Abstract
Nuclear magnetic resonance spectroscopy (NMRS) is a powerful technique that enables continuous monitoring of biochemical processes in tissues and organs in a non-invasive manner. A model of isolated perfused rat pancreas, suitable for NMRS studies, was developed. Acute pancreatitis was induced by injections of either 0.5 ml 5% sodium taurocholate (TC) into the bile duets, or 1.0 ml 10% TC injections into the pancreatic parenchyma. Phosphorous (31P) NMRS of experimental pancreatitis were characterized by a transient signal at -0.18±0.04 ppm which was assigned as solubilized lecithin, and can be used as an indicator of the early phases of the discase. Depletion of the high energy phosphorous compounds, phosphocreatine and ATP, were also found during acute pancreatitis, and paralleled the extension of the pathological damage. The role of NMRS in pancreatic cancer diagnosis and its treatment were assessed in three models of pancreatic neoplasms. Perfused MIA PaCa-2 human pancreatic cancer cells, subcutancously implanted pancreatic tumors in hamsters, and pancreatic tumors induced in-situ in rats by direct appiication of the carcinogen 7,12-dimethyl benzanthracene, were studied by phosphorous (31P), sodium (23Na) and proton (¹H) NMRS. 31P spectra of pancreatic cancer were qualitatively similar to those of intact organs. However, 31P NMRS was found to be useful for monitoring the effects of treatment. Total (infra- and extracellular) sodium concentrations, measured in the solid tumors, were similar in both the normal pancreas and the pancreatic tumors (39-40 mmol/g wet weight). Proton spectra of perchloric acid extracts revealed several differences between tumors and control pancreases. The principal findings were elevated levels of the amino acid taurine, from I.17±O.39 mmol/g wet weight in healthy pancreases, to 2.79±0.71 mmol/g wet weight in pancreatic carcinoma in rats, and lactate levels which increased from 0.92±0.2 to 6.19±1.93 mmol/g wet weight, respectively. On the other hand, creatine and glutamate were higher in the normal pancreases. These studies demonstrated that NMRS is a useful technique for studying fundamental biochemical and metabolic events of acute pancreatitis and pancreatic cancer, and for the development of therapeutical modalities
Nuclear Magnetic Resonance; Spectroscopy; Pancreas
ORIGINAL ARTICLE
Nuclear magnetic resonance spectroscopy in pancreatic disorders1 1 Article from the Dept. of Surgery A, Tel-Aviv Medical Center and Sackler School of Medicine, Tel-Aviv University.
Ofer Kaplan
M.D. Ichilov Hospital, 6 Weizmann st., Tel-Aviv 64239 - Israel
Address for correspondence Address for correspondence: Dr. O. Kaplan Dept. of Surgery A Tel-Aviv Medical Center 6 Weizmann St, Tel-Aviv 64239, Israel Tel: 972-3-6793493 Fax: 972-3-6974621
SUMMARY
Nuclear magnetic resonance spectroscopy (NMRS) is a powerful technique that enables continuous monitoring of biochemical processes in tissues and organs in a non-invasive manner. A model of isolated perfused rat pancreas, suitable for NMRS studies, was developed. Acute pancreatitis was induced by injections of either 0.5 ml 5% sodium taurocholate (TC) into the bile duets, or 1.0 ml 10% TC injections into the pancreatic parenchyma. Phosphorous (31P) NMRS of experimental pancreatitis were characterized by a transient signal at -0.18±0.04 ppm which was assigned as solubilized lecithin, and can be used as an indicator of the early phases of the discase. Depletion of the high energy phosphorous compounds, phosphocreatine and ATP, were also found during acute pancreatitis, and paralleled the extension of the pathological damage. The role of NMRS in pancreatic cancer diagnosis and its treatment were assessed in three models of pancreatic neoplasms. Perfused MIA PaCa-2 human pancreatic cancer cells, subcutancously implanted pancreatic tumors in hamsters, and pancreatic tumors induced in-situ in rats by direct appiication of the carcinogen 7,12-dimethyl benzanthracene, were studied by phosphorous (31P), sodium (23Na) and proton (1H) NMRS. 31P spectra of pancreatic cancer were qualitatively similar to those of intact organs. However, 31P NMRS was found to be useful for monitoring the effects of treatment. Total (infra- and extracellular) sodium concentrations, measured in the solid tumors, were similar in both the normal pancreas and the pancreatic tumors (39-40 mmol/g wet weight). Proton spectra of perchloric acid extracts revealed several differences between tumors and control pancreases. The principal findings were elevated levels of the amino acid taurine, from I.17±O.39 mmol/g wet weight in healthy pancreases, to 2.79±0.71 mmol/g wet weight in pancreatic carcinoma in rats, and lactate levels which increased from 0.92±0.2 to 6.19±1.93 mmol/g wet weight, respectively. On the other hand, creatine and glutamate were higher in the normal pancreases. These studies demonstrated that NMRS is a useful technique for studying fundamental biochemical and metabolic events of acute pancreatitis and pancreatic cancer, and for the development of therapeutical modalities
Subject headings: Nuclear Magnetic Resonance. Spectroscopy. Pancreas.
