Abstract
The chemical study of roots from Azadirachta indica A. Juss., Meliaceae, led to the isolation of two new terpenoids, limonoid morenolide and diterpene 17-hydroxy-sandaracopimar-8,15-dien-11-one, in addition to the four well-known limonoids nimbinene, nimbinal, nimbandiol and salannin, and three diterpenoids nimbidiol, ferruginol, and 6,7-dehydroferruginol. Their structural elucidations were based on one and bidimensional Nuclear Magnetic Resonance and Electrospray ionization mass spectrometry spectra data which was compared to the data found in literature. The anti-inflammatory, cytotoxic and antimycobacterial activities of the identified terpenoids were evaluated.
Keywords
Meliaceae; Limonoids; Diterpenoids; Mycobacterium; Inflammation
Introduction
Azadirachta indica A. Juss., Meliaceae, commonly known as neem, originated from South and Southeast Asia, is also found in tropical and subtropical areas of Africa, America and Australia (Puri, 1999Puri, H.S., 1999. Neem: The Dive Tree Azadirachta indica. Harwood Academic Publishers, Amsterdam.). Neem has been widely used by humankind since prehistoric times to treat diseases, and has been known as "divine tree" (Kumar and Navaratnam, 2013Kumar, V.S., Navaratnam, V., 2013. Neem (Azadirachta indica): prehistory to contemporary medicinal uses to humankind. Asian Pac. J. Trop. Biomed. 3, 505-514.). Biological activities such as antibacterial (Joy Sinha et al., 2017Joy Sinha, D., Nandha, D.S., Jaiswal, K., Vasudeva, N., Prabha Tyagi, A., Pratap Singh, S.U., 2017. Antibacterial effect of Azadirachta indica (neem) or Curcuma longa (turmeric) against Enterococcus faecalis compared with that of 5% sodium hypochlorite or 2% chlorhexidine in vitro. Bull. Tokyo Dent. Coll. 58, 103-109.), antiviral (Ashfaq et al., 2016Ashfaq, U.A., Jalil, A., ul Qamar, M.T., 2016. Antiviral phytochemicals identification from Azadirachta indica leaves against HCV NS3 protease: an in silico approach. Nat. Prod. Res. 30, 1866-1869.), antifungal (Osman Mohamed Ali et al., 2017Osman Mohamed Ali, E., Shakil, N.A., Rana, V.S., Sarkar, D.J., Majumder, S., Kaushik, P., Singh, B.B., Kumar, J., 2017. Antifungal activity of nano emulsions of neem and citronella oils against phytopathogenic fungi, Rhizoctonia solani and Sclerotium rolfsii. Ind. Crops Prod. 108, 379-387.), molluscicidal (Ebenso, 2004Ebenso, I.E., 2004. Molluscicidal effects of neem (Azadirachta indica) extracts on edible tropical land snails. Pest Manag. Sci. 60, 178-182.) and antihyperglycemic (Ezeigwe et al., 2015Ezeigwe, O.C., Ononamadu, C.J., Enemchukwu, B.N., Umeoguaju, U.F., Okoro, J.C., 2015. Antidiabetic and antidiabetogenic properties of the aqueous extracts of Azadirachta indica leaves on alloxan induced diabetic wistar rats. Int. J. Biosci. 7, 116-126.) have been attributed to different parts and extracts of neem, besides the antifeedant activity characteristic of limonoids present in this species (Mordue (Luntz) and Blackwell, 1993Mordue (Luntz), A.J., Blackwell, A., 1993. Azadirachtin: an update. J. Insect Physiol. 39, 903-924.). The limonoids, also known as tetranortripernoids, are the main representatives of the chemical composition of neem. Azadirachtin is considered the most important antifeedant compound, which showed activity in at least 550 insect species (Mondal and Mondal, 2012Mondal, D., Mondal, T., 2012. A review on efficacy of Azadirachta indica A. Juss based biopesticides: an Indian perspective. Res. J. Recent Sci. 1, 94-99.).
Neem compounds it also showed inhibition of nitric oxide (NO) production, characterized in the methanolic extract of neem leaves, which evidenced a strong effect on proinflammatory cell signaling and apoptotic cell death mechanisms (Schumacher et al., 2011Schumacher, M., Cerella, C., Reuter, S., Dicato, M., Diederich, M., 2011. Anti-inflammatory, pro-apoptotic, and anti-proliferative effects of a methanolic neem (Azadirachta indica) leaf extract are mediated via modulation of the nuclear factor-κB pathway. Genes Nutr. 6, 149-160.). Twenty limonoids from A. indica were also evaluated for their inhibitory activity against NO production in mouse macrophage RAW 264.7 cells induced by lipopolysaccharide (LPS) (Akihisa et al., 2017Akihisa, T., Nishimoto, Y., Ogihara, E., Matsumoto, M., Zhang, J., Abe, M., 2017. Nitric oxide production-inhibitory activity of limonoids from Azadirachta indica and Melia azedarach. Chem. Biodivers. 14, e1600468.).
