Abstract
In this study, the adsorption/desorption characteristics of quercetin, luteolin and apigenin from Flos populi extract (Populus tomentosa Carrière, Salicaceae) on twelve macroporous resins (NKA-9, HPD-600, HPD-826, HPD-750, HPD-400, DM-130, AB-8, SP-825, X-5, D-101, HPD-100, HPD-200) were evaluated. Both high adsorption and desorption capacities of quercetin, luteolin and apigenin from Flos populi extract on SP-825 resin indicated that SP-825 resin was appropriate and its data were well fitted to the Langmuir and Freundlich isotherms. To get the optimal separation process, the influences of factors such as flow rates, loading sample volumes, concentrations of desorption solution were further investigated. Column packed with SP-825 resin was used to perform dynamic adsorption and desorption experiments. After one round of treatment, the contents of quercetin, luteolin and apigenin in the final products were 3.75-fold, 3.67-fold and 3.54-fold increased with recovery yields of 87.25, 85.19 and 82.22%, respectively. The results showed that the preparative enrichment of quercetin, luteolin and apigenin was available via adsorption and desorption on SP-825 resin. This method is a promising basis for the large-scale preparation of quercetin, luteolin and apigenin from Flos populi.
Keywords
Quercetin; Luteolin; Apigenin; Flos populi; Enrichment
Introduction
Flos populi, prepared from the male inflorescence of Populus tomentosa Carrière, Salicaceae, is used as a traditional medicine in China (Chinese Pharmacopoeia, 2015Chinese Pharmacopoeia, 2015. Pharmacopoeia of People's Republic of China. Chemical Industry Press, Beijing.). It is indigenous to China and is widely distributed due to its tolerance to a wide range of climatic conditions. Research has indicated that Flos populi contains flavonoids, cardiac glycosides and phenolic compounds (Zhao et al., 2014Zhao, Y., Tang, G.S., Cai, E.B., Liu, S.L., Zhang, L.X., Wang, S.J., 2014. Hypolipidaemic and antioxidant properties of ethanol extract from Flos populi. Nat. Prod. Res. 28, 1467-1470.); it is used in the treatment of a variety of inflammatory diseases (Xu et al., 2014Xu, Q., Wang, Y., Guo, S., Shen, Z., Wang, Y., Yang, L., 2014. Anti-inflammatory and analgesic activity of aqueous extract of Flos populi. J. Ethnopharmacol. 152, 540-545.) and as an antidiarrheal agent in East Asian countries (Xu et al., 2013Xu, Q., Shen, Z., Wang, Y., Guo, S., Li, F., Wang, Y., Zhou, C., 2013. Anti-diarrhoeal and anti-microbial activity of Flos populi (male inflorescence of Populus tomentosa Carrière) aqueous extracts. J. Ethnopharmacol. 148, 640-646.). The medicinal use of Flos populi has attracted scientific interest in the screening of its bioactive constituents for potential pharmacological utilization. Several bioactive compounds have been identified in Flos populi, such as quercetin, luteolin and apigenin (Si et al., 2010Si, C.L., Wu, L., Ni, Y.H., 2010. Isolation and structure elucidation of low molecular weight extractives from wood of triploid Populus tomentosa Carr. In: Research Progress in Paper Industry and Biorefinery (4thIsetpp), vols. 1–3, pp. 197–200.). The pharmacological efficacy and application potential of these compounds were primarily due to their antitumor, anti-inflammation, antioxidant, anti-cancer, anti-diabetic, antiviral and anti-allergic activities (Shao et al., 2013Shao, H.J., Jing, K., Mahmoud, E., Huang, H.H., Fang, X.J., Yu, C.R., 2013. Apigenin sensitizes colon cancer cells to antitumor activity of ABT-263. Mol. Cancer Ther. 12, 2640-2650.; Feng et al., 2015Feng, W.M., Guo, H.H., Xue, T., Wang, X., Tang, C.W., Ying, B., Gong, H., Cui, G., 2015. Anti-inflammation and anti-fibrosis with PEGylated, apigenin loaded PLGA nanoparticles in chronic pancreatitis disease. RSC Adv. 5, 83628-83635.; Wu et al., 2015Wu, W., Li, R., Li, X., He, J., Jiang, S., Liu, S., Yang, J., 2015. Quercetin as an antiviral agent inhibits Influenza A virus (IAV) entry. Viruses, 8.; Chiow et al., 2016Chiow, K.H., Phoon, M.C., Putti, T., Tan, B.K.H., Chow, V.T., 2016. Evaluation of antiviral activities of Houttuynia cordata Thunb. extract, quercetin, quercetrin and cinanserin on murine coronavirus and dengue virus infection. Asian Pac. J. Trop. Med. 9, 1-7.; Xu et al., 2016Xu, H.L., Yang, T., Liu, X.H., Tian, Y., Chen, X.L., Yuan, R., Su, S.N., Lin, X.K., Du, G.H., 2016. Luteolin synergizes the antitumor effects of 5-fluorouracil against human hepatocellular carcinoma cells through apoptosis induction and metabolism. Life Sci. 144, 138-147.). However, owing to the low content of these compounds in Flos populi, poor activities were often observed. As such, it is necessary to develop an effective purification strategy to obtain quercetin, luteolin and apigenin in high purity for potential pharmaceutical applications. Currently, methods for the purification of bioactive compounds - including ion exchange (Smith and Evans, 1995Smith, N.W., Evans, M.B., 1995. Efficient analysis of neutral and highly polar pharmaceutical compounds using reversed-phase and ion-exchange electrochromatography. Chromatographia 41, 197-203.), liquid-liquid extraction (Amelio et al., 2016Amelio, A., Loise, L., Azhandeh, R., Darvishmanesh, S., Calabro, V., Degreve, J., Luis, P., Van der Bruggen, B., 2016. Purification of biodiesel using a membrane contactor: liquid-liquid extraction. Fuel Process. Technol. 142, 352-360.; Mun et al., 2016Mun, L.W., Rafiqul, I.S.M., Sakinah, A.M.M., Zularisam, A.W., 2016. Purification of bioxylitol by liquid-liquid extraction from enzymatic reaction mixture. Sep. Sci. Technol. 51, 2369-2377.), and high-speed counter-current chromatography (Liu et al., 2010aLiu, W., Zhang, S., Zu, Y.-G., Fu, Y.-J., Ma, W., Zhang, D.-Y., Kong, Y., Li, X.-J., 2010. Preliminary enrichment and separation of genistein and apigenin from extracts of pigeon pea roots by macroporous resins. Bioresource Technol. 