INTRODUCTION
The treatment of surgical disorders of the pancreas is a major challenge. The diagnosis is often delayed due to the anatomical location of the pancreas, the non-specific clinical presentation, and the inadequate diagnostic techniques. Inaccurate preoperative assessment and staging may lead to errors in determining the indications for surgery. Moreover, operations of the pancreas are associated with remarkable morbidity and mortality due to the seventy of the disease, the extent of the surgery and the general condition of the patients. Reliable diagnostic and prognostic means, especially non-invasive methods, are therefore essential in order to improve the treatment and outcome of these diseases. Nuclear magnetic resonance spectroscopy (NMRS) presents such a means. NMRS is a powerful technique that provides information on biochemical status and physiological processes both in-vitro and in-vivo (1-3).In this paper we describe NMRS studies on two of me most important pancreatic disorders, acute pancreatitis and pancreatic cancer.
Nuclear magnetic resonance spectroscopy is based on the phenomenon that nuclei of several elements behave like tiny bar magnets when in magnetic fields, and orient in the field directions. Radio frequency irradiation causes re-orientation of the nuclei by the resonance process, which can be detected and presented as a signal. The frequency of this irradiation is proportional to the strength of the magnetic field, and is specific for each nucleus. The different nuclei of each element resonate at slightly different frequencies due to the different distribution of electrons surrounding them (chemical shift). A spectrum of signals for a specific element can be obtained, and assignments of these signals enable chemical analysis of the sample. The relative areas of the signals is proportional to the compounds concentrations; therefore NMRS is a qualitative as well as a quantitative method. Information on intracellular processes can be obtained in a continuous manner, and thus, NMRS is a unique non-invasive research tool enabling detection of physiological and pathological changes as they occur (for comprehensive description of NMR see reviews4,3).
METHOD
Animals, cells and materials: The animals which were used in these studies were kept as outlined in the Guides for the care and use of laboratory animals (N1H publication #85-23, 1985), and were given food and water ad libitum. Prior to surgical procedures they fasted for 8 hours, but had free access to water. All the surgical procedures were performed under general anesthesia induced by ketamine hydrochloride (70 mg/kg, i.m.). Human pancreatic carcinoma cells, MIA PaCa-2, were obtained from the American Type Culture Collection (Rockville MD), and were grown in DMEM, supplemented with penicilin-streptomycin-nystatine, L-glutamine and 10% fetal calf serum, under 5% CO, environment. The pancreatic cancer cell line of hamsters was established from nitrosamine treated CB strain Syrian hamsters at the National Institutes of Health (Bethesda MD). Ali materials were purchased from Sigma Chemicals (St. Louis, USA) unless otherwise indicated.
Acute pancreatitis: The model of isolated perfused rat pancreas was used. Forty four male Sprague-Dawley rats, weighing 300-350 gr. were used as pancreas donors.
Surgical technique for isolation and removal of the pancreas with intact vasculture. Through median laparatomy the small intestines were divided just distal to the take-off of the inferior pancreaticoduodenal artery, and the superior mesenterie artery and mesentery were double ligated and separated. The sigmoid colon was transacted, the transverse colon was carefully dissected from its attachments to the pancreas, and the entire small and large intestines were removed. The splenic vessels were ligated near the hilum of the spleen and it was removed. The gastric branches of the right and left gastroepiploic arteries and veins, the left gastric vessels, the esophagus, and the hepatic artery were ligated and transacted in that order. The next step was dissection of the duodenum from the pancreas with meticulous ligation of the many common vessels near the duodenum. This critical part of the operation was performed with caution to avoid severing the blood supply of the head of the pancreas. The common bile duct was ligated and divided and the duodenum was removed. The posterior peritoneum was opened, the testicular vessels were ligated and transacted bilaterally and both kidneys were removed after ligation of their vasculture. The lower aorta, just above its bifurcation, was cannulated with a plastic catheter (Venflon, Vigo 21G), and 500 units of heparin were flushed into the circulation. The aorta below the entrance of the catheter was double ligated, and by blunt dissection was freed from the inferior vena cava. This vessel was double ligated just under the liver and divided between the ligatures. The aorta below the diaphragm was ligated and proximally transacted, and the arterial catheter was flushed with heparinized perfusion solution. A preformed suture was tied around the portal vein and a hole was made in it for venous drainage, and the liver was removed. The aorta and the pancreas attached to it by the celiac trunk were immediately removed by sharp dissection, connected to the perfusion apparatus and placed in a 20 mm tube. Ischemic time was less than one min., and operative time was approximately 75 min. Successful isolation and perfusion of the pancreas was achieved in 89% (39 out of 44 rats) of the operations.