In Brazil, the presence of pests in agriculture and livestock are limiting to production. Works were been carried out with neem extracts in these cases. For example, neem oil hydroalcoholic extract was highly toxic to the Iphiseiodes zuluagai mite (coffee pests) (Mourao et al., 2004Mourao, S.A., Silva, J.C.T., Guedes, R.N.C., Venzon, M., Jham, G.N., Oliveira, C.L., Zanuncio, J.C., 2004. Selectivity of neem extracts (Azadirachta indica A Juss.) to the predatory mite Iphiseiodes zuluagai (Denmark & Muma) (Acari: Phytoseiidae). Neotrop. Entomol. 33, 613-617.); another work presented an acaricidal effect of neem extracts against Rhipicephalus microplus (cattle tick) (Giglioti et al., 2011Giglioti, R., Forim, M.R., Oliveira, H.N., Chagas, A.C.S., Ferrezini, J., Brito, L.G., Falcoski, T.O.R.S., Albuquerque, L.G., Oliveira, M.C.S., 2011. In vitro acaricidal activity of neem (Azadirachta indica) seed extracts with known azadirachtin concentrations against Rhipicephalus microplus. Vet. Parasitol. 181, 309-315.). In relation to public health, the ethanolic extract of neem oil also showed activity against larvae of Aedes aegypti (mosquito transmitting dengue) (Wandscheer et al., 2004Wandscheer, C.B., Duque, J.E., da Silva, M.A.N., Fukuyama, Y., Wohlke, J.L., Adelmann, J., Fontana, J.D., 2004. Larvicidal action of ethanolic extracts from fruit endocarps of Melia azedarach and Azadirachta indica against the dengue mosquito Aedes aegypti. Toxicon 44, 829-835.).
Due to ethnopharmacological importance and few researches regarding the neem roots, the present study was based on the chemical characterization and biological investigation of the Azadirachta indica roots (dichloromethane and ethyl acetate extracts). It led to the identification of a new C-seco limonoid (1) and a new pimarane diterpene (6), along with seven known additionals terpenoids (2-5, 7-9), which were characterized by spectra data, mainly obtained by 1H and 13C NMR (1D and 2D) and mass spectra (MS), in comparison with literature data. The biological investigations were assessed for their cytotoxic, inhibition of NO production, and antimycobacterial activities.
Materials and methods
General experimental procedures
The NMR analysis was carried out on a Bruker Ascend 500 (500 MHz for 1H and 125 MHz for 13C) in CDCl3, and used TMS as the internal standard. Chemical shifts (δ) in ppm and coupling constants (J) in Hz. HRESI-MS mass spectra were obtained on a micrOTOF-Q II BrukerDaltonics mass spectrometer, with the use of the positive ion mode of analysis. Gas chromatography coupled with low resolution mass (GC/MS) were determined on a GCMS-QP5050A Shimadzu with the use of an ionization energy of 70 eV. Column chromatography (CC) was performed on silica gel 60 (0.063-0.200 mm, MERCK). For the preparative thin layer chromatography (PTLC) silica gel 60 PF254 MERCK was used on glass plates. n-Hexane (98.5%), methanol (MetOH, 99.8%), ethyl acetate (AcOEt, 99.5%), dichloromethane (CH2Cl2, 99.5%) and n-butanol (99.4%) were purchased from Synth (São Paulo, Brazil).
Plant material
The roots of Azadirachta indica A. Juss., Meliaceae, were collected in Cachoeiro de Itapemirim city, Espirito Santo state, Brazil, in December 2015. A voucher specimen (H10154) was deposited in the herbarium of the Centro de Ciências e Biotecnologia of the Universidade Estadual do Norte Fluminense Darcy Ribeiro (UENF).
Extraction and isolation
Dried and powdered roots (2.5 kg) from A. indica were extracted with methanol at room temperature. After solvent evaporation, 148.3 g of crude methanol extract was suspended in a MeOH:H2O solution (1:3), and partitioned with CH2Cl2, EtOAc, and n-butanol, successively. The CH2Cl2 fraction (AID, 38.1 g) was chromatographed on a silica gel column (CC) and eluted with a gradient of CH2Cl2:MeOH, yielding nine subfractions (AID1-AID9). Compounds 8 and 9 were identified in admixture from the AID1-4 (14 mg) fraction. The fraction AID4 (14.4 g) was subjected to CC, with the use of n-hexane:EtOAc gradient as eluent, yielding eight fractions. The AID4-4 (764 mg) fraction was rechromatographed yielding thirteen fractions. The AID4-5 (854 mg) fraction was purified by PTLC with the use of CH2Cl2:MeOH (7:3) as eluent in order to obtain compounds 2 (12 mg) and 6 (2 mg). Similarly, the AID4-4.7 fraction was purified by PTLC (CH2Cl2:MeOH, 7:3) yielding compound 3 (8 mg). Fraction AID4-6 was fractionated by silica gel CC with a n-hexane:EtOAc mixture, yielding eight fractions. The AID4-6.4 and AID4-6.6 fractions yielded compound 7 (405 mg) and 4 (6 mg), respectively. The EtOAc fraction (AIA, 5.6 g) was fractionated by silica gel CC with a gradient of n-hexane:EtOAc to yield nine fractions (AIA1-AIA9). The AIA4 (3.5 g) fraction was rechromatographed similarly in order to yield thirteen fractions. Compound 1 (8 mg) was isolated from fraction AIA4-11. Thereafter, compound 5 (5 mg) was obtained from fraction AID4-13 (822 mg) by silica gel CC (gradient of CH2Cl2:MeOH).