101, 4667-4675.; Liu et al., 2010bLiu, Y.F., Liu, J.X., Chen, X.F., Liu, Y.W., Di, D.L., 2010. Preparative separation and purification of lycopene from tomato skins extracts by macroporous adsorption resins. Food Chem. 123, 1027-1034.) have been developed. However, these established methods possess several disadvantages, such as low recovery, low capacity, solvent waste, high cost, time-consuming processes, the requirement for special instruments and environmental pollution. Therefore, these methods are not suitable for large-scale industrial production (Chang et al., 2012Chang, X.L., Wang, D., Chen, B.Y., Feng, Y.M., Wen, S.H., Zhan, P.Y., 2012. Adsorption and desorption properties of macroporous resins for anthocyanins from the calyx extract of roselle (Hibiscus sabdariffa L.). J. Agric. Food Chem. 60, 2368-2376.; Xi et al., 2015Xi, L., Mu, T., Sun, H., 2015. Preparative purification of polyphenols from sweet potato (Ipomoea batatas L.) leaves by AB-8 macroporous resins. Food Chem. 172, 166-174.). Presently, quercetin, luteolin and apigenin are only obtained in small quantities and at high cost. Due to its significant pharmacological potential, the application of a low-cost technology to enrich quercetin, luteolin and apigenin from Flos populi is a rational strategy. Macroporous resins have a variety of characteristics, including their polarity, material, particle size, large surface area, high stability and pore diameter, and are relatively inexpensive, are easy to preprocess and retrieve and are suitable for large-scale production (Li et al., 2013Li, Y., Liu, J., Cao, R., Deng, S., Lu, X., 2013. Adsorption of myricetrin, puerarin, naringin, rutin, and neohesperidin dihydrochalcone flavonoids on macroporous resins. J. Chem. Eng. Data 58, 2527-2537.; Yang et al., 2016Yang, Q., Zhao, M., Lin, L., 2016. Adsorption and desorption characteristics of adlay bran free phenolics on macroporous resins. Food Chem. 194, 900-907.). Therefore, macroporous resins have been widely used in the separation and purification of many secondary metabolites such as flavonoids, glycosides, saponins, alkaloids, and lignans (Liu et al., 2013Liu, Z., Wang, J.Y., Gao, W.Y., Man, S.L., Wang, Y., Liu, C.X., 2013. Preparative separation and purification of steroidal saponins in Paris polyphylla var. yunnanensis by macroporous adsorption resins. Pharm. Biol. 51, 899-905.; Torres et al., 2014Torres, S., Cerutti, S., Raba, J., Pacheco, P., Silva, M.F., 2014. Preconcentration of seleno-amino acids on a XAD resin and determination in regional olive oils by SPE UPLC-ESI-MS/MS. Food Chem. 159, 407-413.). However, there is no literature reported regarding the use of macroporous resins to enrich and purify quercetin, luteolin and apigenin from Flos populi extracts.
In our previous study, we had optimized the ultrasound-assisted extraction process of quercetin, luteolin and apigenin from Flos populi (Wang et al., 2018Wang, B., Goldsmith, C.D., Zhao, J., Zhao, S., Sheng, Z., Yu, W., 2018. Optimization of ultrasound-assisted extraction of quercetin, luteolin, apigenin, pinocembrin and chrysin from Flos populi by Plackett-Burman design combined with Taguchi method. Chiang Mai J. Sci. 45, 427-439.). Consequently, this work aimed to investigate the adsorption properties of twelve macroporous resins with different polarities for the purification of quercetin, luteolin and apigenin from Flos populi. The adsorption mechanism was optimized by analyzing the adsorption isotherms with Langmuir and Freundlich equations at different temperatures, and the purification parameters were elaborated by static and dynamic adsorption and desorption tests. This study provides a technical method to further utilize this potentially valuable resource.
Materials and methods
Plant materials and chemicals
Flos populi (male inflorescence of Populus tomentosa Carrière, Salicaceae,) was purchased from a medicinal herbs store (Anguo, Hebei Province, China) and authenticated by Associate Professor Junkai Wu (Heilongjiang University of Traditional Chinese Medicine, Harbin, China). The voucher specimen (accession no. 1009015ch) was deposited at the Herbarium in the College of Veterinary Medicine, Northeast Agricultural University.
Standards (quercetin, luteolin and apigenin) were obtained from the Chinese Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). Their purities meet with the standard of the reference material (>98%, w/w). HCl, NaOH, ethanol (analytical grade) and methanol (HPLC grade) were acquired from the Hangzhou Reagent Company (Hangzhou, PR China). Distilled water was purified with a Milli-Q academic water purification system (Millipore, Bedford, MA, USA).
HPD 100, HPD 200, HPD 400, HPD 600, HPD 750, HPD 826 resins were purchased from Cangzhou Bon Adsorber Technology Co., Ltd. (Cangzhou, Hebei Province, China), NKA-9, D-101, X-5 and AB-8 were products of NankaiHecheng S & T Co., Ltd. (Tianjin, China). DM 130 resin was product of Shandong Lukang Co., Ltd. (Shandong, China). SP-825 resin was product of Mitsubishi Chemical Corporation of Japan (Japan). The chemistry and physical characteristics of the twelve different macroporous resins used in this study are summarized in Table 1. To remove undesired substances trapped inside the resin pores during resin manufacturing, such as monomers and porogenic agents, the resins were subjected to pretreatment. Resins were soaked in ethanol overnight, washed with distilled water to completely remove the ethanol, and then treated with two bed volumes (BV) of 5% (v/v) HCl solution and 5% (m/m) NaOH solution, respectively. Finally, the resins were washed in distilled water until the washing fluid is neutralized.
Ultrasonic assisted solvent extraction
Flos populi was ultrasonic extracted twice with 30% ethanol at 60 ºC (liquid-solid ratio, 10 ml/g) for 15 min using a KQ-2200DB ultrasonic bath (Kunshan Ultrasound Instrument Co., Ltd., Jiangsu, China) with frequency 40 kHz and 100 W. The extract was collected and filtered through a 10 µm membrane (Beijing Honghulian Chemical Co. Ltd.), and the filtrate was evaporated to dryness in a rotary evaporator at 60 ºC, and later lyophilized in a freeze-dryer system (Labconco FreeZone 6, USA). The crude extract was dissolved to an appropriate concentration for analysis.
