Abstract
Five compounds were isolated from the peels of chufa (Eleocharis dulcis (Burm.f.) Trin. ex Hensch., Cyperaceae). The chemical structures were determined by various spectroscopic analysis methods, including 1D and 2D NMR, and by comparison with literature data. All compounds were isolated for the first time from the peels of chufa. Compounds orcinol glucoside, leonuriside A, 2-hydroxymethyl-6-(5-hydroxy-2-methyl-phenoxy-methyl)-tetra-hydro-pyran-3,4,5-triol, and 1,4-dihydroxy-3-methoxy-phenyl-4-O-β-D-glucopyranoside showed good acrylamide formation activity, and acrylamide inhibition rates were 30.24, 32.81, 30.53, and 28.18%, respectively.
Keywords:
Chinese water chestnut; Chufa peels; Acrylamide; Antioxidant; HPLC
Introduction
Cooking food has many advantages, including the destruction of microbes, elimination of heat-sensitive toxins, increase in the bioavailability of nutrients, and the development of desirable colors, flavors, and textures (Van et al., 2010Van, B.M., Fogliano, V., Pellegrini, N., Stanton, C., Scolz, G., Lalljie, S., Somoza, V., Knorr, D., Jasti, P.R., Eisenbrand, G., 2010. A review on the beneficial aspects of food processing. Mol. Nutr. Food Res. 54, 1215-1247.). However, food processing can sometimes lead to the formation of toxic compounds. One such compound is acrylamide, a rodent carcinogen and human neurotoxin that is classified as a possible human carcinogen (Mottram and Friedman, 2008Mottram, D.S., Friedman, M., 2008. Symposium on the chemistry and toxicology of acrylamide. J. Pharm. Sci. 56, 5983.). Acrylamide is of particular concern worldwide (Marion and Reinhard, 2018Marion, R., Reinhard, M., 2018. Acrylamide in cocoa: a survey of acrylamide in cocoa and cocoa products sourced from the German market. Eur. Food Res. Technol. 244, 1381-1388.). This compound was first detected in various heat-treated, carbohydrate-rich, and low-moisture containing food items in 2002 (Maurus and Koni, 2008Maurus, B., Koni, G., 2008. In GC-MS, acrylamide from heated foods may be coeluted with 3-hydroxy propionitrile. Eur. Food Res. Technol. 227, 945-948.; Zhang et al., 2008Zhang, Y., Fang, H.R., Zhang, Y., 2008. Study on formation of acrylamide in asparagine–sugar microwave heating systems using UPLC-MS/MS analytical method. Food Chem. 108, 542-550.; Tateo et al., 2010Tateo, F., Bononi, M., Gallone, F., 2010. Acrylamide content in potato chips on the Italian market determined by liquid chromatography tandem mass spectrometry. Int. J. Food Sci. Tech. 45, 629-634.; Bartkiene et al., 2015Bartkiene, E., Jakobsone, I., Pugajeva, I., Bartkevics, V., Vidmantiene, D., Juodeikiene, G., 2015. Influence of the addition of Helianthus tuberosus L. fermented with different lactobacilli on acrylamide content in biscuits. Int. J. Food Sci. Tech. 50, 431-439.).
