economic Co-operation and Development., pp. 1–10.).
In vivo antitumor activity
Eight-day-old sarcoma 180 ascites cells (0.2 ml–25 × 106 cells/ml) were implanted subcutaneously into the left subaxillary region of female mice (n = six/group) (Bezerra et al., 2008Bezerra, D.P., Castro, F.O., Alves, A.P.N.N., Pessoa, C., Moraes, M.O., Silveira, E.R., Lima, M.A.S., Elmiro, F.J.M., de Alencar, N.M.N., Mesquita, R.O., Lima, M.W., Costa-Lotufo, L.V., 2008. In vitro and in vivo antitumor effect of 5-FU combined with piplartine and piperine. J. Appl. Toxicol. 28, 156-163.). One day after inoculation, ALO (50 or 100 mg/kg) was dissolved using Tween-80 5% (v/v) and administered intraperitoneally for 7 days to mice transplanted with sarcoma 180 tumor. 5-FU (25 mg/kg) was used as the standard drug. The healthy group (healthy mice) and tumor control group (mice bearing sarcoma 180) were inoculated with Tween-80 5% in 0.9% (w/v) NaCl. On the eighth day, peripheral blood samples from all mice were collected from the retro-orbital plexus under light sodium thiopental anesthesia (40 mg/kg). The animals were then sacrificed by cervical dislocation. The tumors were excised and weighed, and then fixed in formaldehyde 10% and submitted to histopathological analysis. The tumor growth inhibition in per cent was calculated by the following formula: Inhibition (%) = [(A - B)/A] × 100, where A is the average of the tumor weights of the tumor control group, and B is that of the treated group.
Toxicity in transplanted mice
Body weights were recorded at the beginning and end of treatment, while consumption of water and feed was evaluated daily for the 7 days of treatment. The liver, spleen, thymus and kidneys were excised and weighed for determination of their organ index. For biochemical analysis, levels of urea, creatinine and alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activities were determined. For hematological analysis, the following parameters were determined: hemoglobin (Hb) level, red blood cells (RBC) count, haematocrit (Hct) and the red cell indices mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH) and mean corpuscular hemoglobin concentration (MCHC), as well as total and differential leukocyte counts. After weight determination and fixation using formaldehyde 10% (v/v), portions of the livers and kidneys were cut into small pieces and then into sections of 5 µm thickness, which were stained with hematoxylin-eosin and Masson's trichrome for the liver.
Statistical analysis
Data are presented as mean ± SEM. The HC50 value and their 95% confidence intervals (CI 95%) were obtained by nonlinear regression. From the concentration-response curve for each tumor cell line, TGI was determined through non-linear regression analysis using the software Origin 8.0 (OriginLab Corporation, Northampton, MA, USA). For in vivo assays, the differences between experimental groups were compared by analysis of variance (ANOVA), followed by Tukey's test (p <0.05) using the Graphpad program (Intuitive Software for Science, San Diego, CA, USA). We used Kolmogorov smirnov and Levene tests as prerequisites for the ANOVA test.
Results and discussion
It was identified 37 compounds in ALO, corresponding 98.1% of total oil, being 44.1% monoterpenes and 55.9% sesquiterpenes, corroborating to literature about Annonaceae species (Tavares et al., 2007Tavares, J.F., Silva, M.V.B., Queiroga, K.F., Martins, R.M., Silva, T.M.S., Camara, C.A., Agra, M.F., Barbosa-Filho, J.M., Silva, M.S., Marques, M.O.M., 2007. Composition and molluscicidal properties of essential oils from leaves of Xylopia langsdorffiana A. St. Hil. et Tul.(Annonaceae). J. Essent. Oil Res. 19, 282-284.). Previously, chemical composition for volatile oils from the leaves of A. leptopetala was described (Costa et al., 2008Costa, V.C.O., Tavares, J.F., Agra, M.F., Falcão-Silva, V.S., Facanali, R., Vieira, M.A.R., Marques, M.O.M., Siqueira-Júnior, J.P., Silva, M.S., 2008. Composição química e modulação da resistência bacteriana a drogas do óleo essencial das folhas de Rollinia leptopetala RE Fries. Rev. Bras. Farmacogn. 18, 245-248.; Feitosa et al., 2009Feitosa, E.M.A., Arriaga, A.M.C., Santiago, G.M.P., Lima, J.Q., Malcher, G.T., do Nascimento, R.F., Braz-Filho, R., 2009. Chemical composition and larvicidal activity of Rollinia leptopetala (Annonaceae). J. Braz. Chem. Soc. 20, 375-378.). Nevertheless, our data show spathulenol as major component (12.56%) followed by α-limonene (9.06%), both with reported antitumor activity (Bicas et al., 2011Bicas, J.L., Neri-Numa, I.A., Ruiz, A.L.T.G., de Carvalho, J.E., Pastore, G.M., 2011. Evaluation of the antioxidant and antiproliferative potential of bioflavors. Food Chem. Toxicol. 49, 1610-1615.; Bomfim et al., 2016Bomfim, L.M., Menezes, L.R.A., Rodrigues, A.C.B.C., Dias, R.B., Rocha, C.A.G., Soares, M.B.P., Neto, A.F.S., Nascimento, M.P., Campos, A.F., Silva, L.C.R.C., Costa, E.V., Bezerra, D.P., 2016. Antitumour activity of the microencapsulation of Annona vepretorum essential oil. Basic Clin. Pharmacol. Toxicol. 118, 208-213.). Similarly, (E)-caryophyllene was also identified, since it was reported as a marker in Annonaceae (Valter et al., 2008Valter, J.L., Alencar, K.M.C., Sartori, A.L.B., Nascimento, E.A., Chang, R., Morais, S.A.L., Laura, V.A., Yoshida, N.C., Carollo, C.A., Silva, D.B., Grassi, R.F., Fabri, J.R., Siqueira, J.M., 2008. Variação química no óleo essencial das folhas de seis indivíduos de Duguetia furfuracea (Annonaceae). Rev. Bras. Farmacogn. 18, 373-378.; Palazzo et al., 2009Palazzo, M.C., Wright, H.L., Agius, B.R., Wright, B.S., Moriarity, D.M., Haber, W.A., Setzer, W.N., 2009. Chemical compositions and biological activities of leaf essential oils of six species of Annonaceae from Monteverde. Costa Rica. Rec. Nat. Prod. 3, 153-160.). Other components identified in ALO are shown in Table 1.