HPLC analysis of quercetin, luteolin and apigenin
Quantification of quercetin, luteolin and apigenin was determined by RP-HPLC. A Shimadzu HPLC system equipped with an LC-10ATVP binary pump, SPD-10AVP detector, CTO-10ASVP column oven and N300 workstation was used for quantitative analysis. A C18 column (150 mm × 4.6 mm i.d., 5 µm, Diamonsil, Dikma Technologies, China) was used for separation of the compounds. Elution was performed with mobile phase A (0.1% formic acid aqueous solution) and mobile phase B (methanol). The flow rate was 1 ml min-1 with a 50 min gradient as follows: 0-23 min, 65% B; 23-24 min, 65-90% B; 24-34 min, 90% B; 34-35 min, and 90%-65% B, followed by 15 min of re-equilibration of the column before the next run. The column temperature was maintained at 30 ºC. The detection wavelength was 254 nm. All solutions were filtered through a 0.45 µm membrane (Lab Instrument Co. Ltd.) before injection. Quantification was performed using an external standard method using a five-point calibration curve. The chromatographic peaks of quercetin, luteolin and apigenin in the sample were identified by their retention times.
Static adsorption and desorption tests for screening of resins
Static adsorption and desorption tests were performed as follows: pretreated resins (1 g) were placed into 250 ml conical flasks with stoppers, and 50 ml of sample aqueous solution with an initial quercetin concentration of 70 µg ml-1 was added to each flask. The flasks were shaken at 120 rpm for 12 h at 25 ºC. After adsorption, the resins were filtered and washed with 50 ml of distilled water, and 50 ml of 70% ethanol (v/v) solution was added for desorption. The contents of quercetin, luteolin and apigenin in the desorbed solutions after adsorption and desorption were tested using HPLC. According to their adsorption capacities, desorption capacities and desorption ratios, the candidate resins were selected. The adsorption capacity, desorption capacity and desorption ratio of the resins were calculated according to the following equations:
where Qe represents the adsorption capacity at adsorption equilibrium (mg/g dry resin); Qd represents the desorption capacity after adsorption equilibrium (mg/g dry resin); D represents the desorption ratio (%); C0 and Ce are the initial and equilibrium concentrations of quercetin, luteolin and apigenin in the solutions (mg ml-1), respectively; V0 is the volume of the initial sample solution (ml); W is the weight of the tested dry resin (g); Vd is the volume of the desorption solution (ml); and Cd represents the concentration of quercetin, luteolin and apigenin in the desorption solution (mg ml-1).
The effect of the initial pH on the adsorption of quercetin, luteolin and apigenin in the crude extract on SP-825 resin was studied at pH 3-7 (pH 3, 5 and 7). Pre-weighed amounts of SP-825 resin (equal to 1 g of dry resin) were added to 50 ml crude extract solutions (quercetin 70 µg ml-1) with shaking (120 rpm) for 6 h at 25 ºC. The sample pH was adjusted to the desired value with hydrochloric acid or an ammonia solution.
Adsorption kinetics
To obtain the adsorption kinetics curve of quercetin, luteolin and apigenin on SP-825 resin, the pretreated hydrated resins (2 g, dry weight) and 100 ml sample solutions with an initial quercetin concentration of 70 µg ml-1 were added to 250 ml Erlenmeyer flasks with a stopper. The flasks were continually shaken in a thermostatic oscillator at 25 ºC at a shaking speed of 120 rpm for 24 h. After withdrawing 0.5 ml of each extract solution at the time points of 0.5, 1, 1.5, 2, 2.5, 3, 4, 5, 6, 7, 8, 9, 10 and 24 h, the quercetin, luteolin and apigenin content of each sample were determined.
Adsorption isotherms
To further explore the adsorption properties of quercetin, luteolin and apigenin on the SP-825 resin, the equilibrium adsorption isotherms were studied by adding 50 ml of aqueous solution with different initial concentrations (23-115 µg ml-1, 5.5-27.5 µg ml-1 and 7.7-38.6 µg ml-1 for quercetin, luteolin and apigenin, respectively) to the SP-825 resin at 25, 35 and 45 ºC. After adsorption, the contents of quercetin, luteolin and apigenin in the samples were measured, and their degrees of fitness to the Langmuir and Freundlich equations were evaluated. The Langmuir model can be described as the following mathematical formula:
where Ce and Qe is the same as in Eq. (1); KL (mg ml-1) is the Langmuir constant; and Qm (mg/g resin) is the maximum adsorption capacity.
The Freundlich model can be expressed by the following mathematical formula:
where KF is the Freundlich constant, an indicator of adsorption capacity, and 1/n is an empirical constant related to the magnitude of the adsorption driving force.
Dynamic adsorption/desorption tests
Dynamic adsorption/desorption tests were conducted in a glass column (inner diameter 15 mm, length 30 mm) packed with SP-825 resin (5 g, dry weight). The bed volume (BV) was approximately 28 ml. Sample solution containing 70 µg ml-1 quercetin was carefully loaded onto the resin column at a flow rate of 2 BV/h. The temperature was maintained at 25 ºC. The effect of flow rates on the adsorption capacities and dynamic breakthrough tests were first performed. To investigate the effect of different flow rates and loading sample volumes on dynamic adsorption, flow rates varied from 1 BV/h to 4 BV/h, and loading volumes of sample solutions varied from 5 BV to 35 BV.
After the SP-825 resin was saturated with quercetin, luteolin and apigenin, the resin column was washed with distilled water and 10, 20, 30, 40, 50, 60, 70, and 80% ethanol with in the isocratic mode at a flow rate of 2 BV/h. The elution volume of each concentration was kept constant at 5 BV. The contents of quercetin, luteolin and apigenin in each eluent were detected by HPLC and concentrated to dryness under vacuum. The elution volume of each ethanol concentration was adjusted with monitoring by HPLC.