Perfusion apparatus and solution. A long tubing surrounded by a heated water jacket connected the bottle containing the perfusion solution with the pancreas inside the tube which was placed within the magnet. Perfusion was maintained by peristaltic pump (Cole Parmer), and the venous outflow was suctioned from the tube to a reservoir. The perfusion solution consisted of phosphate-free modified Krebs-Henseleit solution gassed with mixture of 95% O2 and 5% CO2 The solution and magnet were warmed to 37ºC.
Induction of pancreatitis (IOP). The bile salt, sodium taurocholate (TC, Sigma, 98% purity) was used to induce pancreatitis in two experimental models: a) intraparenchymal infiltration using 25G needle, b) injection into the common bile duct12 using a 25G plastic cannula that was inserted into it prior to removal of the pancreas. After recording control spectrum of the perfused pancreas, it was removed from the magnet and the TC was injected. This procedure took approximately two min., and NMRS recording immediately continued under the same conditions. Experiments were performed with various concentrations and volumes of the taurocholate solutions (5% - 0.5 ml, 10% - 1 ml), and at two temperatures (37°C and 22°C). For control studies 0.9% NaCL solution injections, in the appropriate volumes, were used in the two experimental methods.
Pancreatic cancer: Three models of experimental pancreatic cancer were used: 1) perfused human pancreatic carcinoma cells, MIA PaCa-2; 2) subcutaneous implanted pancreatic tumors in hamsters 3) in-situ induction of pancreatic cancer in rats by direct application of the carcinogen 7,12-dimethyl benzanthracene (DMBA).
Perfused human pancreatic cancer cells - The essentials of cellular perfusion are that metabolic events are unhampered; thus, substrates and nutrients can be continuously furnished, and waste products removed, while stable pH levels and a temperature of 37°C are maintained. Perfusion cannot be performed in cells freely suspended in the NMR tube, since the flow would wash them away, or the cells would block filters if used; therefore the cells should be restrained. In the present studies we used the method of cellular embedding in sodium alginate micro capsules6. 1.5-2 x 108 cells were used in each experiment, and the cellular pellet (1.0-1.2 ml) was mixed with an equal volume of 2.5% (w/w in PBS) sodium alginate. The mixture was manually extruded, under minimal pressure, through a 25-gauge needle, on the surface of a 0.1 M CaCl2 solution. The small drops (app. 1 mm diameter) gelled and were immediately washed three times in the growth medium. The capsules were isolated by decantation, transferred to a 10 mm screw cap NMR tube, and perfusion was promptly initiated. The perfusion was performed through an insert with inlet and outlet tubes, and the volume of the perfusion chamber was 2 ml. A constant flow of 0.9 ml/min. in a single pass mode was maintained by a peristaltic pump (Cole Parmer), and the temperature was maintained at 37°C. In each experiment control perfusion with 31P NMRS recording was carried out for about 60 min., to ensure metabolic stability of the cells, before adding the metabolic inhibitor to the perfusion solution
Implanted pancreatic tumors - Twenty four male inbred golden Syrian hamsters (8 to 12 weeks old, 100±20 gr.) were used. 0.5x106 cells of the established line of the hamsters pancreatic cancer cells7 were subcutaneously implanted in the interscapular area of 24 hamsters. Two animals died during the follow-up period. At predetermined time intervals a group of animals were operated upon, the tumors were excised without the skin and fat. Half of the tumors were used for preparation of extracts for proton (1H) NMRS, and the remaining tumors were placed in a chilled (1-2°C) isotonic (310 mM), sodium free, mannitol solution in a 10 mm NMR tube, for sodium (23Na) NMRS measurements. Controls were pancreases of three animal in each group which were excised and treated in an identical manner.