Chemical composition (%) of volatile components from volatile oil from Annona leptopetala leaves.
Regarding to antiproliferative assay, ALO showed greater selectivity for leukemia cell line K-562 with TGI of 0.64 µg/ml. In addition, ALO showed no cytotoxic effect to non-tumor cell line HaCat, suggesting specificity to tumor cells. Date for TGI on other tumor cell lines are shown on Table 2.
Antiproliferative effect of volatile oil from Annona leptopetala leaves and doxorubicin against human cancer cell lines.a a Assessed by the SRB assay.
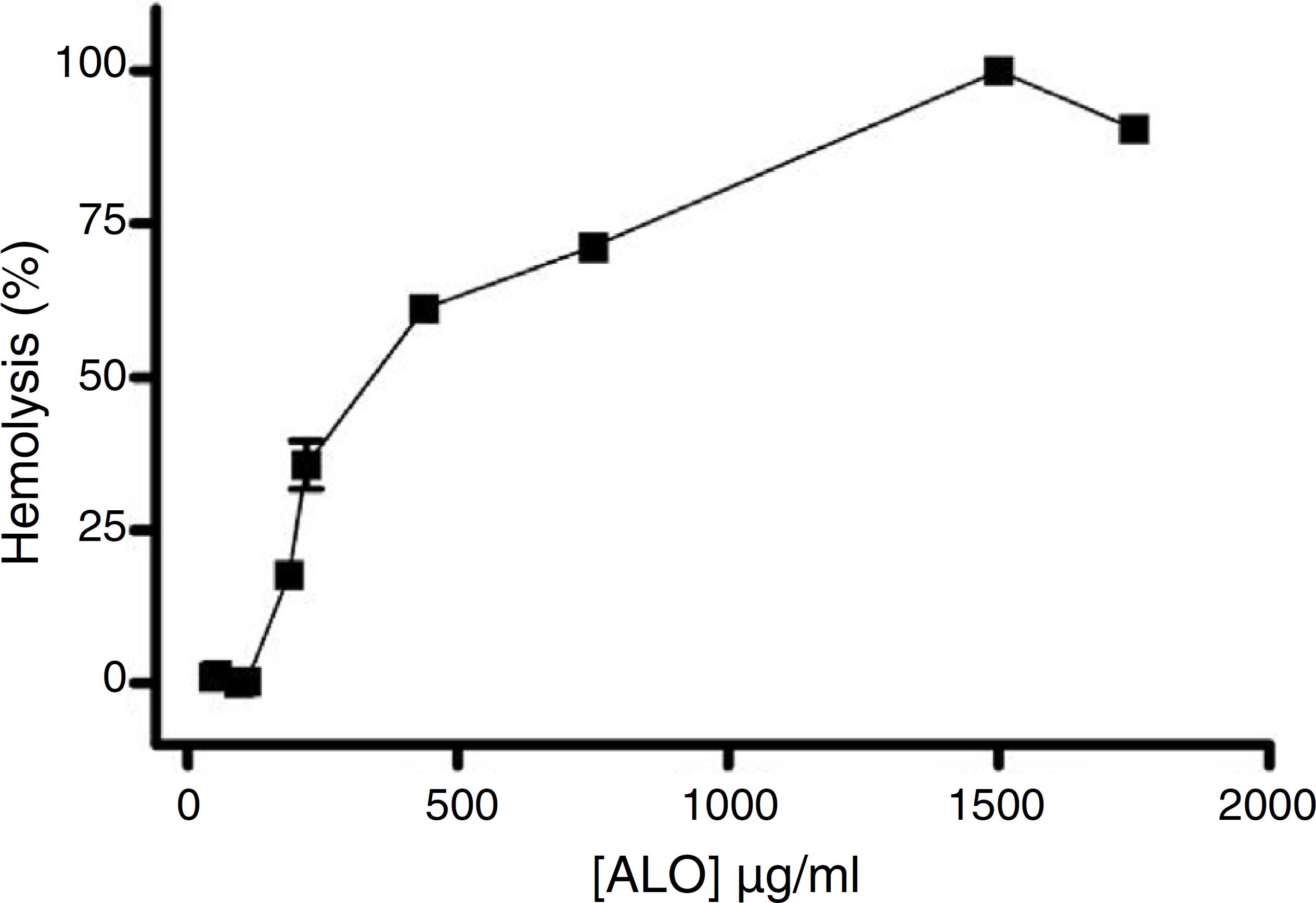
Antitumor agents are limited due to their serious side effects. The incidence of anemia increases with the administration of chemotherapy/radiation therapy due to the destruction and/or inability of the bone marrow to produce red blood cells and hemoglobin. Moreover, studies have documented that volatile oils and their chemical constituents may have hemolytic effects (Mendanha et al., 2013Mendanha, S.A., Moura, S.S., Anjos, J.L., Valadares, M.C., Alonso, A., 2013. Toxicity of terpenes on fibroblast cells compared to their hemolytic potential and increase in erythrocyte membrane fluidity. Toxicol. In Vitro 27, 323-329.). ALO exhibited a concentration-dependent hemolytic effect, with HC50 of about 372.8 µg/ml (344.8–403.1 µg/ml) (Fig. 1). Considering much lower concentrations that produced antiproliferative effect in SRB assay, higher concentrations were required to induce hemolysis, suggesting low toxicity to erythrocytes.