Laboratory preparative-scale separation
Flos populi extract (150 g) was dissolved in distilled water. The sample solution (quercetin 70 µg ml-1, pH 5.0) was applied to a glass column (inner diameter 7.5 cm, length 100 cm) packed with SP-825 resin (500 g, dry weight). The bed volume (BV) was approximately 2.8 ml. The column was washed with 5 BV of distilled water, and 5 BV of 20% ethanol was used to remove highly polar impurities. Quercetin, luteolin and apigenin were eluted from the column using 5 BV of 50% ethanol. The flow rate of each gradient elution was set at 2 BV/h, and the 50% (v/v) ethanol desorption solution was collected, concentrated and dried.
Results and discussion
Adsorption and desorption capacities, desorption ratio of the resins
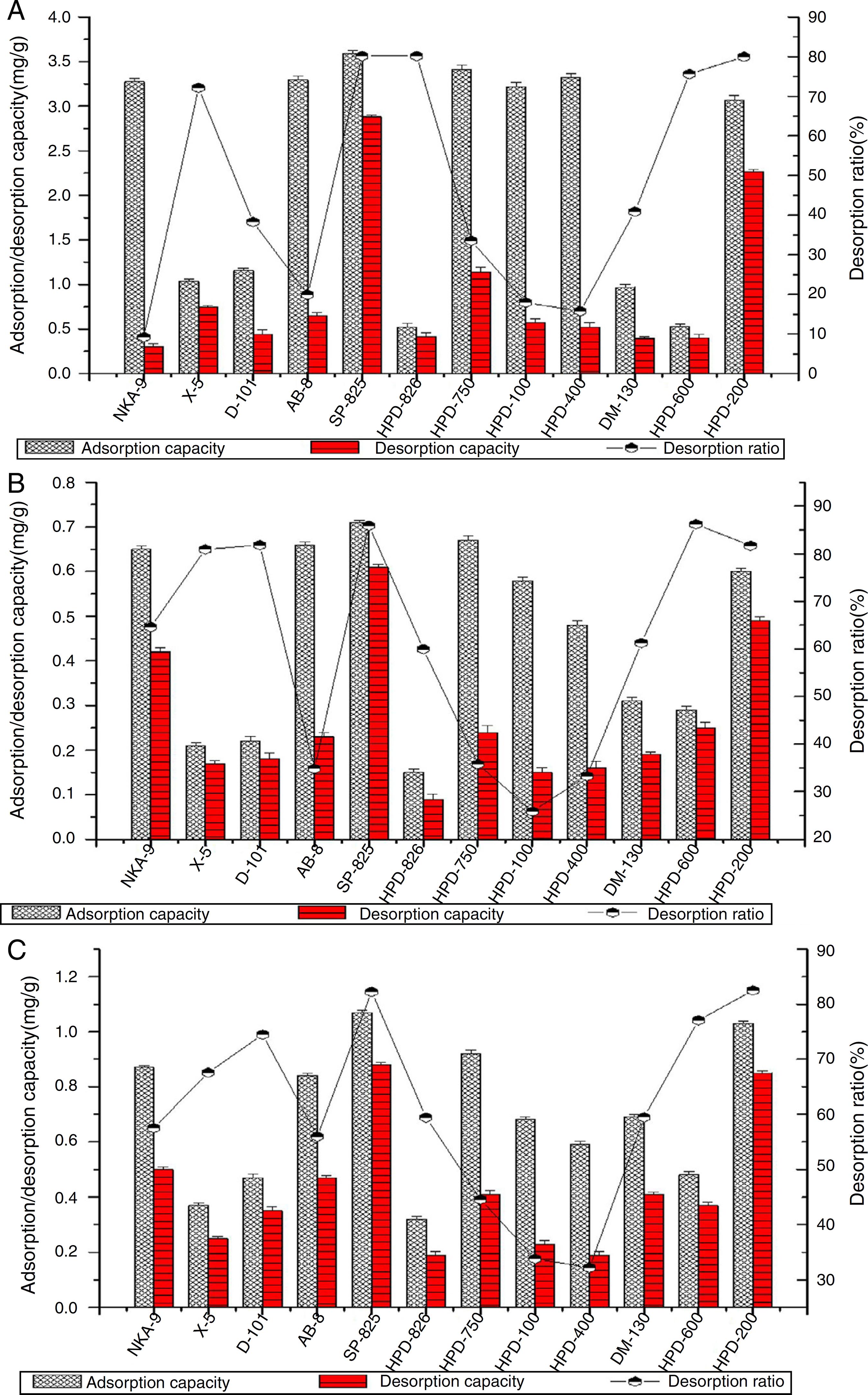
The equations of regression for quercetin, luteolin and apigenin were Y = 6 × 107X - 328010 (R2 = 0.9969), Y = 6 × 107X + 32740 (R2 = 0.9991) and Y = 5 × 107X + 62543 (R2 = 0.9990), respectively, where Y is the peak area and X is the concentration (µg ml-1). The contents of quercetin, luteolin and apigenin in the Flos populi extract were 13.40 mg/g, 3.20 mg/g and 4.50 mg/g, respectively. To select the appropriate resin for enriching quercetin, luteolin and apigenin from Flos populi extract, twelve macroporous resins ranging from non-polar to polar were tested and their adsorption/desorption capacities and desorption ratios are shown in Fig. 1. Compared to other resins, NKA-9, AB-8, SP-825, HPD-750, HPD-100, HPD-400 and HPD-200 had much higher adsorption capacities (3.07-3.59 mg/g for quercetin, 0.48-0.71 mg/g for luteolin and 0.59-1.07 mg/g for apigenin). NKA-9, AB-8, HPD-750, HPD-100 and HPD-400 did not show good desorption capacities (0.30-1.14 mg/g for quercetin, 0.15-0.42 mg/g for luteolin, 0.19-0.50 mg/g for apigenin). Only SP-825 and HPD-200 resins exhibited desorption characteristics (2.88-2.26 mg/g for quercetin, 0.49-0.61 mg/g for quercetin, 0.85-0.88 mg/g for apigenin). This correlates with the physical features (polarity, surface area, average pore diameter of the resins, etc.) and chemical capability (the synergistic effect of hydrogen bond interactions) (Sandhu and Gu, 2013Sandhu, A.K., Gu, L., 2013. Adsorption/desorption characteristics and separation of anthocyanins from muscadine (Vitis rotundifolia) juice pomace by use of macroporous adsorbent resins. J. Agric. Food Chem. 61, 1441-1448.).