In-situ induction of pancreatic tumors - Sixty male Sprague-Dawley rats (150±30 gr.) were operated under general anesthesia, the pancreases were exposed, and 1-2 mg (crystalline) of DMBA were directly applied to the pancreases parenchyma through a superficial cut in the anterior surface8,9. The small incision was covered by the pancreatic capsule and omentum. Since we used a solid carcinogen the operations were performed in a biological hood using strict no-touch techniques. The controls were 8 animals in which physiological solutions instead of DMBA were used. The animals were kept for a period from four to fifteen months. At predetermined time intervals laparatomies and thoracotomies were performed. The pancreases, and all other tissues which appeared neoplastic, were excised, and prepared for NMRS measurements as outlined in implanted pancreatic tumors section. The pancreases of control animals were excised and examined by NMRS in an identical manner.
Preparations of tissue extracts: Since almost all intracellular compounds contain several hydrogen molecules, the NMRS of the protons (1H) in biological systems is very crowded, with overlapping signals. Extraction of cellular metabolites are often essential for the interpretation of results of NMRS studies of intact cells. The excellent resolution of extracts spectra enables assignments of proximate signals with the aid of pH titration curves, and addition of known compounds to the extract solution. In contrast to living cells, there are no time constrains, and with prolonged data accumulation, compounds present at low concentrations may be observed and quantitated. In these studies we used perchloric acid extraction10,11 for studying proton (1H) spectra of the solid tumors. Excised pancreases were divided, and one part was kept for histological examination. The other was weighed and promptly freeze-clamped and minced under liquid nitrogen using a precooled mortar and pestle. 4 ml of precooled (-10ºC) 0.5 M perchloric acid were added to every gr. of tissue, and the mixture was stirred mechanically for five min. at -4°C. Samples were centrifuged at 2000 x g and -4°C for 10 min. and the pH of the supernatant was adjusted to 6.5 with KOH in an ice water bath. Potassium perchlorate was removed by a second centrifugation at 3500 x g and -4nC for 15 min. The supernatant was lyophilized to dryness, dissolved in D2O, adjusted to pH 7.0, and kept at -20°C for less than 24 hours before the NMRS measurements were taken.
Histopathological assessment: Upon completion of each experiment the pancreas was macroscopically examined for the presence of edema, hemorrhages and necrosis (In acute pancreatitis studies) or tumors (in pancreatic cancer studies). Then, the tissues were weighed and fixed in 10% formaldehyde, embedded in paraffin, cut to 5µ thick slices and stained with hematoxylin-eosin. Seven-micron-thick longitudinal sections of the whole pancreas were stained with hematoxylin and eosin. The intensity of the pancreatic injury in acute pancreatitis was evaluated and graded, by a pathologist who was unaware of the experimental conditions and the NMRS spectrum of each pancreas.
The microscopical evaluation of the pancreatic tumors was similarly performed.
Magnetic Resonance Spectroscopy: Acute pancreatitis was studied by phosphorous (3'P) and sodium (23Na) NMRS. Pancreatic tumors studies included 31P NMRS of perfused cells and their extracts, 31P and 23Na NMRS of solid tumors, and proton (1H) measurements of extracts of solid tumors. For each nucleus the acquisition and processing parameters were identical throughout all the experiments. Spectra were recorded on a Bruker AM-360 WB spectrometer equipped with a variable frequency 10 mm probe, and were analyzed on a SGI Data Station. 31P and 23Na spectra of solid tumors were sequentially recorded, after which the tissues were weighed and processed for histological assessment. 31P spectra were measured at 145.78 MHz by applying 45º radio frequency pulses; each spectrum of the whole organ was a collection of 400 scans and a relaxation delay of 2 sec, while in the extracts the parameters were 700 scans and 10 sec. Sodium spectra were recorded at 95.26 MHz using 90° radio-frequency pulses and 200 scans with no relaxation delay. In experiments where tuning for 31P and 23Na was alternately performed, a Standard sample containing 30 mM NaCl was used as a reference. The level of sodium in the pancreas or tumor was calculated by comparing the integrated area of the 23Na peak to the integral of the reference signal. For 1H NMRS 1.0 ml of D2O dissolved extracts of normal pancreases or tumors were placed in a 5 mm tube. A five sec repetition time and a 90° flip angle were used, and 1600 transients were accumulated for each spectrum.