Previous studies have demonstrated the effectiveness of various antioxidants and antioxidant extracts in reducing acrylamide production (Bassama et al., 2010Bassama, J., Brat, P., Bohuon, P., Boulanger, R., Günata, Z., 2010. Study of acrylamide mitigation in model system: effect of pure phenolic compounds. Food Chem. 123, 558-562.; Jin et al., 2013Jin, C., Wu, X.Q., Zhang, Y., 2013. Relationship between antioxidants and acrylamide formation: a review. Food Res. Int. 51, 611-620.; Zhan et al., 2016Zhan, G., Pan, L.Q., Tu, K., Jiao, S.S., 2016. Antitumor, antioxidant, and nitrite scavenging effects of Chinese Water Chestnut (Eleocharis dulcis) peel flavonoids. J. Food Sci. 81, 2578-2586.). The Chinese water chestnut or chufa (Eleocharis dulcis (Burm.f.) Trin. ex Hensch.), which belongs to the family Cyperaceae, is widespread in southern China, particularly in the Guangxi Province (Li et al., 2013Li, X.R., Luo, Y.H., He, J., Peng, L.Y., Wu, X.D., Du, R.N., Zhao, Q.S., 2013. Phenolic constituents and antioxidant activity of Eleocharis tuberosa peels. Nat. Prod. Res. 25, 1615-1620.), and is often used in Chinese folk medicine for the treatment of pharyngitis, laryngitis, enteritis, cough, hepatitis, and hypertension (Luo et al., 2014Luo, Y.H., Li, X.R., He, Juan, Peng, L.Y., Wu, X.D., Du, R.N., Zhao, Q.S., 2014. Isolation, characterisation, and antioxidant activities of flavonoids from chufa (Eleocharis tuberosa) peels. Food Chem. 164, 30-35.). It is one of the most popular hydrophytic vegetables in China due to its unique taste (Li et al., 2016Li, Y.X., Pan, Y.G., He, F.P., Yuan, M.Q., Li, S.B., 2016. Pathway analysis and metabolites identification by metabolomics of etiolation substrate from fresh-cut Chinese water chestnut (Eleocharis tuberosa). Molecules 21, .
https://doi.org/10.3390/molecules2112164...
). Chufa peels are often discarded; however, previous studies have demonstrated that they exhibit good antioxidant activities (Li et al., 2013Li, X.R., Luo, Y.H., He, J., Peng, L.Y., Wu, X.D., Du, R.N., Zhao, Q.S., 2013. Phenolic constituents and antioxidant activity of Eleocharis tuberosa peels. Nat. Prod. Res. 25, 1615-1620.; Luo et al., 2014Luo, Y.H., Li, X.R., He, Juan, Peng, L.Y., Wu, X.D., Du, R.N., Zhao, Q.S., 2014. Isolation, characterisation, and antioxidant activities of flavonoids from chufa (Eleocharis tuberosa) peels. Food Chem. 164, 30-35.), hence, they could be used to inhibit natural acrylamide formation during food processing.
Therefore, to clarify and inhibit acrylamide formation, it is necessary to identify the chemical compounds in chufa peels. In this study, HPLC, column chromatography and NMR analysis were used to purify and identify the chemical constituents of chufa peels. Compounds 1 and 4 showed good inhibitory activity with an inhibitory concentration of 1 × 10−3 mg/ml. Compounds 2 and 5 showed good inhibitory activity with an inhibitory concentration of 1 × 10−2 mg/ml.
Materials and methods
Chemicals and materials
1D and 2D NMR spectra were obtained on a Bruker Advance 400-MHz spectrometer. The HRESIMS data were carried out on a MAT 95XP mass spectrometer. Semi-preparative HPLC was performed using a reverse-phase column (Apollo C18 column, 5 µm, 10 mm × 250 mm, detection at UV 210 nm). For column chromatography (CC), Sephadex LH-20 (Amersham Pharmacia Biotech AB) and RP-C18 (LiChroprep 40-63 µm, Merck) were used. Centrifugal preparative TLC was performed using an apparatus from Qingdao HAIYANG (PR China). Analytical HPLC was conducted on an Agilent 1260 instrument (PDA detector, Agilent Zorbax SB-C18 column, 4.6 mm × 150 mm, 5 µm).
Materials
Fresh chufa peels (Eleocharis dulcis (Burm.f.) Trin. ex Hensch., Cyperaceae) were collected from Guangxi Province in China in March 2016 and identified by Professor Baiming Pan, Hezhou University (Hezhou, Guangxi, China).
Acrylamide, >99% (CAS registry number 79-06-1), was supplied by Sigma-Aldrich (St. Louis, MO, USA) and deuterium labeled acrylamide-d3 (99%) by LGC Standards (Teddington, Middlesex, UK).