Percentage of haemolysis in red blood cells of Swiss mice upon treatment with volatile oil from Annona leptopetala leaves (ALO) (µg/ml). Data presented as mean ± SEM of three experiments with three replicates, with a 95% confidence interval.
Acute preclinical toxicity was used to determine safe doses to be used in pharmacological tests in mice. ALO caused animal death in a dose-dependent manner, with LD50 of about 447.2 mg/kg. Ptosis, sedation, and other CNS depressive effects were observed (Table 3). Only at 500 mg/kg these effects remained after 4 h of treatment. In general, if LD50 of test substance is three times more than the minimum effective dose, the substance is considered a good candidate for further studies (Auletta, 1995Auletta, C.S., 1995. Acute, subchronic, and chronic toxicology. In: Derelanko, M.J., Hollinger, M.A. (Eds.), Handbook of Toxicology. CRC Press, London, pp. 51–162.; Mangueira et al., 2017Mangueira, V.M., Batista, T.M., Brito, M.T., Sousa, T.K.G., Cruz, R.M.D., Abrantes, R.A., Veras, R.C., Medeiros, I.A., Medeiros, K.K.P., Pereira, A.L.C., Serafim, V.L., Moura, R.O., Sobral, M.V., 2017. A new acridine derivative induces cell cycle arrest and antiangiogenic effect on Ehrlich ascites carcinoma model. Biomed. Pharmacother. 90, 253-261.).
Effect the single doses (i.p.) of volatile oil from Annona leptopetala leaves in mice (n = 6).
Micronucleus testing is widely used and accepted by international and governmental agencies as part of assays needed to evaluate genotoxicity of new chemicals (Choy, 2001Choy, W.N., 2001. Regulatory genetic toxicology tests. In: Choy, W.N. (Ed.), Genetic Toxicology and Cancer Risk Assessment. CRC Press, London, pp. 107–128.). ALO did not increase number of micronucleated erythrocytes in peripheral blood (Table 4), suggesting no genotoxicity.
Number of micronucleated erythrocytes in peripheral boold of mice treated with different doses of the volatile oil from Annona leptopetala leaves (ALO) and cyclophosphamide (i.p.) (n = 6).
A significant reduction in tumor weight was observed in ALO 100 and 150 mg/kg-treated groups, being 0.93 ± 0.09 and 0.95 ± 0.10 g, respectively, in comparison to tumor control group. Inhibition rates of sarcoma 180 tumor were 59.3%, 58.8% and 67.6% at doses of 100 and 150 mg/kg ALO and 25 mg/kg 5-FU, respectively (Fig. 2).
Effects of volatile oil from Annona leptopetala leaves (ALO) and 5-FU on sarcoma 180 tumor growth in mice. The graph shows the tumor weight (g) and the inhibition rate of tumor growth (%) of the different experimental groups. Data are expressed as mean ± SEM of six animals analyzed by ANOVA followed by Tukey. a p < 0.05 compared to tumor control.
In the histopathological analysis of tumors, tissue infiltration, areas of coagulative tumor necrosis, desmoplasia and asymmetric distribution of mitoses were observed, with variations between groups (Fig. 3A and B). In tumor control group, desmoplastic reaction presented moderate level with areas of necrosis corresponding to about 60% of neoplastic growth. Tumors of animals treated with 5-FU (25 mg/kg), in turn, showed areas of necrosis corresponding to about 60% of neoplastic growth (Fig. 3C). Asymmetric mitosis which accounted for about three per high power field (data not shown). Architectural and cytological aspects of tumor in ALO-treated groups were similar to those observed in tumor control group (Fig. 3B and D). ALO 150 mg/kg treated tumors had the characteristics infiltration of adipose tissue and muscle limited, areas of coagulative tumor necrosis, about 35%, and asymmetric mitosis count, about three per high power field (Fig. 3D).