Quantitative results are expressed as means ± SD. Statistical analyses were performed with the paired, double-tailed, Students t test (p<0.05).
RESULTS
Acute pancreatitis: The 31P NMR spectrum of the normal perfused rat pancreas is shown in Fig. 1a. The spectrum is dominated by the signals of the high energy phosphorous compounds, phosphocreatine (PCr), and ATP. PCr is the major energy reserve compound, and is usually very high in striated and cardíac muscles. Since the ATP molecule contains three phosphorous atoms, there are three signals of ATP. ADP levels are usually very low in physiological conditions, and cannot be observed in NMR spectrum of cells and tissues, but only in spectra of extracts where the signals are better resolved. The two peaks in the phsophomonoester (PME) region at 4.2 and 3.7 ppm were assigned as phosphoethanolamine (PEA), and phosphocholine (PCh), respectively. These compounds are the precursors of the phospholipds in the cells membranes, and are usually elevated in highly proliferating tissues (i. e. tumors), and changes in their levels may be used for monitoring cell proliferation as well as the effects of nutrients and drugs on the cells. Glycerophosphocholine (GPC) is the degradative product of the phosphatidylcholine (see below). The chemical shift (location in the spectrum) of the inorganic phosphate provides the exact intracellular pH.
Perfusion rate had a profound influence on the spectra; in order to maintain cellular homeostasis and the initial levels of PCr and ATP, perfusion rate of 5±1 ml/min, was required. At these high flow rates the metabolic status of the perfused pancreas was very stable at 37ºC, and no spectral changes were noted for three hours. GPC levels under these conditions were negligible. In control studies we found that the average pancreatic weight of these rats was 1.2±0.2 gr. (in these studies we could not obtain the net weight of the pancreases before connecting to the perfusion cannula). Therefore it can be calculated that the optimal perfusion rate is 4.2±1 ml/min./gr. pancreatic weight. Very high perfusion rates, i.e. 8-12 ml/min., were characterized by unstable preparation; though the initial PCr and ATP were high, they rapidly declined. Perfusion rates of 3±1 ml/min, yielded a depletion of high energy phosphorous compounds (Fig. 1b), concomitant with increased signals of GPC and inorganic phosphate (Pi). Following complete ischemia high energy compounds completely depleted, GPC signal declined, and Pi signal markedly increased (Fig. 1c). Thus, under no circumstances both PCr and GPC levels were high.
Pancreatic reperfusion (5±I ml/min.) after complete ischemia for 15 min. was followed by recovery of PCr and ATP, concomitant with decrease of GPC and Pi signals to pre-ischemic levels (Fig. 1d). The preparation remained stable and at the end of the experiment there was no histopathological damage.
In control experiments injections of physiological solutions, either into the parenchyma or into the bile duct, were followed by no NMRS changes during three hours of perfusion; the 31P NMR. spectra were identical to those of intact perfused pancreases (Fig. 1a), and there were no macroscopically or histological damage (Fig. 2a).
3IP NMR. spectral changes of taurocholate induced acute pancreatitis were characterized by a new signal at -0.18±0.04 ppm (arrow at Fig. 3a), which appeared following TC injections to both pancreatic parenchyma and into the bile duct. The new signal was noted 10-20 min. after induction of pancreatitis, and disappeared 30-40 min. afterwards. This signal was identified as solubilized lecithin12, and was a characteristic NMRS marker of early acute pancreatitis.
31P NMRS also demonstrated depletion of the high energy phosphorous compounds following TC induced acute pancreatitis. The effects of 1 ml 10% sodium taurocholate into pancreatic parenchyma, and 0.5 ml 5% sodium taurocholate into the common bile duct (evaluated on six preparations in each model), on PCr and ATP were similar. However, The two compounds depleted at different rates at 37ºC. PCr declined very quickly, and 20-25 min. after IOP iís signal was invisible (Fig. 3a, compare to Fig. 1a). ATP decrease was much more gradual and complete depletion was observed only 150-160 min. after IOP (Fig. 3b). The time course of the depletion of PCr and ATP is shown in Fig. 4. Another feature of acute pancreatitis which was detected by 3IP NMRS was a moderate increase (43±14 % of initial level) of GPC in the early phases of the disease in both models (Fig. 3a). At the end of these experiments the TC injected pancreases were macroscopically edematous, and on histopathological examination acute pancreatitis was found (Fig. 2b). The gradual PCr and ATP depletion paralleled the extension of the pathological damage.