Extraction and isolation of the compounds
Dry chufa peels (10 kg) were subjected to extraction three times with 70% aqueous acetone (32 l × 3), each for 24 h, at room temperature. The extract was evaporated in vacuo to give a crude extract. The residue was separated into petroleum ether (60–90%, 3 × 6 l), EtOAc (3 × 6 l), and n-BuOH (3 × 6 l), and H2O layers using liquid–liquid partitioning. Each fraction was subjected to an acrylamide formation assay to determine their acrylamide reduction activities. The n-BuOH extract had the highest activity (Table 1). The n-BuOH extract (92.5 g) was divided into three fractions (A–C) using D101 macroporous resin chromatography (8 × 60 cm) eluting with a gradient of H2O/EtOH (75:25, 45:55, 10:90, v/v). Fraction A (15 g) was subjected to RP-C18 column chromatography with H2O/MeOH (90:10–0:100, v/v) to give four fractions A-(1-4). Subfraction A-1 was fractionated by TLC to give three fractions A-1-(1-3). Fraction A-1-1 (230 mg) was subjected to semi-preparative HPLC and eluted with H2O/MeOH (95:5; flow rate: 2.5 ml/min) to yield compound 1 (17 mg, tR = 25.0 min) and compound 2 (22 mg, tR = 87.0 min). Fraction A-1-2 (890 mg) was purified using Sephadex LH-20 column chromatography (3 × 180 cm). The column was eluted with MeOH to yield compound 3 (4 mg). Fraction A-1-3 (910 mg) was subjected to semi-preparative HPLC and eluted with H2O/MeOH (95:5; flow rate: 2 ml/min) to yield compound 4 (36 mg, tR = 36.0 min) and compound 5 (5 mg, tR = 87.0 min). The structures of all isolates were characterized based on their NMR data.
Inhibition of acrylamide formation by extracts from chufa peels.a a All values are means of three independent experiments.
Acrylamide formation assays
Sample preparation
Potato tubers, purchased from a local market, were peeled, and cut into little cubes. The cubes were air-dried and ground into potato powder. Chips prepared from potato powder were shaped as circles with a thickness of 1 mm and a diameter of 4 cm, and baked using an electric oven (Ukeo HBD-5002, Zhuhai Jiabao de Science & Technology Co, Zhuhai, Guangdong Province, China) at 150 ºC for 5 min. Each potato chip weighed 1.12 g.
Standard preparation
Stock solutions of acrylamide standard and the internal standard acrylamide-d3, 1 mg ml−1, were prepared in H2O/MeOH (95/5, v/v). Acrylamide was further separated by HPLC using an elution with H2O/MeOH (95:5) and a flow rate of 0.8 ml/min. It eluted at 8.53 min.
Sample extraction
Potato chips were analyzed according to the LCI-internal validated method (Matissek et al., 2005Matissek, R., Raters, M., Friedman, M., Mottram, D., 2005. Analysis of acrylamide in food chemistry and safety of acrylamide in food. Adv. Exp. Med. Biol. 561, 293-302.; Carrieri et al., 2010Carrieri, G., Anese, M., Quarta, B., Bonis, M., Ruocco, G., 2010. Evaluation of acrylamide formation in potatoes during deep-frying: the effect of operation and configuration. J. Food Eng. 98, 141-149.). Potato chips were homogenized and 20 ml of water and 400 µl of internal standard acrylamide-d3 (5 × 10−6 g ml−1) were added to 2 g of the homogenized sample. Potato chips were extracted by ultrasonic treatment (15 min, 60 ºC) and 20 ml of acetonitrile were added. Clean-up of the extracts was performed using 500 µl of Carrez I (K4Fe(CN)6·3H2O, 150 g l−1) and Carrez II (ZnSO4·7H2O, 300 g l−1), and the samples were then centrifuged at 3155× ɡ for 10 min at 4 ºC. The supernatant was then passed through a syringe filter prior to injection into the HPLC system (0.22 µm, Millex® Syringe Filter Unite).
Analysis of acrylamide
Acrylamide determination was carried out via water extraction, purification via solid phase extraction (Isolute Env+, 1 g) and quantitation via HPLC with acrylamide-d3 as the internal standard, following a method similar to that proposed by Vural Gökmen (Vural and Hamide, 2007Vural, G., Hamide, Z.S., 2007. Effects of some cations on the formation of acrylamide and furfurals in glucose–asparagine model system. Eur. Food Res. Technol. 225, 815-820.). The absorbance of the mixture was measured at 210 nm (tR = 8.53 min). These tests were carried out in triplicate. The inhibition of acrylamide activity was expressed as the acrylamide inhibition rate (AIR) and was calculated as follows:
where AM, acrylamide content in the experimental group; AM0, acrylamide content in the blank control group.