Histopathology of tumors of the different experimental groups: peritumoral desmoplastic reaction in different treatments. A. 5-FU, moderate; B. ALO (150 mg/kg) discrete. Coagulative necrosis areas associated with the treatment in C 5-FU, D. ALO 150 mg/kg. A (×400), B (×100) – Masson; C, D: H.E. ×100, ALO: volatile oil from Annona leptopetala leaves.
Concerning to toxicity analysis, ALO caused reduction in food consumption when compared to tumor control and healthy groups (Table 5), which was accompanied of decrease on body weights. It is already observed in literature for other chemotherapeutic drugs (Sánchez-lara et al., 2013Sánchez-lara, K., Ugalde-Morales, E., Motola-Kuba, D., Green, D., 2013. Gastrointestinal symptoms and weight loss in cancer patients receiving chemotherapy. Br. J. Nutr. 109, 894-897.; Caillet et al., 2016Caillet, P., Liuu, E., Simon, A.R., Bonnefoy, M., Guerin, O., Berrut, G., Lesoourd, B., Jeandel, C., Ferry, M., Rolland, Y., Paillaud, E., 2016. Association between cachexia, chemotherapy and outcomes in older cancer patients: a systematic review. Clin. Nutr. 36, 1473-1482.).
Feed and water consumption and weight of animals (n = 6) subjected to different treatments (7 days).
Main organ of metabolism, liver is susceptible to effects of antitumor drugs. Major markers of cell injury are cytoplasmic and mitochondrial enzymes, aspartate aminotransferase (AST) and alanine aminotransferase (ALT). ALO induced an increase on AST activity without changed ALT activity (Table 6), which may be related to non-severe liver damage (Henry, 2008Henry, J.B., 2008. Diagnósticos clínicos e tratamento por métodos laboratoriais. Manole, São Paulo.) or damage to tissues near tumor, such as, skeletal muscle. On the other hand, ALO did not induce changes in urea and creatinine levels (Table 6), suggesting no renal toxicity.
Effects of 5-FU and volatile oil from Annona leptopetala leaves (ALO) on biochemical parameters of peripheral blood of mice (n = 6) subjected to different treatments (7 days).
To assess tissue damage induced by ALO, histopathological evaluation was performed. No histopathological changes were observed in kidneys of animals treated with ALO (data not shown).
In animals treated with ALO (100 mg/kg) there was hypertrophy of Kupffer cells (Fig. 4A), focal hepatic necrosis, and hepatocytes associated with lymph-histiocytic flow (Fig. 4B). At 150 mg/kg ALO, it was also observed microvesicular (Fig. 4C) and macrovesicular (Fig. 4D) steatosis, and hepatocellular regenerative atypia (Fig. 4F). In addition, there was cyst formation of bile nature, multilocular, with coating cylindrical epithelium simple cuboid and walls of fibrous connective tissue (Fig. 4E). Same changes, albeit more frequent, were observed in animals treated with 5-FU accompanied by periportal lymphocytes presence (data not shown).
Histopathology of liver of different groups: A. ALO (100 mg/kg), hypertrophy of Kupffer cells (short arrows) in zone 1; B. ALO (100 mg/kg), focal hepatic necrosis (thick arrow) hepatocyte associated with lymph-histiocytic flow in zone 2, accompanied by hypertrophy of Kupffer cells (short arrows); C. ALO (150 mg/kg) predominantly microvesicular steatosis in zone 3 (short arrow); D. and ALO (150 mg/kg) macrovesicular microvesicular steatosis in zone 2 (short arrows); E. ALO (150 mg/kg) cyst formation of bile nature, multilocular, with coating cylindrical epithelium simple cuboid and walls of fibrous connective tissue; F. ALO (150 mg/kg) Hepatocellular regenerative atypia. HTV: Hepatic Terminal Vein; A, B, C, E, F: H.E. ×400; D: Masson, ×400; ALO: volatile oil from Annona leptopetala leaves.
Microgoticular steatosis observed is a lesion of metabolic etiology, usually reaction to drug therapy, but of reversible nature and rapid improvement after treatment discontinuation (Torti et al., 2001Torti, V.R., Cobb, A.J., Everitt, J.I., Marshall, M.W., Boorman, G.A., Butterworth, B.E., 2001. Nephrotoxicity and hepatotoxicity induced by inhaled bromodichloromethane in wild-type and p53-heterozygous mice. Toxicol. Sci. 64, 269-280.; De Vasconcellos et al., 2007De Vasconcellos, M.C., Bezerra, D.P., Fonseca, A.M., Pereira, M.R.P., Lemos, T.L.G., Pessoa, O.D.L., Pessoa, C., Moraes, M.O., Alves, A.P.N.N., Costa-Lotufo, L.V., 2007. Antitumor activity of biflorin, an o-naphthoquinone isolated from Capraria biflora. Biol. Pharm. Bull. 30, 1416-1421.; Montenegro et al., 2008Montenegro, R.C., Farias, R.A.F., Pereira, M.R.P., Alves, A.P.N.N., Bezerra, F.S., Andrade-Neto, M., Pessoa, C., Moraes, M.O., Costa-Lotufo, L.V., 2008. Antitumor activity of pisosterol in mice bearing with S180 tumor. Biol. Pharm. Bull. 31, 454-457.). Another finding in livers were bile cysts, found in ALO treated group. There was no cholestatic histological effect in these animals neither to other bile ducts, nor to the hepatic terminal vein (HTV). These cysts are therefore a causal found and located.