Induction of acute pancreatitis by intraparenchymal injections of 0.5 mi 5% TC (four pancreases) was associated with a much slower rate of high energy compounds depletion. PCr decreased to 50±6% of its initial levels 30 min. after IOP, and after three h of perfusion ATP was depleted to 40±8% of its initial value. When 1.0 ml 10% TC was used in the intraductal injections model the depletion of both PCr and ATP was very quick, and within 58 min. their signals became invisible.
One of the most frequently used models of experimental pancreatitis is intravascular infusion of fatty acids13. This model was used for studying the perfused canine pancreas, and in order to evaluate whether it is also applicable to the perfused rat pancreas we tried to add oleic acid (2% final concentration) to the perfusion solution. However, five to ten min. after the beginning of the experiment the perfusion stopped, followed by a very quick and complete depletion of PCr and ATP (spectra identical to Fig 3b). It is assumed that the high viscosity perfusion solution clogged the small-diameter tubing, or that severe endothelial damage was caused by this perfusion Therefore, the hyperlipidemic model of acute pancreatitis can not be applied to experiments using perfused rat pancreas.
Pancreatic cancer:
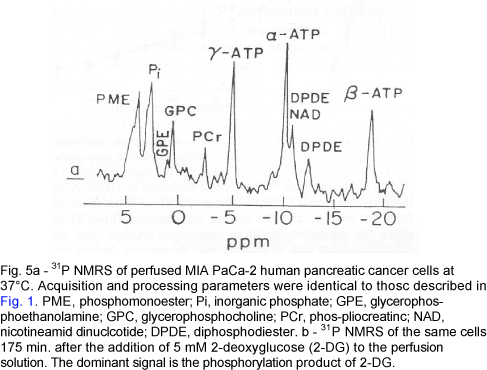
NMRS of pancreatic cancer:31P NMR spectra of perfused MIA PaCa-2 human pancreatic cancer cells are shown in Fig. 5a, and were qualitatively similar to those of intact hamsters pancreases, which were used for comparison since there is no immortal normal human pancreatic cell line. In both models of solid tumors the spectra were generally similar to those of the normal pancreas with high levels of the precursors of phospholipids. There were changes in the phospholipid metabolism compounds; however, these changes were inconsistent, and were also associated with cellular proliferation rate. This inconsistency is demonstrated by the lack of PDE signals in 31P NMRS of pancreatic tumor implanted subcutaneously in hamsters compared to the normal hamster pancreas (Fig. 6a and 6b), and by the abundance of these signals in the spectra of pancreatic tumors which were induced in-situ by DMBA. The spectra of the subcutaneous tumors did not change significantly while the tumors grew from 19 to 108 days after implantation.
Most of the sodium in tissues is extracellular and the 23Na spectra are characterized by a dominant extracellular peak (since sodium is an ion all the nuclei in the extracellular space resonate in the same chemical shift) and a very small intracellular signal, both of which were integrated to determine sodium concentrations (Table 1). Contrary to a previous hypothesis which assumed that neoplastic changes are associated with increased sodium content14,15, 23Na NMRS of both models of solid tumors showed that it was similar to normal pancreases, and total sodium content did not change during tumor growth until necrosis occurred.
Proton spectra of perchloric acid extracts of an intact pancreas and of a pancreatic tumor are shown in Fig. 7. These spectra demonstrated consistent NMRS differences between intact pancreases and pancreatic cancer, which appeared in both models of solid tumors. The principal features were elevated signals of the amino acid taurinc and of the lactate in the tumors, and the high creatinc, and also glutamine/glutamate, signals in the healthy pancreases. The concentrations of the metabolites are shown in Table 2. Taurine, lactate and creatinePCr levels in the two models of tumors were all significantly different from those of normal pancreases (p<0.05) These differences were noted as early as 19 days after tumor implantation in hamsters, and were persistent, i. e. the proton spectra after 19 and 108 days were similar. There were intermediates of the glycolytic pathway, in which lactate is the end product, but at low concentrations. They were represented by pyruvic acid at 2.48 ppm. The most abundant amino acids in the spectra, except for taurine, were alanine, glutamic acid and glycine. Valine, leucine, proline and aspartic acid appeared at low concentrations. The dominant peaks centered at 3.20 ppm represented choline, PC and PE, and the differences between healthy pancreases and tumors were not significant. The spectra of the necrotic inner parts of the overgrowing tumors revealed only very high signals of lactate (data not shown).