The acrylamide inhibition rate of compounds 1-5, and using quercetin, catechin and epicatechin as positive controls, is shown in Fig. 1.
Results and discussion
The structures of all of the compounds were elucidated by NMR spectroscopy (1H, 13C, and HMBC) and comparison with previously reported values in the literature. They are orcinol glucoside (1), leonuriside A (2), L-tryptophan (3), 2-hydroxymethyl-6-(5-hydroxy-2-methyl-phenoxy-methyl)-tetra-hydro-pyran-3,4,5-triol (4), and 1,4-dihydroxy-3-methoxy-phenyl-4-O-β-D-glucopyranoside (5).
Compound 1 (17 mg): C13H18O7, colorless acicular. 1H NMR (400 MHz, CD3OD): 6.42 (1H, s, H-2), 6.36 (1H, s, H-4), 6.29 (1H, s, H-6), 4.59 (1H, s, H-1′), 3.36-3.45 (4H, m, glc. H), 3.70 (1H, dd, J = 12.0, 5.0 Hz, Ha-6′), 3.89 (1H, dd, J = 12.1, 1.8 Hz, Hb-6′), 2.22 (3H, s, –CH3); 13C NMR (101 MHz, CD3OD): 160.1 (C-1), 102.2 (C-2), 159.2 (C-3), 109.8 (C-4), 141.3 (C-5), 111.2 (C-6), 102.1 (C-1′), 74.9 (C-2′), 78.0 (C-3′), 71.4 (C-4′), 77.9 (C-5′), 62.5 (C-6′), 21.6. Compound 1 was identified as orcinol glucoside by comparison with the spectral data in the literature (Li et al., 2003Li, N., Tan, N.H., Zhou, J., 2003. A new lignan glycoside from Curculigo capitulata. Acta Botanica Yunn Anica 25, 711-715.). Orcinol glucoside has been reported to be an antioxidant (Wu et al., 2008Wu, Z.J., Ouyang, M.A., Wang, S.B., 2008. Two new phenolic water-soluble constituents from branch bark of Davidia involucrata. Nat. Prod. Res. 22, 483-488.) and antidepressant (Ge et al., 2014Ge, J.F., Gao, W.C., Cheng, W.M., Lu, W.L., Tang, J., Peng, L., Li, N., Chen, F.H., 2014. Orcinol glucoside products antidepressant effects by blocking the behavioural and neuronal deficits caused by chronic stress. Eur. Neuropsychopharm. 24, 172-180.).
Compound 2 (22 mg): C14H20O9, white amorphous powder. 1H NMR (400 MHz, DMSO-d6): 6.06 (2H, s, H-3/5), 3.68 (6H, s, –OCH3), 4.64 (1H, d, J = 7.2 Hz, H-1′), 3.59 (1H, d, J = 11.3 Hz, H-6′), 3.40-3.45 (4H, m, glc. H), 9.24 (1H, s); 13C NMR (101 MHz, DMSO-d6): 127.5 (C-1), 153.2 (C-2/6), 93.8 (C-3/5), 153.9 (C-4), 103.5 (C-1′), 74.2 (C-2′), 77.1 (C-3′), 70.0 (C-4′), 76.5 (C-5′), 61.1 (C-6′), 56.1 (C-OCH3). Compound 2 was identified as leonuriside A by comparison with spectral data in the literature (Wu et al., 2008Wu, Z.J., Ouyang, M.A., Wang, S.B., 2008. Two new phenolic water-soluble constituents from branch bark of Davidia involucrata. Nat. Prod. Res. 22, 483-488.). The phenolic glucoside leonuriside A has been evaluated for antioxidant activity (Sugaya et al., 1998Sugaya, K., Hashimoto, F., Ono, M., Ito, Y., Masuoka, C., Nohara, T., 1998. Antioxidative constituents from Leonurii Herba (Leonurus japonicas). Food Sci. Technol. Int. 4, 278-281.).