Adsorption, desorption capacities and desorption ratio of quercetin (A), luteolin (B) and apigenin (C) on different resins.
X-5 and D-101 resins exhibited low adsorption capacities due to their lower surface areas. The middle-polar HPD-826 and DM-130 resins and the polar HPD-600 resin also exhibited poor adsorption capacities not only because of their lower surface areas, but also because of their different polarity with the weak-polar compounds quercetin, luteolin and apigenin. The NKA-9, AB-8, HPD-750, HPD-100 and HPD-400 resins also had good adsorption capability. However, they possessed a strong affinity for solute so that the desorption capacities of quercetin, luteolin and apigenin on these resin was actually not notable. The weak-polar SP-825 and non-polar HPD-200 resins exhibited better adsorption and desorption capacities due to their higher surface areas and their similar polarity with the weak-polar compounds quercetin, luteolin and apigenin. In addition, the synergistic effect of hydrogen bond interactions may play an important role in the adsorption of the two resins. The adsorption capacities of quercetin, luteolin and apigenin on SP-825 resins were 3.59, 0.71 and 1.07 mg/g dry resin, with desorption ratios of 80.15%, 85.92% and 82.24%, respectively. The adsorption capacities of quercetin, luteolin and apigenin on HPD-200 resins were 3.07, 0.60 and 1.03 mg/g dry resin, with desorption ratios of 96.54, 81.67 and 82.52%, respectively. Thus, adsorption kinetics experiments were carried out on SP-825 and HPD-200 resins.
Adsorption kinetics
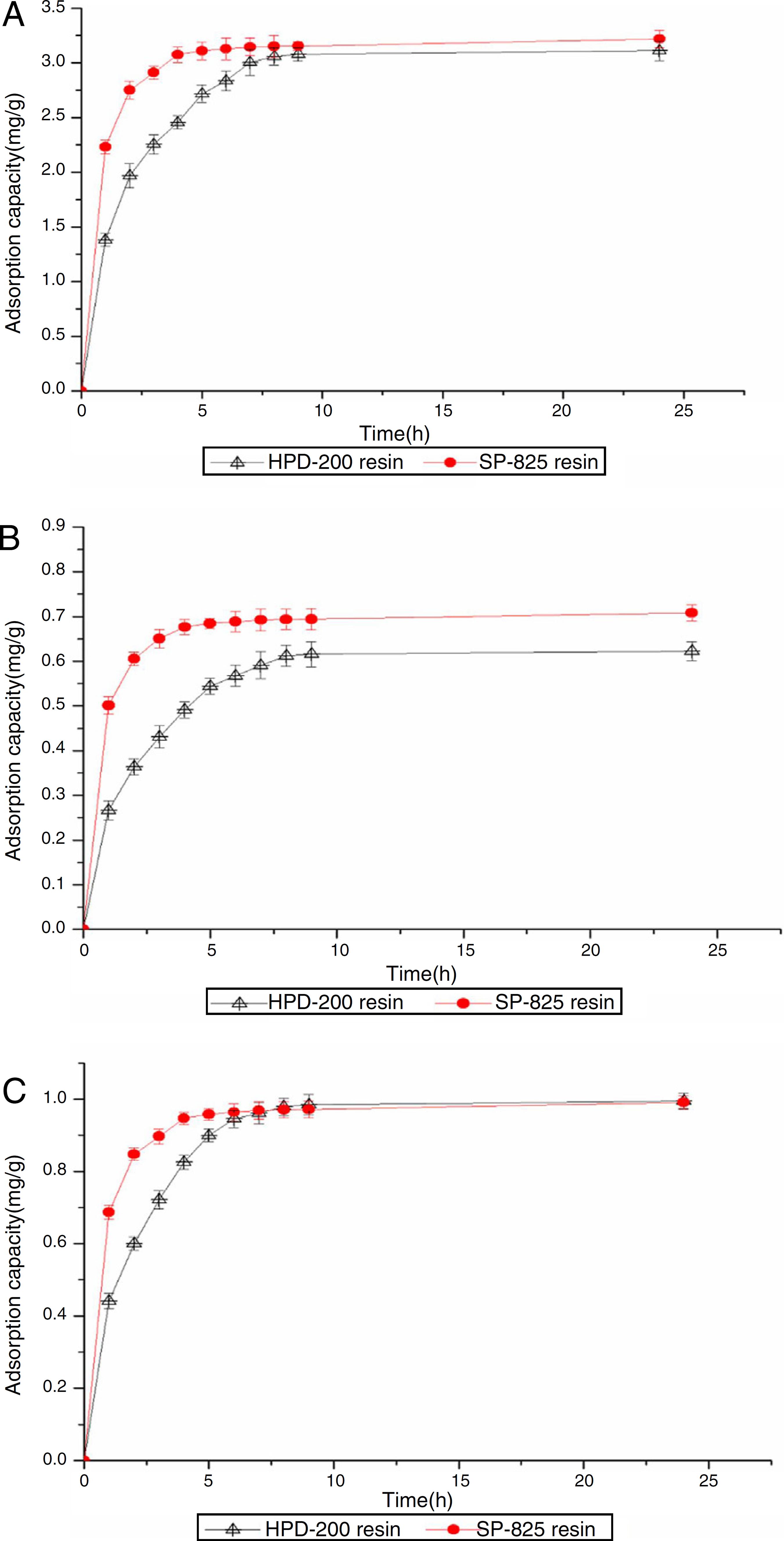
Adsorption equilibrium time was obtained via static adsorption kinetics for quercetin, luteolin and apigenin on HPD-200 and SP-825 resins at 25 ºC. As illustrated in Fig. 2, the equilibrium time for quercetin, luteolin and apigenin was 5 h on SP-825, and 8 h on HPD-200 resin. Moreover, the adsorption capacities of quercetin and luteolin on SP-825 resin were slightly higher than the HPD-200 resin. Comparing the two resins for their adsorption/desorption capacities and desorption ratios, the SP-825 resin possessed many advantages over the HPD-200 resin for quercetin, luteolin and apigenin. The adsorption equilibrium for the three compounds was observed at approximately 5 h on the SP-825 resin. Therefore, 5 h was sufficient to successfully reach adsorption equilibrium over the entire system, and the SP-825 resin was selected as a suitable resin for the enrichment of quercetin, luteolin and apigenin in the following experiments.