Cell culture and treatments
The RAW 264.7 macrophage cell line was obtained from the ATCC (VA, USA) and cultured under previously described condition (Ventura et al., 2015Ventura, T.L.B., Calixto, S.D., De Azevedo Abrahim-Vieira, B., De Souza, A.M.T., Mello, M.V.P., Rodrigues, C.R., De Mariz, E., Miranda, L.S., De Souza, R.O.M.A., Leal, I.C.R., Lasunskaia, E.B., Muzitano, M.F., 2015. Antimycobacterial and anti-inflammatory activities of substituted chalcones focusing on an anti-tuberculosis dual treatment approach. Molecules 20, 8072-8093.).
Quantification of NO production
NO production by macrophages was estimated indirectly by the Griess method to measure nitrites concentration and performed as described previously (Park et al., 2009Park, P.-H., Kim, H.S., Jin, X.Y., Jin, F., Hur, J., Ko, G., Sohn, D.H., 2009. KB-34, a newly synthesized chalcone derivative, inhibits lipopolysaccharide-stimulated nitric oxide production in RAW 264.7 macrophages via heme oxygenase-1 induction and blockade of activator protein-1. Eur. J. Pharmacol. 606, 215-224.). Fifty microliters of each supernatant (obtained as above) were added to Griess reagent (Sigma Aldrich). After 10 min, the absorbance was measured at 570 nm in plate spectrophotometer (Dinatech MR5000). The nitrite concentration in the supernatant was determined in µg/ml using as reference a sodium nitrite curve decreased from the value obtained with the cell-free additives. L-NMMA (NG-methyl-L-arginine acetate) was used as reference standards.
Culture of mycobacteria and evaluation of bacterial growth
Two strains of mycobacteria, which differ in degree of virulence, were used in this study: strains of Mycobacterium tuberculosis (virulent laboratory strain H37Rv, ATCC 27294; and highly virulent Mtb strain Beijing M299 isolated from a TB patient in Mozambique) evaluated for virulence in a previous study (Ribeiro et al., 2014Ribeiro, S.C.M., Gomes, L.L., Amaral, E.P., Andrade, M.R.M., Almeida, F.M., Rezende, A.L., Lanes, V.R., Carvalho, E.C.Q., Suffys, P.N., Mokrousov, I., Lasunskaia, E.B., 2014. Mycobacterium tuberculosis strains of the modern sublineage of the Beijing family are more likely to display increased virulence than strains of the ancient sublineage. J. Clin. Microbiol. 52, 2615-2624.). The mycobacterial strains were grown in suspension of Middlebrook 7H9 broth, containing 10% dextrose albumin complex (ADC), 0.5% glycerol and 0.05% Tween-80 at 37 ºC under conditions of containment of Biosafety 3. To study the antimycobacterial activity of the samples, we used the MTT assay to quantify bacterial growth in liquid medium (Moodley et al., 2014Moodley, S., Koorbanally, N.A., Moodley, T., Ramjugernath, D., Pillay, M., 2014. The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay is a rapid, cheap, screening test for the in vitro anti-tuberculous activity of chalcones. J. Microbiol. Methods 104, 72-78.). Bacterial suspensions and MTT proceeding were described previously (Ventura et al., 2015Ventura, T.L.B., Calixto, S.D., De Azevedo Abrahim-Vieira, B., De Souza, A.M.T., Mello, M.V.P., Rodrigues, C.R., De Mariz, E., Miranda, L.S., De Souza, R.O.M.A., Leal, I.C.R., Lasunskaia, E.B., Muzitano, M.F., 2015. Antimycobacterial and anti-inflammatory activities of substituted chalcones focusing on an anti-tuberculosis dual treatment approach. Molecules 20, 8072-8093.). The resulting samples were measured by optical density at 570 nm. Untreated bacterial suspensions were used to control the spontaneous growth of bacteria. Rifampicin was used as a positive control.
Cytotoxicity assay
The MTT assay was used in the cytotoxicity test (Muzitano et al., 2006Muzitano, M.F., Cruz, E.A., de Almeida, A.P., Da Silva, S.A., Kaiser, C.R., Guette, C., Rossi-Bergmann, B., Costa, S.S., 2006. Quercitrin: an antileishmanial flavonoid glycoside from Kalanchoe pinnata. Planta Med. 72, 81-83.). Cells were plated at the above concentration and culture condition, in a final volume of 100 µl. After 2 h, for macrophage adhesion, the cells were stimulated or not with 1 µg/ml LPS (Escherichia coli Lipopolysaccharide) alone or in combination with the samples evaluated for 24 h. At the end of the incubation, 5 µl of MTT solution (5 mg/ml) were added to each one and 2 h later the plate supernatant was removed, the crystals formed were solubilized by HCl (4 mM) and added to isopropanol. The reading was performed on a plate spectrophotometer at 570 nm.
Statistical analyses
The analysis of variance (ANOVA) was defined, followed by the test of means of Newman-Keuls and Bonferroni, with index of reliability of 95%.