Histopathology: Ali the tumors which developed in the hamsters after subcutaneous cancer cell implantation were papillary ductal adenocarcinoma. After 2 weeks the size of the nodules was app. 0.5 cm, and after a month their diameter was app. 1.5 cm. The tumoral mass seemed to be encapsulated by connective tissues, and most of the blood supply was from vessels which originated from the thoracic wall. The super mature tumors with no macroscopic dimension increase, skin and thoracic wall invasion, togcther with central degeneration, were observed, and the amount of necrosis correlated with the size of the tumors.
Two of the rats in the DMBA model died in the immediate post-operative period, and 3 rats died during the ensuing 15 months. In all pancreases there were marked adhesions to neighbouring organs, fibrosis and foreign body granuloma. However, malignant changes were observed only after 10 months, and after 15 months 70% of the animals had tumors. Macroscopically most tumors were hard gray-white nodules of 2-4 cm in diameter, and in some animals there was hemorrhagic ascites. The pancreatic tumors were papillary adenocarcinoma, some of them with cyst formation. Most tumors were of acinar rather than of ductal origin, and in almost all pancreases areas of ductal hyperplasia were found after 12 months. Two of the tumors which were detected after 12 months, and 6 of the tumors found after 15 months, reached large sizes of 5 to 8 cm and invaded surrounding organs, the diaphragm and the abdominal wall, and had inner necrotic areas. In five of the adenocarcinoma detected after 15 months there were distant metastases; 2 in the liver, 2 in the lungs and one in the right thigh. Four of the tumors were of mesenchymal origin; two of them were in the laparatomy incision, one was between the pancreas and the spleen, and one was in the lung. In the control group neither tumors, nor inflammatory or reactive changes, were noted after 12 months.
Effects of metabolic inhibitors:31P NMRS was used for monitoring the effects of two metabolic inhibitors on perfused MIA PaCa-2 human pancreatic cancer cells. 2-deoxy-glucose is a glycolysis inhibitor which causes cell starvation and death16. Following the addition of 5 mM 2-DG to the perfusion solution there was a gradual accumulation of its phosphorylation product 2-DG-6P, concomitant with a decrease in the levels of the high energy compounds, PCr and ATP (Fig. 5b). When lonidamine, another metabolic inhibitor17, was added to the perfusion solution, pH decreased to 6.76±0.12. This finding was in accordance with our previous data that the mechanism of action of LND is through intracellular acidification due to inhibition of extracellular lactate transport18.
DISCUSSION
Nuclear magnetic resonance has been discovered some fifty years ago, and NMRS has become one of the most important analytical methods, which is extensively used in chemical and pharmaceutical industry, as well as in academic research. In the last two decades magnetic resonance techniques has evolved as an important method in bio-medicine (1-3). The revolutionary contribution of magnetic resonance imaging (MRI) to clinical diagnosis is well recognized. Perhaps less well appreciated is the potential of NMRS as a research tool to study physiology, metabolism and diseases, The main advantage of NMRS in this respect is that it can provide biochemical Information on intracellular processes in a non-invasive manner, and these processes can be continuously monitored as they occur.
Several nuclei can be studied and, thus, the data that can be obtained from a single experiment is enormously increased. The choice of an element depends on its NMRS properties, and the required data. The most widely used nucleus in NMRS studies of metabolism has been 3IP. 31P NMRS furnishes data on cellular energetics, precise intracellular pH, phospholipid pathways, and membrane permeability and ion and water distribution. The spectrum is easy to interpret, but the number of compounds which are detectable is limited. Proton has the highest NMRS sensitivity, and is the most abundant nucleus in biological molecules. However, this may cause difficulties in interpretation and assignment of the 1H NMRS spectrum. Since metabolic studies are usually performed in aqueous solutions the huge signal from the water protons should be suppressed. Similarly, the wide signals arising from proteins and membrane components should be suppressed. These problems can be addressed now by several NMRS innovative methods. Carbon NMRS is also useful since it is found in most biological compounds; however, I3C has a natural abundance of only 1.1% (the most abundant 12C isotope has no magnetic resonance properties), and 13C enrichment is mandatory. Other nuclei which are used less often in NMRS studies of biological systems are 23Na, 19F, and rarely 15N and 39K.