Compound 3 (4 mg): C11H12N2O2, yellow solid. 1H NMR (400 MHZ, DMSO-d6), 10.93 (1H, s, NH), 7.20 (1H, s, H-2), 7.56 (1H, d, J = 7.8 Hz, H-4), 6.97 (1H, t, J = 7.3 Hz, H-5), 7.06 (1H, t, J = 7.3 Hz, H-6), 7.34 (1H, d, J = 8.0 Hz, H-7); 13C NMR (101 MHz, DMSO-d6), 124.1 (C-2), 109.6 (C-3), 118.3 (C-4), 118.4 (C-5), 120.9 (C-6), 111.4 (C-7), 136.4 (C-8), 127.3 (C-9), 27.1 (C-10), 54.8 (C-11), 170.3 (C-COOH). This compound was identified as L-tryptophan by comparison with spectral data in the literature (Li et al., 2004Li, G.Q., Deng, Z.W., Li, J., Fu, H.Z., Lin, W.H., 2004. Chemical constituents from Starfish Asterias rollestoni. J. Pharm. Sci. 13, 81-86.). L-Tryptophan has been reported to have antifungal properties (Diego et al., 2016Diego, Q., Lili, D.B., John, S.B., Nathali, V., Ericsson, C.B., 2016. Synthesis and antifungal activity against Fusarium oxysporum of some Brassinin analogs derived from L-tryptophan: a DFT/B3LYP study on the reaction mechanism. Molecules , 21.
https://doi.org/10.3390/molecules2110134...
).
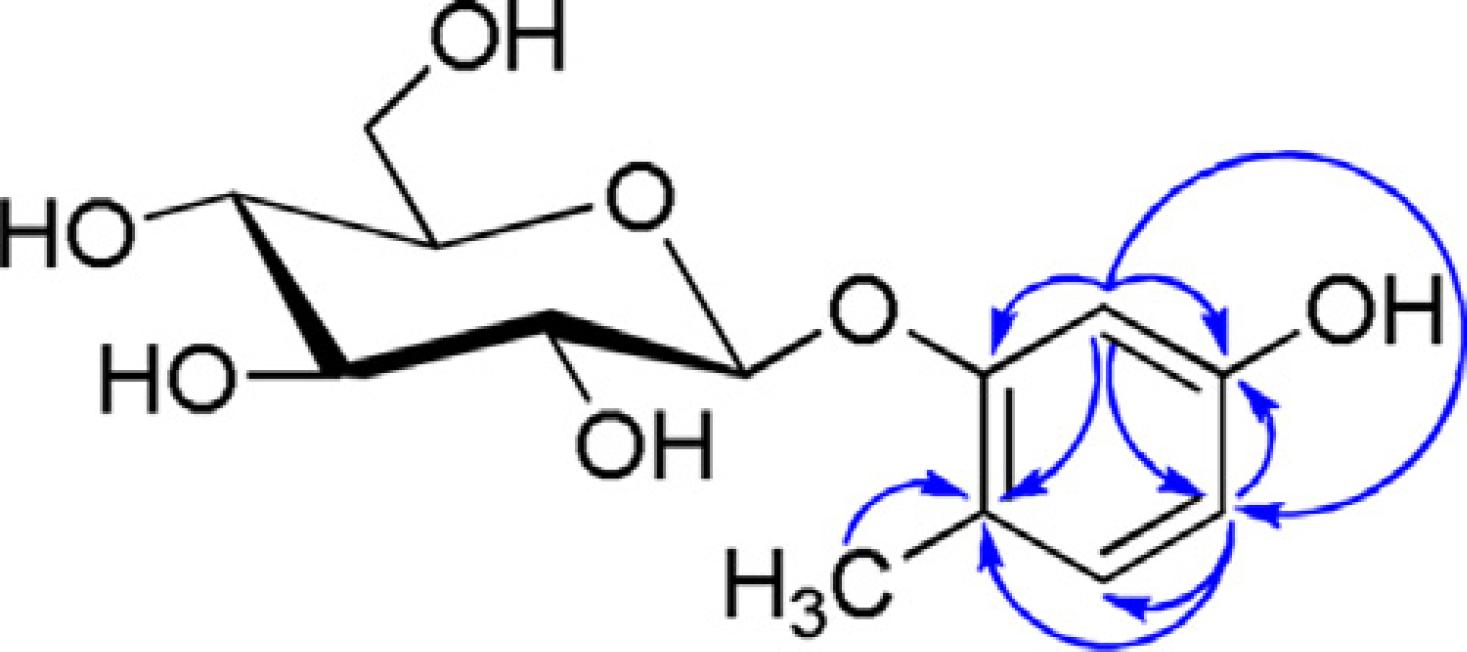
Compound 4 (36 mg): C13H18O7, yellow oil, HRSEI-MS m/z 309.0941 [M+Na]+ (calcd for C13H18O7Na, 309.0941). 1H NMR (400 MHz, CD3OD): 6.28 (1H, s, H-3), 6.24 (1H, d, H-5), 6.24 (1H, H-6), 2.16 (3H, s, –CH3), 3.31-3.33 (4H, m, glc. H); 13C NMR (101 MHz, CD3OD): 168.1 (C-1), 148.8 (C-2), 110.4 (C-3), 167.6 (C-4), 119.2 (C-5), 117.4 (C-6), 110.1 (C-1′), 82.8 (C-2′), 86.1 (C-3′), 79.2 (C-4′), 86.5 (C-5′), 70.2 (C-6′), 30.9 (C-CH3). The 1H and 13C NMR spectroscopic data for this compound include peaks for one 1,2,4-trisubstituted aromatic ring system. Its HMBC spectrum shows