Adsorption kinetics curves of quercetin (A), luteolin (B) and apigenin (C) on HPD-200 resin and SP-825 resin at 25 ºC.
Effect of sample solution pH on adsorption capacity
The initial pH of an adsorption solution is an important parameter that can influence adsorption capacity (Zhang et al., 2008Zhang, B., Yang, R.Y., Zhao, Y., Liu, C.Z., 2008. Separation of chlorogenic acid from honeysuckle crude extracts by macroporous resins. J. Chromatogr. B 867, 253-258.). The pH determines the extent of ionization of quercetin, luteolin and apigenin molecules, thereby affecting their adsorption affinity. As shown in Table 2, for the SP-825 resin, the adsorption capacity increased first, reached its peak at pH 5.0, and then decreased with further increasing pH. These results indicated that hydrogen bonding may play an important role in the adsorption process on the SP-825 resin. At a higher pH, hydrogen bonding interactions between flavonoids and the macroporous resin were reduced because the phenolic hydroxyl groups in flavonoids dissociated to H+ and the corresponding anions, resulting in a lower adsorption capacity. Therefore, the pH of the sample solution was adjusted to 5.0 for the further tests.
Effect of sample solution pH value on the adsorption capacities of quercetin, luteolin and apigenin on SP-825 resin.
Adsorption isotherms
The Langmuir and Freundlich equations are frequently used to describe adsorption isotherms, which are relative simple and reasonably accurate (Lin et al., 2012Lin, L., Zhao, H., Dong, Y., Yang, B., Zhao, M., 2012. Macroporous resin purification behavior of phenolics and rosmarinic acid from Rabdosia serra (MAXIM.) HARA leaf. Food Chem. 130, 417-424.). As seen in Fig. 3, the adsorption capacities increased with increasing equilibrium concentration and reached a saturation plateau when the initial concentrations of quercetin, luteolin and apigenin were 70, 16.5 and 23.2 µg ml-1. Thus, the initial quercetin, luteolin and apigenin concentrations of 70, 16.5 and 23.2 µg ml-1 in the sample solutions were used in the following tests.
Adsorption isotherms for quercetin (A), luteolin (B) and apigenin (C) on SP-825 resin at 25, 35 and 45 ºC.
The Langmuir equation well describes the adsorption behavior of a monomolecular layer, whereas the Freundlich equation describes the equilibrium conditions on heterogeneous surfaces (Liu et al., 2016Liu, B., Dong, B., Yuan, X., Kuang, Q., Zhao, Q., Yang, M., Liu, J., Zhao, B., 2016. Enrichment and separation of chlorogenic acid from the extract of Eupatorium adenophorum Spreng by macroporous resin. J. Chromatogr. B 1008, 58-64.). The Langmuir and Freundlich parameters at different temperatures (25, 35 and 45 ºC) are listed in Table 3. This table lists the two isotherm equations at different temperatures and two parameters: Qm (obtained from the Langmuir isotherm) and 1/n (obtained from the Freundlich isotherm). The correlation coefficients of Langmuir equations (0.9915-0.9978) and Freundlich equations (0.9698-0.9962) for quercetin, luteolin and apigenin on SP-825 resin were rather high, which showed that the two models were suitable to describe the tested adsorption system in the concentration ranges studied. In the Freundlich equation, adsorption occurs easily when 1/n is between 0.1 and 0.5, and does not occur easily if 1/n is above 1 (Liu et al., 2010aLiu, W., Zhang, S., Zu, Y.-G., Fu, Y.-J., Ma, W., Zhang, D.-Y., Kong, Y., Li, X.-J., 2010. Preliminary enrichment and separation of genistein and apigenin from extracts of pigeon pea roots by macroporous resins. Bioresource Technol. 101, 4667-4675.). In Table 3, 1/n ranged between 0.1 and 0.5, indicating that the adsorption of flavonoids on the SP-825 resin occurred easily. Therefore, the SP-825 resin was appropriate for enriching and separating quercetin, luteolin and apigenin.
Langmuir and Freundlich adsorption parameters of quercetin, luteolin and apigenin on SP-825 resin at different temperatures.
As seen from Fig. 3, at the same concentration, the adsorption capacities decreased as temperature increased from 25 to 45 ºC, implying that the adsorption process was a thermopositive process. Similar results were obtained for the adsorption and desorption of other flavonoids using a macroporous resin (Du et al., 2012Du, Z., Wang, K., Tao, Y., Chen, L., Qiu, F., 2012. Purification of baicalin and wogonoside from Scutellaria baicalensis extracts by macroporous resin adsorption chromatography. J. Chromatogr. B 908, 143-149.). Therefore, 25 ºC was selected as the adsorption temperature in the following experiments.
Effect of sample concentration on adsorption/desorption capacities
The initial concentration of the sample solution of the Flos populi extract has an important effect on the affinity of quercetin, luteolin and apigenin to the SP-825 resin. The effect of the initial sample solution of the Flos populi extract on static adsorption is shown in Fig. 4.
Effects of initial concentration of sample solution on adsorption capacities and adsorption ratios of quercetin (A), luteolin (B) and apigenin (C) on SP-825 resin.
The adsorption capacity of the resin increases with the concentration of the sample solution, but the adsorption ratio decreases. The adsorption capacities increased directly with increasing concentration and reached a saturation plateau when the initial concentration of Flos populi extract was 45 mg ml-1. However, the adsorption ratio decreased at higher concentrations. When the concentration of the sample solution was low, the adsorption capacity increased because the number of active sites related to flavonoids increased. When the concentration of the sample solution of Flos populi extract increased, more impurities were adsorbed on the SP-825 resin, resulting in competition for active sites between flavonoids and impurities, which led to a slight drop in the adsorption ratio.