Regarding to hematological parameters, there were only a decrease on MCV and MCHC, comparing with tumor control and health groups (Table 7). Nevertheless, these isolated changes have not any clinical significance. Animals transplanted with sarcoma 180 tumor showed a significant increase in total numbers of leukocytes compared to healthy animals. There was also an increase of neutrophils and decreased of lymphocytes in peripheral blood of animals transplanted with sarcoma 180, compared to healthy group (Table 7), corroborating to literature (Pita et al., 2012Pita, J.C.L.R., Xavier, A.L., Sousa, T.K.G., Mangueira, V.M., Tavares, J.F., Júnior, R.J.O., Veras, R.C., Pessoa, H.L.F.P., Silva, M.S., Morelli, S., Ávila, V.M.R., Silva, T.G., Diniz, M.F.F.M., Castello-Branco, M.V.S., 2012. In vitro and in vivo antitumor effect of trachylobane-360, a diterpene from Xylopia langsdorffiana. Molecules 17, 9573-9589.; Moura et al., 2016Moura, A.P.G., Beltrão, D.M., Pita, J.C.L.R., Xavier, A.L., Brito, M.T., Sousa, T.K.G., Batista, L.M., Carvalho, J.E., Ruiz, A.L.T.G., Torre, A.D., Duarte, M.C., Tavares, J.F., Silva, M.S., Sobral, M.V., 2016. Essential oil from fruit of Xylopia langsdorffiana: antitumour activity and toxicity. Pharm. Biol. 54, 3093-3102.). 5-FU treatment led to a decrease in total leukocytes and neutrophils number, as well as an increase in lymphocytes count, when compared to tumor control group. ALO treatment induced no significant change in the leucogram parameters, when compared to tumor control group (Table 7). These data suggest that, unlike most chemotherapy, this volatile oil does not alter the number of hematopoietic cells, which represents one of main effects cancer chemotherapy treatment (Liu et al., 2013Liu, W., Zhang, C., Li, K., 2013. Prognostic value of chemotherapy-induced leukopenia in small-cell lung cancer. Cancer Biol. Med. 10, 92-98.; Campos et al., 2015Campos, M.I.C., Vieira, W.D.A., Campos, C.N., Aarestrup, F.M., Aarestrup, B.J.V., 2015. Atorvastatin and trans-caryophyllene for the prevention of leukopenia in an experimental chemotherapy model in Wistar rats. Mol. Clin. Oncol. 3, 825-828.; Chopra et al., 2016Costa, E.V., Dutra, L.M., Salvador, M.J., Ribeiro, L.H.G., Gadelha, F.R., Carvalho, J.E., 2013. Chemical composition of the essential oils of Annona pickelii and Annona salzmannii (Annonaceae), and their antitumour and trypanocidal activities. Nat. Prod. Res. 27, 997-1001.).
Effects of 5-FU and volatile oil from Annona leptopetala leaves on hematological parameters of peripheral blood of mice (n = 6) subjected to different treatments (7 days).
In conclusion, ALO shows in vitro and in vivo antitumor activity, without major changes in toxicity parameters evaluated. The results provide essential information, making possible further investigations of antitumor activity and action mechanisms of ALO.
Ethical disclosures
-
Protection of human and animal subjects. The authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
-
Confidentiality of data. The authors declare that no patient data appear in this article.
-
Right to privacy and informed consent. The authors declare that no patient data appear in this article.
References
- Adams, R.P., 2001. Identification of Essential Oil Components by Gas Chromatography/Quadrupole Mass Spectroscopy. Allured Publishing Corporation, Carol Stream (IL).
- Anvisa, 2013. Guia para a condução de estudos não clínicos de toxicologia e segurança farmacológica necessários ao desenvolvimento de medicamentos. Agência Nacional de Vigilância Sanitária. Ministério da Saúde, Brasília.
- Agra, M.F., Freitas, P.F., Barbosa-Filho, J.M., 2007. Synopsis of the plants known as medicinal and poisonous in Northeast of Brazil. Rev. Bras. Farmacogn. 17, 114-140.
- Agu, K.C., Okolie, P.N., 2017. Proximate composition, phytochemical analysis, and in vitro antioxidant potentials of extracts of Annona muricata (Soursop). Food Sci. Nutr. 5, 1029-1036.
- Almeida, R.N., Falcão, A.C.G.M., Diniz, R.S.T., Quintans-Júnior, L.J., Polari, R.M., Barbosa-Filho, J.M., Agra, M.F., Duarte, J.C., Ferreira, C.D., Antoniolli, A.R., Araújo, C.C., 1999. Metodologia para avaliação de plantas com atividade no Sistema Nervoso Central e alguns dados experimentais. Rev. Bras. Farm. 80, 72-76.