the following correlations: H-3/C-2, H-3/C-4, H-3/C-5, H-5/C-3, H-5/C-4, H-5/C-6, H-6/C-1, H-6/C-2, H-6/C-5 (Fig. 2). We identified this compound as 2-hydroxymethyl-6-(5-hydroxy-2-methyl-phenoxy-methyl)-tetra-hydro-pyran-3,4,5-triol by comparison with spectral data in the literature (Takashi et al., 2007Takashi, S., Ayashi, N., Satoru, T., Yasuo, S., Makoto, T., Masayuki, N., 2007. Preparation of alkylresorcinol glycosides and their use in cosmetics. Patent WO 2007/077770, A1.).
Compound 5 (5 mg): C13H17O7, colorless amorphous powder. 1H NMR (400 MHz, DMSO-d6): 7.09 (1H, s, H-2), 6.66 (1H, s, H-5), 6.44 (1H, s, H-6), 4.93 (1H, d, J = 7.2 Hz, H-1′), 3.90 (1H, s, H-6′), 3.72 (3H, s, –OCH3), 3.60-3.68 (4H, m, glc. H), 13C NMR (101 MHz, DMSO-d6): 147.8 (C-1), 107.9 (C-2), 151.0 (C-3), 152.4 (C-4), 115.2 (C-5), 107.9 (C-6), 102.5 (C-1′), 74.7 (C-2′), 78.2 (C-3′), 70.4 (C-4′), 79.7 (C-5′), 67.3 (C-6′), 55.5 (C-OCH3). We identified compound 5 as 1,4-dihydroxy-3-methoxy-phenyl-4-O-β-D-glucopyranoside by comparison with spectral data in the literature (Wu et al., 2008Wu, Z.J., Ouyang, M.A., Wang, S.B., 2008. Two new phenolic water-soluble constituents from branch bark of Davidia involucrata. Nat. Prod. Res. 22, 483-488.; Li et al., 2017Li, S., Wan, C.X., He, L.L., Yan, Z.G., Wang, K.S., Yuan, M.H., Zhang, Z.F., 2017. Rapid identification and quantitative analysis of chemical constituents of Gentiana veitchiorum by UHPLC-PDA-QTOF-MS. Rev. Bras. Farmacogn. 27, 188-194.).
Compounds 1, 2, 4, and 5 exhibited good inhibition of acrylamide formation activity, inhibiting acrylamide formation better than quercetin, catechin, and epicatechin.