Dynamic breakthrough curve
In general, solutes will leak from the resin due to a decrease in adsorption affinity when the break point occurs (Zhao et al., 2015Zhao, P., Qi, C., Wang, G., Dai, X., Hou, X., 2015. Enrichment and purification of total flavonoids from Cortex juglandis Mandshuricae extracts and their suppressive effect on carbon tetrachloride-induced hepatic injury in mice. J. Chromatogr. B 1007, 8-17.). Therefore, it is necessary to establish breakthrough curves to determine the quantity of resin and the feed volume of the sample solution. As shown in Fig. 5, luteolin and apigenin in solution were almost absorbed by the resin before 10 BV, and then the concentrations of luteolin and apigenin in the breakthrough solution increased rapidly until a steady plateau at 27 BV. In contrast, with luteolin and apigenin, the steady plateau (30 BV) of quercetin occurred much later. Generally, breakthrough points are defined as 10% of the ratio of the concentration in the effluent to the original concentration (Sun et al., 2015Sun, P.C., Liu, Y., Yi, Y.T., Li, H.J., Fan, P., Xia, C.H., 2015. Preliminary enrichment and separation of chlorogenic acid from Helianthus tuberosus L. leaves extract by macroporous resins. Food Chem. 168, 55-62.). However, the breakthrough points (10%) were not the same for the three compounds, due to the differences in retention time. Luteolin and apigenin were leaked much earlier than quercetin (Fig. 5). Quercetin could not reach adsorption saturation if the feed volume of the sample solution was selected in accordance with luteolin and apigenin. Hence, considering all three compounds, the feed volume of the sample solution on SP-825 resin was determined to be 10 BV.
The effect of flow rate on dynamic adsorption capacities was studied with a constant loading volume of 10 BV. As shown in Table 4, an increase in flow rate had a negative effect on adsorption capacity. The adsorption capacities decreased little as the flow rate increased from 1 to 2 BV/h, but decreased greatly as the flow rate increased from 2 to 4 BV/h. A fast flow rate may result in insufficient time for quercetin, luteolin and apigenin to contact the active sites of the SP-825 resin surface. Therefore, considering this efficiency, the flow rate for loading the sample was maintained at a constant 2 BV/h.
Effect of flow rate on the adsorption capacities of quercetin, luteolin and apigenin on SP-825 resin.
Dynamic desorption of the SP-825 resin
Different concentrations of ethanol solution were used for the dynamic desorption of quercetin, luteolin and apigenin at a flow rate of 2 BV/h. Fig. 6 shows the profile of the desorption of quercetin, luteolin and apigenin with different concentrations of ethanol solution, with the same volume of 5 BV, when the sample loading was 10 BV. At 20% ethanol, luteolin and apigenin were hardly desorbed, but a small amount of quercetin was detected. When the ethanol concentration was over 20%, the desorption ability increased and reached a peak value at 50% ethanol. Thus, 50% ethanol was deemed optimal to desorb quercetin, luteolin and apigenin. When the ethanol concentration was 60%, 70% and 80%, more impurities were desorbed. Hence, the final separation and purification conditions for the three compounds were determined as follows:
Dynamic desorption curves of quercetin, luteolin and apigenin by different elution solvents with same volume, 5 BV.
Adsorption: concentrations of quercetin, luteolin and apigenin sample solutions of 70, 16.5 and 23.2 µg ml-1, respectively; pH 5; sample loading amount, 10 BV; flow rate, 2 BV/h; and temperature, 25 ºC.
Desorption: distilled water and 20% ethanol, each 5 BV; then 50% ethanol, 5 BV; and flow rate, 2 BV/h.
Laboratory preparative-scale separation
Laboratory preparative-scale separation was performed on a SP-825 resin (500 g, wet weight) column using the optimized conditions, and 34.86 g of the 50% ethanol fraction was obtained from a Flos populi extract. As shown in Table 5, elution with 5 BV 50% ethanol gave the quercetin-rich fraction with a content of 50.31% and recovery yield of 87.25%, the luteolin-rich fraction with a content of 11.73% and recovery yield of 85.19%, and the apigenin-rich fraction with a content of 15.92% and recovery yield of 82.22%.
Purification result of quercetin, luteolin and apigenin on column packed with SP-825 resin by final modified conditions.
Conclusions
In the current study, the enrichment process of quercetin, luteolin and apigenin with macroporous resins from Flos populi extract were successfully achieved. Based on the static experimental results, SP-825 was selected as a suitable resin for quercetin, luteolin and apigenin enrichment, owing to its higher adsorption/desorption capacity. According to the static experimental results with SP-825, it was found that the experimental data fitted best to Langmuir and Freundlich isotherms. The most effective resin (SP-825) was successfully applied to obtain a product of quercetin, luteolin and apigenin with higher contents. This study may serve as a reference for separating and enriching other bioactive components from crude extracts of raw herbal materials using macroporous resins.
-
AuthorshipYZ and BW carried out most of the studies. ZS designed the study and wrote the manuscript. CS improved the manuscript language. All authors have read and approved the final version.
Acknowledgements
This research was supported by National Natural Science Foundation of China (Grant No. 31572559), Academic Backbone Project of Northeast Agricultural University (Grant No. 16XG16).
References
- Amelio, A., Loise, L., Azhandeh, R., Darvishmanesh, S., Calabro, V., Degreve, J., Luis, P., Van der Bruggen, B., 2016. Purification of biodiesel using a membrane contactor: liquid-liquid extraction. Fuel Process. Technol. 142, 352-360.
- Chang, X.L., Wang, D., Chen, B.Y., Feng, Y.M., Wen, S.H., Zhan, P.Y., 2012. Adsorption and desorption properties of macroporous resins for anthocyanins from the calyx extract of roselle (Hibiscus sabdariffa L.). J. Agric. Food Chem. 60, 2368-2376.
- Chinese Pharmacopoeia, 2015. Pharmacopoeia of People's Republic of China. Chemical Industry Press, Beijing.
- Chiow, K.H., Phoon, M.C., Putti, T., Tan, B.K.H., Chow, V.T., 2016. Evaluation of antiviral activities of Houttuynia cordata Thunb. extract, quercetin, quercetrin and cinanserin on murine coronavirus and dengue virus infection. Asian Pac. J. Trop. Med. 9, 1-7.
- Du, Z., Wang, K., Tao, Y., Chen, L., Qiu, F., 2012. Purification of baicalin and wogonoside from Scutellaria baicalensis extracts by macroporous resin adsorption chromatography. J. Chromatogr. B 908, 143-149.
- Feng, W.M., Guo, H.H., Xue, T., Wang, X., Tang, C.W., Ying, B., Gong, H., Cui, G., 2015. Anti-inflammation and anti-fibrosis with PEGylated, apigenin loaded PLGA nanoparticles in chronic pancreatitis disease. RSC Adv. 5, 83628-83635.