- Auletta, C.S., 1995. Acute, subchronic, and chronic toxicology. In: Derelanko, M.J., Hollinger, M.A. (Eds.), Handbook of Toxicology. CRC Press, London, pp. 51–162.
- Bezerra, D.P., Castro, F.O., Alves, A.P.N.N., Pessoa, C., Moraes, M.O., Silveira, E.R., Lima, M.A.S., Elmiro, F.J.M., de Alencar, N.M.N., Mesquita, R.O., Lima, M.W., Costa-Lotufo, L.V., 2008. In vitro and in vivo antitumor effect of 5-FU combined with piplartine and piperine. J. Appl. Toxicol. 28, 156-163.
- Bhalla, Y., Gupta, V.K., Jaitak, V., 2013. Anticancer activity of essential oils: a review. J. Sci. Food Agric. 93, 3643-3653.
- Bicas, J.L., Neri-Numa, I.A., Ruiz, A.L.T.G., de Carvalho, J.E., Pastore, G.M., 2011. Evaluation of the antioxidant and antiproliferative potential of bioflavors. Food Chem. Toxicol. 49, 1610-1615.
- Bomfim, L.M., Menezes, L.R.A., Rodrigues, A.C.B.C., Dias, R.B., Rocha, C.A.G., Soares, M.B.P., Neto, A.F.S., Nascimento, M.P., Campos, A.F., Silva, L.C.R.C., Costa, E.V., Bezerra, D.P., 2016. Antitumour activity of the microencapsulation of Annona vepretorum essential oil. Basic Clin. Pharmacol. Toxicol. 118, 208-213.
- Caillet, P., Liuu, E., Simon, A.R., Bonnefoy, M., Guerin, O., Berrut, G., Lesoourd, B., Jeandel, C., Ferry, M., Rolland, Y., Paillaud, E., 2016. Association between cachexia, chemotherapy and outcomes in older cancer patients: a systematic review. Clin. Nutr. 36, 1473-1482.
- Campos, M.I.C., Vieira, W.D.A., Campos, C.N., Aarestrup, F.M., Aarestrup, B.J.V., 2015. Atorvastatin and trans-caryophyllene for the prevention of leukopenia in an experimental chemotherapy model in Wistar rats. Mol. Clin. Oncol. 3, 825-828.
- Chopra, D., Rehan, H.S., Sharma, V., Mishra, R., 2016. Chemotherapy-induced adverse drug reactions in oncology patients: a prospective observational survey. Indian J. Med. Paediatr. Oncol. 37, 42-46.
- Choy, W.N., 2001. Regulatory genetic toxicology tests. In: Choy, W.N. (Ed.), Genetic Toxicology and Cancer Risk Assessment. CRC Press, London, pp. 107–128.
- Costa, V.C.O., Tavares, J.F., Agra, M.F., Falcão-Silva, V.S., Facanali, R., Vieira, M.A.R., Marques, M.O.M., Siqueira-Júnior, J.P., Silva, M.S., 2008. Composição química e modulação da resistência bacteriana a drogas do óleo essencial das folhas de Rollinia leptopetala RE Fries. Rev. Bras. Farmacogn. 18, 245-248.
- Costa, E.V., Dutra, L.M., Salvador, M.J., Ribeiro, L.H.G., Gadelha, F.R., Carvalho, J.E., 2013. Chemical composition of the essential oils of Annona pickelii and Annona salzmannii (Annonaceae), and their antitumour and trypanocidal activities. Nat. Prod. Res. 27, 997-1001.
- Costa, V.C.O., Tavares, J.F., Queiroga, C.S., Castello-Branco, M.V.S., Diniz, M.F.F.M., Lima, C.U.G.B., Santos, B.V.O.S., Pita, J.C.L.R., Silva, M.S., 2012. Constituintes químicos das folhas de Rollinia leptopetala RE Fries. Quim. Nova 22, 1-5.
- David, J.P., Meira, M., David, J.M., Brandão, H.N., Branco, A., Agra, M.F., Barbosa, M.R.V., de Queiroz, L.P., Giulietti, A.M., 2007. Radical scavenging, antioxidant and cytotoxic activity of Brazilian Caatinga plants. Fitoterapia 78, 215-218.
- De Vasconcellos, M.C., Bezerra, D.P., Fonseca, A.M., Pereira, M.R.P., Lemos, T.L.G., Pessoa, O.D.L., Pessoa, C., Moraes, M.O., Alves, A.P.N.N., Costa-Lotufo, L.V., 2007. Antitumor activity of biflorin, an o-naphthoquinone isolated from Capraria biflora Biol. Pharm. Bull. 30, 1416-1421.
- Feitosa, E.M.A., Arriaga, A.M.C., Santiago, G.M.P., Lima, J.Q., Malcher, G.T., do Nascimento, R.F., Braz-Filho, R., 2009. Chemical composition and larvicidal activity of Rollinia leptopetala (Annonaceae). J. Braz. Chem. Soc. 20, 375-378.