Results and discussion
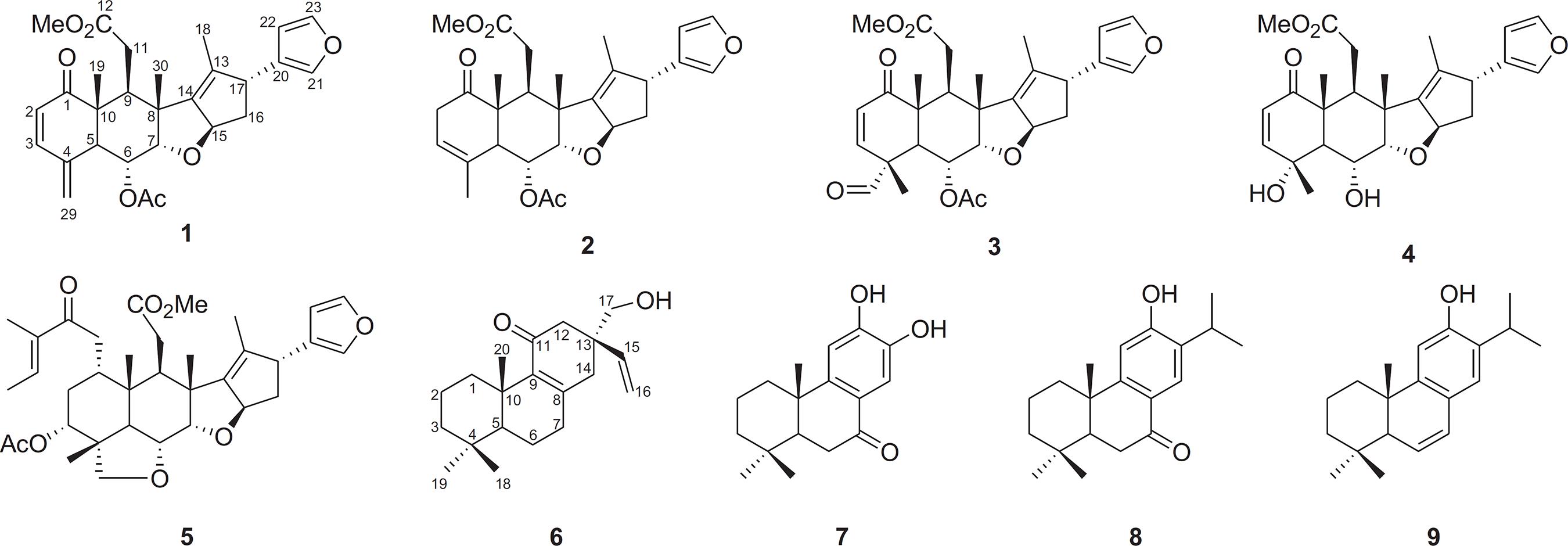
The limonoid morenolide (1) was isolated as a yellow, amorphous powder. Its molecular formula C28H32O7 was deduced by HR-ESI-MS (m/z 503.2033 [M+Na]+, C28H32NaO7, calc. m/z 503.2046). The 13C-DEPTQ NMR spectrum of 1 displayed carbon signals attributed to five methyls [including one of carbomethoxyl (δC 51.7) and one of acetyl (δC 21.3) groups], three methylenes (including one terminal methylene sp2 at δC 119.0), eleven methines [including five sp2 at δC 125.4 (CH-2), 147.0 (CH-3), 139.0 (CH-21), 110.5 (CH-22) and 143.0 (CH-23) and three sp3 monooxygenated at δC 68.0 (CH-6), 84.3 (CH-7) and 86.9 (CH-15)], three carbonyl [one conjugated carbonyl ketonic at δC 202.5 (C-1), one carbonyl carbomethoxy at δC 173.6 (C-12) and one carbonyl acetyl at δC 171.1 (C-1′) groups] and six quaternary [including seven sp2 corresponding to four olefinics at δC 140.4 (C-4), 135.2 (C-13), 146.3 (C-14) and 126.8 (C-20)] carbon atoms.
1H NMR spectrum showed three signals corresponding to methyl groups linked to sp3 carbon atoms at δH 1.70 (3H-18, d, 1.5 Hz), 1.38 (3H-30, s) and 1.12 (3H-19, s), one singlet regarding to acetyl group at δH 2.16 (3H-2′, s) and one singlet at δH 3.64 (MeO-12, s) attributed to the methoxyl group. The singlet signals at δH 5.45 and 5.13 were attributed to a terminal methylene group and the signals at δH 7.05 (H-3, d, 10.2 Hz) and 5.77 (H-2, d, 10.2 Hz) were also used to characterize α,β-unsaturated carbonyl. The presence of hydrogen signals at δH 7.34 (H-23, t, 1.5 Hz), 7.27 (H-21, m), and 6.36 (H-22, dd, 1.8, 0.8 Hz) correlated, respectively, in the HSQC with carbon signals at δC 143.0 (CH-23), 139.0 (CH-21) and 110.5 (CH-22) were attributed to the furan ring characteristic in limonoids C-seco (Ara et al., 1989Ara, I., Siddiqui, B.S., Faizi, S., Siddiqui, S., 1989. Two new terpenoids from root bark of Azadirachta indica. J. Nat. Prod. 52, 1209-1213.).