In the present study, we isolated and identified five compounds, orcinol glucoside, leonuriside A, L-tryptophan, 2-hydroxymethyl-6-(5-hydroxy-2-methyl-phenoxy-methyl)-tetra-hydro-pyran-3,4,5-triol, and 1,4-dihydroxy-3-methoxy-phenyl-4-O-β-D-glucopyranoside from chufa peels for the first time. Compounds 1, 2, 4, and 5 exhibited good inhibition of acrylamide formation activity (Table 2). Compounds 1 and 4 exhibited inhibitory concentrations of 1 × 10−3 mg/ml, and acrylamide inhibition rates of 30.24%, 30.53%. Compounds 2 and 5 exhibited inhibitory concentration of 1 × 10−2 mg/ml, and acrylamide inhibition rate are 32.81% and 28.18%. Interestingly, the compounds with a glucose group exhibited greater activity than the one that lacked. Compounds 1, 2, 4, and 5 inhibited acrylamide formation more strongly than 3. Therefore, our study provides a good starting point for a theoretical basis for a better understanding of how to effectively inhibit acrylamide formation. Chufa peels are a valuable natural resource rich in beneficial bioactive compounds, and should be further developed as a functional food.
Inhibition of acrylamide formation by compounds 1-5.a a All values are means of three independent experiments.
Ethical disclosures
Protection of human and animal subjects
The authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of data
The authors declare that no patient data appear in this article.
Right to privacy and informed consent
The authors declare that no patient data appears in this article.
-
1
These authors contributed equally to this work.
Acknowledgments
This project was supported by the National Key R&D Progrom of China (2018YFD0901003), the National Natural Science Foundation of China (21365011), the Youth Teachers’ Ability Promotion Project of Guangxi (2018KY0561), the Open Research Fund Program of the State Key Laboratory for the Food Science and Technology (1541001), the Doctor Scientific Research Foundation of Hezhou University (HZUBS201608), the Professor Scientific Research Foundation of Hezhou University (HZUJS201613), the Food Science and Engineering First-rate discipline in Guangxi (cultivation, GXYLXKP1802), the Students’ Platform for Innovation and Entrepreneurship Training Program (201811838140), and the Open Research Fund Program of the Guangxi Talent Highland of Preservation (2017XJSS02).
References
- Bassama, J., Brat, P., Bohuon, P., Boulanger, R., Günata, Z., 2010. Study of acrylamide mitigation in model system: effect of pure phenolic compounds. Food Chem. 123, 558-562.
- Bartkiene, E., Jakobsone, I., Pugajeva, I., Bartkevics, V., Vidmantiene, D., Juodeikiene, G., 2015. Influence of the addition of Helianthus tuberosus L. fermented with different lactobacilli on acrylamide content in biscuits. Int. J. Food Sci. Tech. 50, 431-439.
- Carrieri, G., Anese, M., Quarta, B., Bonis, M., Ruocco, G., 2010. Evaluation of acrylamide formation in potatoes during deep-frying: the effect of operation and configuration. J. Food Eng. 98, 141-149.
- Diego, Q., Lili, D.B., John, S.B., Nathali, V., Ericsson, C.B., 2016. Synthesis and antifungal activity against Fusarium oxysporum of some Brassinin analogs derived from L-tryptophan: a DFT/B3LYP study on the reaction mechanism. Molecules , 21.
» https://doi.org/10.3390/molecules21101349 - Jin, C., Wu, X.Q., Zhang, Y., 2013. Relationship between antioxidants and acrylamide formation: a review. Food Res. Int. 51, 611-620.
- Ge, J.F., Gao, W.C., Cheng, W.M., Lu, W.L., Tang, J., Peng, L., Li, N., Chen, F.H., 2014. Orcinol glucoside products antidepressant effects by blocking the behavioural and neuronal deficits caused by chronic stress. Eur. Neuropsychopharm. 24, 172-180.
- Li, X.R., Luo, Y.H., He, J., Peng, L.Y., Wu, X.D., Du, R.N., Zhao, Q.S., 2013. Phenolic constituents and antioxidant activity of Eleocharis tuberosa peels. Nat. Prod. Res. 25, 1615-1620.