- Fevier, A., Ferreira, M.E., Fournet, A., Valuff, G., Inchausti, A., De Arias, A.R., Hocquemiller, R., Waechter, A., 1999. Acetogenins and other compounds from Rollinia emarginata and their antiprotozoal activities. Plant. Med. 65, 47-49.
- Hadri, A., Rio, M.A.G., Sanz, J., Coloma, A.G., Idaomar, M., Ozonas, B.R., Gonzales, J.B., Reus, M.I.S., 2010. Cytotoxic activity of α-humulene and trans-caryophyllene from Salvia officinallis in animal and human tumor cells. Ann. R. Acad. Nac. Farm. 76, 343-356.
- Hassan, S.B., Gali-Muhtasib, H., Goransson, H., Larsson, R., 2010. Alpha terpineol: a potential anticancer agent which acts through suppressing NF-kappa B signalling. Anticancer Res. 30, 1911-1919.
- Henry, J.B., 2008. Diagnósticos clínicos e tratamento por métodos laboratoriais. Manole, São Paulo.
- Júnior, J.G.A.S., Coutinho, H.D.M., Boris, T.C.C., Cristo, J.S., Pereira, N.L.F., Figueiredo, F.G., Cunha, F.A.B., Aquino, P.E.A., Nascimento, P.A.C., Mesquita, F.J.C., Moreira, P.H.F., Coutinho, S.T.B., Souza, I.T., Teixeira, G.C., Ferreira, N.M.N., Farina, E.O., Torres, C.M.G., Holanda, V.N., Pereira, V.S., Guedes, M.I.F., 2016. Chemical characterization and cytoprotective effect of the hydroethanol extract from Annona coriacea Mart (Araticum). Pharmacognosy Res. 8, 253-325.
- Kang, C., Munawir, A., Cha, M., Sohn, E.-T., Lee, H., Kim, J.S., Yoon, W.D., Lim, D., Kim, E., 2009. Cytotoxicity and hemolytic activity of jellyfish Nemopilema nomurai (Scyphozoa: Rhizostomeae) venom. Comp. Biochem. Physiol. 150, 85-90.
- Kossouoh, C., Moudachirou, M., Adjakidje, V., Chalchat, J., Figuérédo, G., 2007. Essential oil chemical composition of Annona muricata L. leaves from Benin. J. Essent. Oil Res. 19, 307-309.
- Kuo, R.Y., Chang, F.R., Chen, C.Y., Teng, C.M., Yen, H.F., Wu, Y.C., 2001. Antiplatelet activity of N-methoxycarbonyl aporphines from Rollinia mucosa Phytochemistry 57, 421-425.
- Liu, W., Zhang, C., Li, K., 2013. Prognostic value of chemotherapy-induced leukopenia in small-cell lung cancer. Cancer Biol. Med. 10, 92-98.
- Mangal, M., Imran, K.M., Mohan, A.S., 2016. Acetogenins as potential anticancer agents. Anticancer Agents Med. Chem. 16, 138-159.
- Mangueira, V.M., Batista, T.M., Brito, M.T., Sousa, T.K.G., Cruz, R.M.D., Abrantes, R.A., Veras, R.C., Medeiros, I.A., Medeiros, K.K.P., Pereira, A.L.C., Serafim, V.L., Moura, R.O., Sobral, M.V., 2017. A new acridine derivative induces cell cycle arrest and antiangiogenic effect on Ehrlich ascites carcinoma model. Biomed. Pharmacother. 90, 253-261.
- Meira, C.S., Guimarães, E.T., Macedo, T.S., Silva, T.B., Menezes, L.R.A., Costa, E.V., Soares, M.B.P., 2014. Chemical composition of essential oils from Annona vepretorum Mart. and Annona squamosa L, (Annonaceae) leaves and their antimalarial and trypanocidal activities. J. Essent. Oil Res. 27, 160-168.
- Mendanha, S.A., Moura, S.S., Anjos, J.L., Valadares, M.C., Alonso, A., 2013. Toxicity of terpenes on fibroblast cells compared to their hemolytic potential and increase in erythrocyte membrane fluidity. Toxicol. In Vitro 27, 323-329.
- Monks, A., Scudiero, D., Skehan, P., Shoemaker, R., Paull, K., Vistica, D., Hose, C., Langley, J., Cronise, P., Vaigro-Wolff, A., Gray-Goodrich, M., Campbell, H., Mayo, J., Boyd, M., 1991. Feasibility of a high-flux anticancer drug screen using a diverse panel of cultured human tumor cell lines. J. Natl. Cancer. Inst. 83, 757-766.
- Monteiro, F.S., Costa, V.C.O., Queiroga, C.S., Tavares, J.F., Santos, B.V.O., Silva, M.S., Silva, B.A., 2008. Comparação do efeito antiespasmódico de Rollinia leptopetala R.E. In: Fries e Rollinia exsucca A. DC. em íleo isolado de cobaia. Anais do XX Simpósio de Plantas Medicinais do Brasil. X Congresso Internacional de Etnofarmacologia, São Paulo.