The HMBC spectrum revealed cross-peaks corresponding to heteronuclear long-range coupling (2JCH and 3JCH) of 2H-29 with both CH-3 (δC 147.0) and CH-5 (δC 44.2); H-3 with C-1 (δC 202.5), C-4 (δC 140.4) and CH-5 (δC 44.2); and H-2 with both C-4 (δC 140.4) and C-10 (δC 49.3) which enabled us to establish the presence of a carbonyl α,β-unsaturated and terminal methylene group into six-membered ring (ring A). The HMBC spectrum and also cross-peaks of MeO-12 (δH 3.64)/δC 173.6 (C-12); 2H-11 (δH 2.97, 2.31)/δC 173.6 (C-12), 36.7 (CH-9); 3H-19 (δH 1.12)/δC 202.5 (C-1), 49.3 (C-10), 44.2 (C-5) and 36.7 (CH-9); and 3H-30 (δH 1.38/δC 84.3 (C-7), 47.8 (C-8), 36.7 (C-9) and 146.3 (C-14) indicated ring C cleavage involving the bond C-12 and C-13 to produce the ester function (COOMe). Additional cross-peaks involving H-21/CH-22 and C-20; H-22/C-20, CH-21 and CH-23; H-23/CH-22 and C-20; and H-17/C-13, CH-15, CH2-16, C-20, CH-21, and CH-22 were also revealed by HMBC, justifying the presence of the furan ring linked to ring D. Additional heteronuclear long-range couplings observed in the HMBC spectrum are presented in Table 1. HSQC and 1H-1H-COSY (Fig. 1) were also used for the complete and unambiguous 1H and 13C chemical shifts assignments.
1H (500 MHz) and 13C (125 MHz) NMR data for 1 and 6, including results obtained by heteronuclear 2D shift-correlated HSQC (1 J HC) and HMBC (n J HC, n = 2 and 3), in CDCl3 as solvent. Chemical shifts (δ, ppm) and coupling constants (J in Hz, in parentheses).
The relative stereochemistry of 1 was determined through 1H-1H-NOESY spectrum analysis, which revealed significant dipolar-dipolar interactions (NOE) between H-9 and H-5, H-9 with H-15, 3H-19 with H-6, H-6 with 3H-30, and 3H-30 with H-7 summarized in Fig. 1.
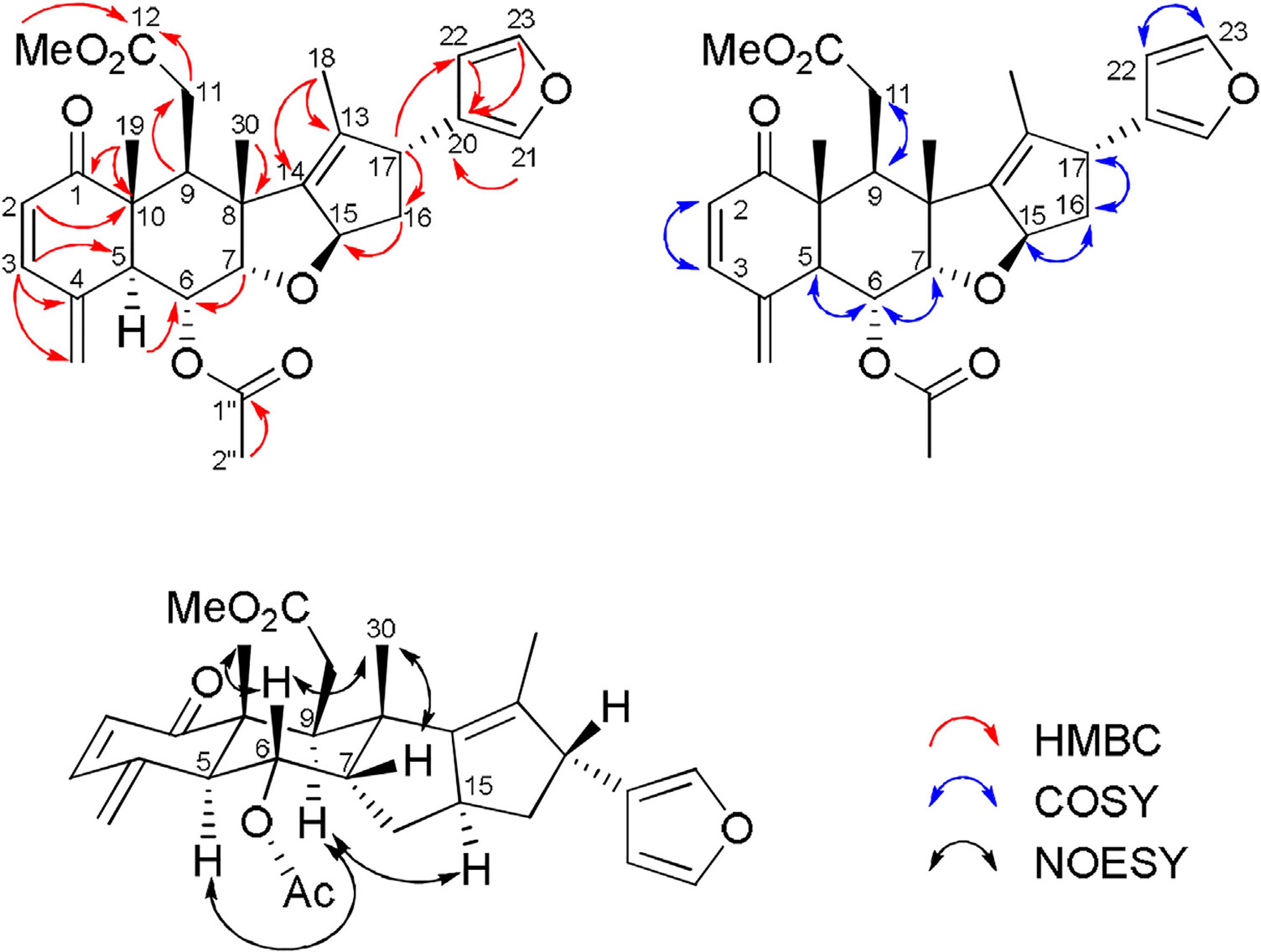
17-Hydroxy-sandaracopimar-8,15-dien-11-one (6) was obtained as a white amorphous powder. Its molecular formula C20H30O2 was deduced based on LREIMS and HRESIMS spectra which showed, respectively, molecular ion peak at m/z 302 [M+.] and a sodium adduct ion at m/z 325.2064 [M+Na]+ (C20H30NaO2, calculated value m/z 325.2143). The 13C-DEPTQ NMR spectrum of 6 showed carbon signals attributed to three methyl, nine methylene (including one sp2 at δC 116.4 and one sp3 carbinolic at δC 68.8), two methine (including one sp2 at δC 140.7), one carbonyl (conjugated carbonyl ketonic at δC 196.8) and five quaternary (including three sp2 corresponding to two olefinics at δC 154.8 and 142.8) carbon atoms. Thus, the expanded molecular formula (CH3)3(CH2)7(= CH2)(CH2OH)(= CH)(CH)(C = C)(C = O)(C)3 is in agreement with the molecular C20H30O2 (six degree of unsaturation), deducted by the LRIEMS e HRESIMS spectra and compatible with a tricyclic pimarane-type skeleton sustaining one conjugated carbonyl group and two double bonds.