- Luo, Y.H., Li, X.R., He, Juan, Peng, L.Y., Wu, X.D., Du, R.N., Zhao, Q.S., 2014. Isolation, characterisation, and antioxidant activities of flavonoids from chufa (Eleocharis tuberosa) peels. Food Chem. 164, 30-35.
- Li, Y.X., Pan, Y.G., He, F.P., Yuan, M.Q., Li, S.B., 2016. Pathway analysis and metabolites identification by metabolomics of etiolation substrate from fresh-cut Chinese water chestnut (Eleocharis tuberosa). Molecules 21, .
» https://doi.org/10.3390/molecules21121648 - Li, N., Tan, N.H., Zhou, J., 2003. A new lignan glycoside from Curculigo capitulata Acta Botanica Yunn Anica 25, 711-715.
- Li, G.Q., Deng, Z.W., Li, J., Fu, H.Z., Lin, W.H., 2004. Chemical constituents from Starfish Asterias rollestoni J. Pharm. Sci. 13, 81-86.
- Li, S., Wan, C.X., He, L.L., Yan, Z.G., Wang, K.S., Yuan, M.H., Zhang, Z.F., 2017. Rapid identification and quantitative analysis of chemical constituents of Gentiana veitchiorum by UHPLC-PDA-QTOF-MS. Rev. Bras. Farmacogn. 27, 188-194.
- Mottram, D.S., Friedman, M., 2008. Symposium on the chemistry and toxicology of acrylamide. J. Pharm. Sci. 56, 5983.
- Marion, R., Reinhard, M., 2018. Acrylamide in cocoa: a survey of acrylamide in cocoa and cocoa products sourced from the German market. Eur. Food Res. Technol. 244, 1381-1388.
- Maurus, B., Koni, G., 2008. In GC-MS, acrylamide from heated foods may be coeluted with 3-hydroxy propionitrile. Eur. Food Res. Technol. 227, 945-948.
- Matissek, R., Raters, M., Friedman, M., Mottram, D., 2005. Analysis of acrylamide in food chemistry and safety of acrylamide in food. Adv. Exp. Med. Biol. 561, 293-302.
- Sugaya, K., Hashimoto, F., Ono, M., Ito, Y., Masuoka, C., Nohara, T., 1998. Antioxidative constituents from Leonurii Herba (Leonurus japonicas). Food Sci. Technol. Int. 4, 278-281.
- Takashi, S., Ayashi, N., Satoru, T., Yasuo, S., Makoto, T., Masayuki, N., 2007. Preparation of alkylresorcinol glycosides and their use in cosmetics. Patent WO 2007/077770, A1.
- Tateo, F., Bononi, M., Gallone, F., 2010. Acrylamide content in potato chips on the Italian market determined by liquid chromatography tandem mass spectrometry. Int. J. Food Sci. Tech. 45, 629-634.
- Van, B.M., Fogliano, V., Pellegrini, N., Stanton, C., Scolz, G., Lalljie, S., Somoza, V., Knorr, D., Jasti, P.R., Eisenbrand, G., 2010. A review on the beneficial aspects of food processing. Mol. Nutr. Food Res. 54, 1215-1247.
- Vural, G., Hamide, Z.S., 2007. Effects of some cations on the formation of acrylamide and furfurals in glucose–asparagine model system. Eur. Food Res. Technol. 225, 815-820.
- Wu, Z.J., Ouyang, M.A., Wang, S.B., 2008. Two new phenolic water-soluble constituents from branch bark of Davidia involucrata Nat. Prod. Res. 22, 483-488.
- Zhang, Y., Fang, H.R., Zhang, Y., 2008. Study on formation of acrylamide in asparagine–sugar microwave heating systems using UPLC-MS/MS analytical method. Food Chem. 108, 542-550.
- Zhan, G., Pan, L.Q., Tu, K., Jiao, S.S., 2016. Antitumor, antioxidant, and nitrite scavenging effects of Chinese Water Chestnut (Eleocharis dulcis) peel flavonoids. J. Food Sci. 81, 2578-2586.
Publication Dates
-
Publication in this collection
17 Oct 2019 -
Date of issue
Jul-Aug 2019
History
-
Received
30 Aug 2018 -
Accepted
21 Nov 2018