- Montenegro, R.C., Farias, R.A.F., Pereira, M.R.P., Alves, A.P.N.N., Bezerra, F.S., Andrade-Neto, M., Pessoa, C., Moraes, M.O., Costa-Lotufo, L.V., 2008. Antitumor activity of pisosterol in mice bearing with S180 tumor. Biol. Pharm. Bull. 31, 454-457.
- Moura, A.P.G., Beltrão, D.M., Pita, J.C.L.R., Xavier, A.L., Brito, M.T., Sousa, T.K.G., Batista, L.M., Carvalho, J.E., Ruiz, A.L.T.G., Torre, A.D., Duarte, M.C., Tavares, J.F., Silva, M.S., Sobral, M.V., 2016. Essential oil from fruit of Xylopia langsdorffiana: antitumour activity and toxicity. Pharm. Biol. 54, 3093-3102.
- Novaes, P., Ferreira, M.J.P., Santos, D.Y.A.C., 2018. Flavonols from Annona coriacea Mart, (Annonaceae). Biochem. Syst. Ecol. 78, 77-80.
- OECD, 1997. Guideline for Testing of Chemicals n. 474: Mammalian erythrocyte micronucleus test, Organization for Economic Co-operation and Development., pp. 1–10.
- Palazzo, M.C., Wright, H.L., Agius, B.R., Wright, B.S., Moriarity, D.M., Haber, W.A., Setzer, W.N., 2009. Chemical compositions and biological activities of leaf essential oils of six species of Annonaceae from Monteverde. Costa Rica. Rec. Nat. Prod. 3, 153-160.
- Pita, J.C.L.R., Xavier, A.L., Sousa, T.K.G., Mangueira, V.M., Tavares, J.F., Júnior, R.J.O., Veras, R.C., Pessoa, H.L.F.P., Silva, M.S., Morelli, S., Ávila, V.M.R., Silva, T.G., Diniz, M.F.F.M., Castello-Branco, M.V.S., 2012. In vitro and in vivo antitumor effect of trachylobane-360, a diterpene from Xylopia langsdorffiana Molecules 17, 9573-9589.
- Ribeiro, L.R., 2003. Teste do micronúcleo em medula óssea de roedores in vivo In: Ribeiro, L.R., Salvadori, D.M.F., Marques, E.K. (Eds.), Mutagênese ambiental. ULBRA, Canoas, pp. 173–198.
- Salvador, M.J., Carvalho, J.E.D., Wisniewski-Jr, A., Kassuya, C.A., Santos, É.P., Riva, D., Stefanello, M.E.A., 2011. Chemical composition and cytotoxic activity of the essential oil from the leaves of Casearia lasiophylla Rev. Bras. Farmacogn. 21, 864-868.
- Sánchez-lara, K., Ugalde-Morales, E., Motola-Kuba, D., Green, D., 2013. Gastrointestinal symptoms and weight loss in cancer patients receiving chemotherapy. Br. J. Nutr. 109, 894-897.
- Santana, K.L., Galvão, M.S., Jesus, M.S., Nogueira, J.P., Narain, N., 2017. HS-SPME optimization and extraction of volatile compounds from soursop (Annona muricata L.) pulp with emphasis on their characteristic impact compounds. Food Sci. Technol. 37, 250-260.
- Siqueira, C.A.T., Oliani, J., Sartoratto, A., Queiroga, C.L., Moreno, P.R.H., Reimão, J.Q., Tempone, A.G., Fischer, D.C.H., 2011. Chemical constituents of the volatile oil from leaves of Annona coriacea and in vitro antiprotozoal activity. Rev. Bras. Farmacogn. 21, 33-40.
- Tavares, J.F., Silva, M.V.B., Queiroga, K.F., Martins, R.M., Silva, T.M.S., Camara, C.A., Agra, M.F., Barbosa-Filho, J.M., Silva, M.S., Marques, M.O.M., 2007. Composition and molluscicidal properties of essential oils from leaves of Xylopia langsdorffiana A. St. Hil. et Tul.(Annonaceae). J. Essent. Oil Res. 19, 282-284.
- Torti, V.R., Cobb, A.J., Everitt, J.I., Marshall, M.W., Boorman, G.A., Butterworth, B.E., 2001. Nephrotoxicity and hepatotoxicity induced by inhaled bromodichloromethane in wild-type and p53-heterozygous mice. Toxicol. Sci. 64, 269-280.
- Valter, J.L., Alencar, K.M.C., Sartori, A.L.B., Nascimento, E.A., Chang, R., Morais, S.A.L., Laura, V.A., Yoshida, N.C., Carollo, C.A., Silva, D.B., Grassi, R.F., Fabri, J.R., Siqueira, J.M., 2008. Variação química no óleo essencial das folhas de seis indivíduos de Duguetia furfuracea (Annonaceae). Rev. Bras. Farmacogn. 18, 373-378.
- Van den Dool, H., Kratz, P.D., 1963. A generalization of the retention index system including linear temperature programmed gas liquid partition chromatography. J. Chromatogr. A 11, 463-471.
Publication Dates
-
Publication in this collection
Sep-Oct 2018
History
-
Received
27 Mar 2018 -
Accepted
13 June 2018 -
Published
21 July 2018