1H-NMR spectrum of 6 showed three singlet signals corresponding to methyl groups linked to sp3 carbon atoms at δH 1.18 (3H-20, s), 0.92 (3H-18, s) and 0.88 (3H-19, s). The signals at δH 5.67 (H-15, dd, 17.5, 10.4 Hz), 5.23 (1H-16a, d, 10.4 Hz), and 5.06 (1H-16b, d, 17.5 Hz), which revealed respectively heteronuclear correlation via one bond in the HSQC spectrum with carbon signals at δC 140.7 (CH-15) and 116.4 (CH2-16), were used to characterize the presence of a terminal vinyl group; the signal at δH 3.41-4.44 (m)/δC 68.8 was attributed to an oxygenated methylene (CH2-17). The presence of hydrogens at δ H 2.51 (1H-12a, dd, 16.5, 2.2 Hz) and 2.34 (1H-12b, d, 16.5 Hz) were used to postulate the existence of hydrogens attached to a α-carbon of carbonyl group.
The signals at δC 196.8 (C-11), 152.8 (C-8), and 142.8 (C-9) indicated a α,β-unsaturated carbonyl group, which was confirmed and located by HMBC spectrum through heteronuclear long-range interactions between the 2H-12 and C-11, C-13, CH-15 and CH2-17; 3H-20 with both C-9 and C-10 (δC 47.3); and 2H-14 with C-8, C-9 and CH-15. The presence of a vinyl group (CH-15 and CH2-16) attached to C-13 was confirmed by HMBC correlations of 2H-16 with C-13, CH-15 and CH2-16. Additional heteronuclear long-range couplings observed in the HMBC spectrum are shown in Table 1.
For the complete and unambiguous 1H and 13C chemical shifts assignments (Table 1) were also used 2D HSQC and 1H-1H-COSY. The relative stereochemistry of 6 was determined through NOESY spectrum analysis in which strong NOEs between H-20 with H-19 and between H-17 with H-12 were observed (Fig. 2).
The seven known additional terpenoids, with four C-seco limonoids, nimbinene (2) (Kraus and Cramer, 1981Kraus, W., Cramer, R., 1981. Pentanortriterpenoide aus Azadirachta indica A. Juss (Meliaceae). Chem. Ber. 114, 2375-2381.), nimbinal (3) (Rojatkar et al., 1989Rojatkar, S.R., Bhat, V.S., Kulkarni, M.M., Joshi, V.S., Nagasampagi, B.A., 1989. Tetranortriterpenoids from Azadirachta indica. Phytochemistry 28, 203-205.), nimbandiol (4) (Kraus and Cramer, 1981Kraus, W., Cramer, R., 1981. Pentanortriterpenoide aus Azadirachta indica A. Juss (Meliaceae). Chem. Ber. 114, 2375-2381.), and salannin (5) (Henderson et al., 1964Henderson, R., McCrindle, R., Overton, K.H., 1964. Salannin. Tetrahedron Lett. 5, 3969-3974.), and three abiatane diterpenes, nimbidiol (7) (Majumder et al., 1987Majumder, P.L., Maiti, D.C., Kraus, W., Bokel, M., 1987. Nimbidiol, a modified diterpenoid of the root-bark of Azadirachta indica. Phytochemistry 26, 3021-3023.), ferruginol (8) (Lee et al., 2005Lee, S.-Y., Choi, D.-Y., Woo, E.-R., 2005. Inhibition of osteoclast differentiation by tanshinones from the root of Salvia miltiorrhiza bunge. Arch. Pharm. Res. 28, 909-913.), and 6,7-dehydroferruginol (9) (Katoh et al., 2007Katoh, T., Akagi, T., Noguchi, C., Kajimoto, T., Node, M., Tanaka, R., Nishizawa (née Iwamoto), M., Ohtsu, H., Suzuki, N., Saito, K., 2007. Synthesis of dl-standishinal and its related compounds for the studies on structure-activity relationship of inhibitory activity against aromatase. Bioorg. Med. Chem. 15, 2736-2748.) were characterized through the comparison of their NMR data with literature values.