- Li, Y., Liu, J., Cao, R., Deng, S., Lu, X., 2013. Adsorption of myricetrin, puerarin, naringin, rutin, and neohesperidin dihydrochalcone flavonoids on macroporous resins. J. Chem. Eng. Data 58, 2527-2537.
- Lin, L., Zhao, H., Dong, Y., Yang, B., Zhao, M., 2012. Macroporous resin purification behavior of phenolics and rosmarinic acid from Rabdosia serra (MAXIM.) HARA leaf. Food Chem. 130, 417-424.
- Liu, B., Dong, B., Yuan, X., Kuang, Q., Zhao, Q., Yang, M., Liu, J., Zhao, B., 2016. Enrichment and separation of chlorogenic acid from the extract of Eupatorium adenophorum Spreng by macroporous resin. J. Chromatogr. B 1008, 58-64.
- Liu, W., Zhang, S., Zu, Y.-G., Fu, Y.-J., Ma, W., Zhang, D.-Y., Kong, Y., Li, X.-J., 2010. Preliminary enrichment and separation of genistein and apigenin from extracts of pigeon pea roots by macroporous resins. Bioresource Technol. 101, 4667-4675.
- Liu, Y.F., Liu, J.X., Chen, X.F., Liu, Y.W., Di, D.L., 2010. Preparative separation and purification of lycopene from tomato skins extracts by macroporous adsorption resins. Food Chem. 123, 1027-1034.
- Liu, Z., Wang, J.Y., Gao, W.Y., Man, S.L., Wang, Y., Liu, C.X., 2013. Preparative separation and purification of steroidal saponins in Paris polyphylla var. yunnanensis by macroporous adsorption resins. Pharm. Biol. 51, 899-905.
- Mun, L.W., Rafiqul, I.S.M., Sakinah, A.M.M., Zularisam, A.W., 2016. Purification of bioxylitol by liquid-liquid extraction from enzymatic reaction mixture. Sep. Sci. Technol. 51, 2369-2377.
- Sandhu, A.K., Gu, L., 2013. Adsorption/desorption characteristics and separation of anthocyanins from muscadine (Vitis rotundifolia) juice pomace by use of macroporous adsorbent resins. J. Agric. Food Chem. 61, 1441-1448.
- Shao, H.J., Jing, K., Mahmoud, E., Huang, H.H., Fang, X.J., Yu, C.R., 2013. Apigenin sensitizes colon cancer cells to antitumor activity of ABT-263. Mol. Cancer Ther. 12, 2640-2650.
- Si, C.L., Wu, L., Ni, Y.H., 2010. Isolation and structure elucidation of low molecular weight extractives from wood of triploid Populus tomentosa Carr. In: Research Progress in Paper Industry and Biorefinery (4thIsetpp), vols. 1–3, pp. 197–200.
- Smith, N.W., Evans, M.B., 1995. Efficient analysis of neutral and highly polar pharmaceutical compounds using reversed-phase and ion-exchange electrochromatography. Chromatographia 41, 197-203.
- Sun, P.C., Liu, Y., Yi, Y.T., Li, H.J., Fan, P., Xia, C.H., 2015. Preliminary enrichment and separation of chlorogenic acid from Helianthus tuberosus L. leaves extract by macroporous resins. Food Chem. 168, 55-62.
- Torres, S., Cerutti, S., Raba, J., Pacheco, P., Silva, M.F., 2014. Preconcentration of seleno-amino acids on a XAD resin and determination in regional olive oils by SPE UPLC-ESI-MS/MS. Food Chem. 159, 407-413.
- Wang, B., Goldsmith, C.D., Zhao, J., Zhao, S., Sheng, Z., Yu, W., 2018. Optimization of ultrasound-assisted extraction of quercetin, luteolin, apigenin, pinocembrin and chrysin from Flos populi by Plackett-Burman design combined with Taguchi method. Chiang Mai J. Sci. 45, 427-439.
- Wu, W., Li, R., Li, X., He, J., Jiang, S., Liu, S., Yang, J., 2015. Quercetin as an antiviral agent inhibits Influenza A virus (IAV) entry. Viruses, 8.
- Xi, L., Mu, T., Sun, H., 2015. Preparative purification of polyphenols from sweet potato (Ipomoea batatas L.) leaves by AB-8 macroporous resins. Food Chem. 172, 166-174.
- Xu, H.L., Yang, T., Liu, X.H., Tian, Y., Chen, X.L., Yuan, R., Su, S.N., Lin, X.K., Du, G.H., 2016. Luteolin synergizes the antitumor effects of 5-fluorouracil against human hepatocellular carcinoma cells through apoptosis induction and metabolism. Life Sci. 144, 138-147.
- Xu, Q., Shen, Z., Wang, Y., Guo, S., Li, F., Wang, Y., Zhou, C., 2013. Anti-diarrhoeal and anti-microbial activity of Flos populi (male inflorescence of Populus tomentosa Carrière) aqueous extracts. J. Ethnopharmacol. 148, 640-646.
- Xu, Q., Wang, Y., Guo, S., Shen, Z., Wang, Y., Yang, L., 2014. Anti-inflammatory and analgesic activity of aqueous extract of Flos populi J. Ethnopharmacol. 152, 540-545.
- Yang, Q., Zhao, M., Lin, L., 2016. Adsorption and desorption characteristics of adlay bran free phenolics on macroporous resins. Food Chem. 194, 900-907.
- Zhang, B., Yang, R.Y., Zhao, Y., Liu, C.Z., 2008. Separation of chlorogenic acid from honeysuckle crude extracts by macroporous resins. J. Chromatogr. B 867, 253-258.
- Zhao, P., Qi, C., Wang, G., Dai, X., Hou, X., 2015. Enrichment and purification of total flavonoids from Cortex juglandis Mandshuricae extracts and their suppressive effect on carbon tetrachloride-induced hepatic injury in mice. J. Chromatogr. B 1007, 8-17.
- Zhao, Y., Tang, G.S., Cai, E.B., Liu, S.L., Zhang, L.X., Wang, S.J., 2014. Hypolipidaemic and antioxidant properties of ethanol extract from Flos populi Nat. Prod. Res. 28, 1467-1470.
Publication Dates
-
Publication in this collection
Jan-Feb 2019
History
-
Received
14 Aug 2018 -
Accepted
10 Sept 2018 -
Published
5 Oct 2018