In order to verify the biological potential of the samples, the inhibition of NO production, cytotoxic and antimycobacterial activities of compounds 1-5, 7-9 were evaluated and the results are shown in Table 2. Compound 6 was not tested as there was no enough mass.
Primarily, the inhibitory effects on nitric oxide (NO) production by LPS-stimulated macrophages were evaluated. The results showed that all the compounds studied act as potent inhibitors of NO (with IC50 values below 15 µg/ml), with compounds 1, 3, 5 and 7 showing more pronounced inhibitory activity.
To verify if the samples showed cytotoxicity, the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide] assay was performed with the use of commercial lactate dehydrogenase (LDH) kit. The results showed that compounds 1-4 caused low cytotoxicity, while the others were cytotoxic. Of particular note are the compounds 1 and 3 which, besides being strong anti-inflammatory agents, due to the fact that they can significantly inhibit NO production, they also have almost no cytotoxic effect.
The compounds were further evaluated for antimycobacterial activity against the strains of Mycobacterium tuberculosis H37Rv (virulent laboratory strain) and M299 (isolated hypervirulent Beijing). Compound 1 was shown to be promising for growth inhibition of the Mtb strain H37Rv, showing low MIC50 (48.7 µg/ml) and no cytotoxicity interference, exhibiting IC50 of 224.7 µg/ml. Compounds 5, 7-9, despite having a good response in inhibition of both virulent and super virulent strains, were highly cytotoxic according to the MTT assay.
-
Ethical disclosuresProtection of human and animal subjects. The authors declare that no experiments were performed on humans or animals for this study.Confidentiality of data. The authors declare that no patient data appear in this article.Right to privacy and informed consent. The authors declare that no patient data appear in this article.
Acknowledgment
The authors are grateful to Fundação de Amparo à Pesquisado Estado do Rio de Janeiro, to CNPq, and to CAPES - Finance Code 001.
References
- Akihisa, T., Nishimoto, Y., Ogihara, E., Matsumoto, M., Zhang, J., Abe, M., 2017. Nitric oxide production-inhibitory activity of limonoids from Azadirachta indica and Melia azedarach Chem. Biodivers. 14, e1600468.
- Ara, I., Siddiqui, B.S., Faizi, S., Siddiqui, S., 1989. Two new terpenoids from root bark of Azadirachta indica J. Nat. Prod. 52, 1209-1213.
- Ashfaq, U.A., Jalil, A., ul Qamar, M.T., 2016. Antiviral phytochemicals identification from Azadirachta indica leaves against HCV NS3 protease: an in silico approach. Nat. Prod. Res. 30, 1866-1869.
- Ebenso, I.E., 2004. Molluscicidal effects of neem (Azadirachta indica) extracts on edible tropical land snails. Pest Manag. Sci. 60, 178-182.
- Ezeigwe, O.C., Ononamadu, C.J., Enemchukwu, B.N., Umeoguaju, U.F., Okoro, J.C., 2015. Antidiabetic and antidiabetogenic properties of the aqueous extracts of Azadirachta indica leaves on alloxan induced diabetic wistar rats. Int. J. Biosci. 7, 116-126.
- Giglioti, R., Forim, M.R., Oliveira, H.N., Chagas, A.C.S., Ferrezini, J., Brito, L.G., Falcoski, T.O.R.S., Albuquerque, L.G., Oliveira, M.C.S., 2011. In vitro acaricidal activity of neem (Azadirachta indica) seed extracts with known azadirachtin concentrations against Rhipicephalus microplus. Vet. Parasitol. 181, 309-315.
- Henderson, R., McCrindle, R., Overton, K.H., 1964. Salannin. Tetrahedron Lett. 5, 3969-3974.
- Joy Sinha, D., Nandha, D.S., Jaiswal, K., Vasudeva, N., Prabha Tyagi, A., Pratap Singh, S.U., 2017. Antibacterial effect of Azadirachta indica (neem) or Curcuma longa (turmeric) against Enterococcus faecalis compared with that of 5% sodium hypochlorite or 2% chlorhexidine in vitro Bull. Tokyo Dent. Coll. 58, 103-109.
- Katoh, T., Akagi, T., Noguchi, C., Kajimoto, T., Node, M., Tanaka, R., Nishizawa (née Iwamoto), M., Ohtsu, H., Suzuki, N., Saito, K., 2007. Synthesis of dl-standishinal and its related compounds for the studies on structure-activity relationship of inhibitory activity against aromatase. Bioorg. Med. Chem. 15, 2736-2748.
- Kraus, W., Cramer, R., 1981. Pentanortriterpenoide aus Azadirachta indica A. Juss (Meliaceae). Chem. Ber. 114, 2375-2381.
- Kumar, V.S., Navaratnam, V., 2013. Neem (Azadirachta indica): prehistory to contemporary medicinal uses to humankind. Asian Pac. J. Trop. Biomed. 3, 505-514.
- Lee, S.-Y., Choi, D.-Y., Woo, E.-R., 2005. Inhibition of osteoclast differentiation by tanshinones from the root of Salvia miltiorrhiza bunge. Arch. Pharm. Res. 28, 909-913.
- Majumder, P.L., Maiti, D.C., Kraus, W., Bokel, M., 1987. Nimbidiol, a modified diterpenoid of the root-bark of Azadirachta indica Phytochemistry 26, 3021-3023.
- Mondal, D., Mondal, T., 2012. A review on efficacy of Azadirachta indica A. Juss based biopesticides: an Indian perspective. Res. J. Recent Sci. 1, 94-99.
- Moodley, S., Koorbanally, N.A., Moodley, T., Ramjugernath, D., Pillay, M., 2014. The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay is a rapid, cheap, screening test for the in vitro anti-tuberculous activity of chalcones. J. Microbiol. Methods 104, 72-78.
- Mordue (Luntz), A.J., Blackwell, A., 1993. Azadirachtin: an update. J. Insect Physiol. 39, 903-924.
- Mourao, S.A., Silva, J.C.T., Guedes, R.N.C., Venzon, M., Jham, G.N., Oliveira, C.L., Zanuncio, J.C., 2004. Selectivity of neem extracts (Azadirachta indica A Juss.) to the predatory mite Iphiseiodes zuluagai (Denmark & Muma) (Acari: Phytoseiidae). Neotrop. Entomol. 33, 613-617.
- Muzitano, M.F., Cruz, E.A., de Almeida, A.P., Da Silva, S.A., Kaiser, C.R., Guette, C., Rossi-Bergmann, B., Costa, S.S., 2006. Quercitrin: an antileishmanial flavonoid glycoside from Kalanchoe pinnata Planta Med. 72, 81-83.
- Osman Mohamed Ali, E., Shakil, N.A., Rana, V.S., Sarkar, D.J., Majumder, S., Kaushik, P., Singh, B.B., Kumar, J., 2017. Antifungal activity of nano emulsions of neem and citronella oils against phytopathogenic fungi, Rhizoctonia solani and Sclerotium rolfsii Ind. Crops Prod. 108, 379-387.
- Park, P.-H., Kim, H.S., Jin, X.Y., Jin, F., Hur, J., Ko, G., Sohn, D.H., 2009. KB-34, a newly synthesized chalcone derivative, inhibits lipopolysaccharide-stimulated nitric oxide production in RAW 264.7 macrophages via heme oxygenase-1 induction and blockade of activator protein-1. Eur. J. Pharmacol. 606, 215-224.
- Puri, H.S., 1999. Neem: The Dive Tree Azadirachta indica. Harwood Academic Publishers, Amsterdam.
- Ribeiro, S.C.M., Gomes, L.L., Amaral, E.P., Andrade, M.R.M., Almeida, F.M., Rezende, A.L., Lanes, V.R., Carvalho, E.C.Q., Suffys, P.N., Mokrousov, I., Lasunskaia, E.B., 2014. Mycobacterium tuberculosis strains of the modern sublineage of the Beijing family are more likely to display increased virulence than strains of the ancient sublineage. J. Clin. Microbiol. 52, 2615-2624.
- Rojatkar, S.R., Bhat, V.S., Kulkarni, M.M., Joshi, V.S., Nagasampagi, B.A., 1989. Tetranortriterpenoids from Azadirachta indica Phytochemistry 28, 203-205.
- Schumacher, M., Cerella, C., Reuter, S., Dicato, M., Diederich, M., 2011. Anti-inflammatory, pro-apoptotic, and anti-proliferative effects of a methanolic neem (Azadirachta indica) leaf extract are mediated via modulation of the nuclear factor-κB pathway. Genes Nutr. 6, 149-160.
- Ventura, T.L.B., Calixto, S.D., De Azevedo Abrahim-Vieira, B., De Souza, A.M.T., Mello, M.V.P., Rodrigues, C.R., De Mariz, E., Miranda, L.S., De Souza, R.O.M.A., Leal, I.C.R., Lasunskaia, E.B., Muzitano, M.F., 2015. Antimycobacterial and anti-inflammatory activities of substituted chalcones focusing on an anti-tuberculosis dual treatment approach. Molecules 20, 8072-8093.
- Wandscheer, C.B., Duque, J.E., da Silva, M.A.N., Fukuyama, Y., Wohlke, J.L., Adelmann, J., Fontana, J.D., 2004. Larvicidal action of ethanolic extracts from fruit endocarps of Melia azedarach and Azadirachta indica against the dengue mosquito Aedes aegypti Toxicon 44, 829-835.
Publication Dates
-
Publication in this collection
Jan-Feb 2019
History
-
Received
8 Oct 2018 -
Accepted
6 Dec 2018 -
Published
21 Dec 2018