Bio-medicine NMRS studies can be performed with cells, intact and perfused tissues and organs, and also in-vivo NMRS, a technique which is termed localized spectroscopy, and is use now also in the clinical setting19. NMRS studies of cells include cellular extracts, cell suspensions, and perfused intact cells. Perfused ceils represent perhaps the best approach to the non-invasive study of metabolism. In contrast to the in-vivo situation, these cells are homogenous, and there are no "artifact" data from connective tissues and blood vessels. The cells are metabolically stable for prolonged periods during perfusion under physiological conditions. Thus, the effects on metabolism following manipulation with nutrients, drugs, hormones and growth factors can be monitored20,22.
In this presentation the applications of NMRS for studies of two of the most important "surgical" disorders of the pancreas, acute pancreatitis and pancreatic cancer, were demonstrated. Experiments with perfused pancreas offer the advantage of continuous monitoring of the endocrine, as well as exocrine, functions of the pancreas23. In order to avoid artifact NMRS signals from neighboring tissues and organs only the pancreas should be perfused. Ensuring adequate pancreatic perfusion is essential, and it may be difficult considering the complex anatomy and blood supply of the pancreas and accompanying duodenum. We found that the metabolic status of the isolated rat pancreas can be continuously monitored by 31P NMRS. Determining the optimal perfusion rates is essential for sound physiological studies. We found that the optimal metabolic status of the pancreas was maintained with perfusion rate of 4.2±1 mlmin. gr. pancreatic weight at 37°C. Several reports described models of perfused pancreas for endocrinological studies employing lower perfusion rates24,25 , but the metabolic status of the pancreas was not evaluated.
Acute pancreatitis is characterized by a broad pathological spectrum, and the correlation between the clinical presentation and the pathological damage is ill-defíne26,27. There are no unambiguous quantitative parameters to assess the severity and prognosis. Such an assessment is essential for choosing the optimal therapeutic management and for studying the efficacy of the treatment. NMRS revealed a characteristic 31P NMRS signal in the phosphodiester region at -0.18±0.04 ppm, which was diagnosed as solubilized lecithin. This signal could be detected shortly after induction of acute pancreatitis and later on it disappeared. Lecithin is most abundant in membranes, but can not be detected there by 31P NMRS. After solubilization of the membranes phospholipid moiety by a detergent (for example TC) lecithin becomes NMRS visible. Our findings support the theory that the initial step of bile salts pancreatitis is detergent induced membranous damage26. Lysolecithin, which has a direct toxic effect on pancreatic cells, is then formed, and the disease progresses. Solubilized lecithin signal can therefore be used as an indicator of the early phases of the disease, and with the advent and improvement of localized NMRS perhaps it could be applicable in humans.
The incidence of adenocarcinoma of the pancreas has steadily increased over the last several decades, and it is now the fourth most common cause of cancer deaths28,29. Accurate and reliable diagnostic means are essential to improve the outcome of the patients. Imaging diagnostic modalities are based on spatial resolution of neoplastic tissues from their normal counterparts, and since a certain size of tumor is required for its detection, their usefulness for early cancer diagnosis is somehow limited. The genetic events which are associated with carcinogenesis are often accompanied by metabolic alterations. A technique which may discover biochemical features associated with the malignant changes may be an important tool for cancer research.
During the last two decades there has been ongoing NMRS research in malignant diseases. These studies provided valuable data on the biochemistry and metabolism of tumors, and on the effects of nutrients, hormones and growth factors30,32. The mechanisms of action of anti-cancer drugs and the acquired resistance to these agents were delineated". NMRS was used also for monitoring the response to therapy34,35. Numerous studies have focused on the value of NMRS in cancer detection36,39, but its efficacy as a diagnostic tool is still controversial. Size, location and especially heterogeneity of tumors are major obstacles for clinical interpretations of experimental findings. Also, previous attempts to define characteristic and diagnostic NMRS features in the plasma of cancer patients were found to be futile40,41.
In order to assess the potential role of NMRS in the diagnosis and treatment of pancreatic cancer, and to address the problem of diversity and heterogeneity of tumors, we performed multinuclear NMRS in three different models of pancreatic cancer. 31P NMRS studies showed that there were no consistent spectral features of pancreatic neoplasms, and there were no characteristic signals of the malignant processes. Only in the model of subcutaneous implantation of human pancreatic cancer cells in hamsters was there a qualitative difference between normal pancreases and tumors, namely the absence of PDE signals in spectra of the latter. It seems that the main role of 3lP NMRS in pancreatic cancer research will be in monitoring the effects of anti-cancer agents, and in the development of new therapeutical modalities, as was also shown in our studies with 2-DG.
Increased sodium concentrations are associated with several pathological processes, especially when there is remarkable tissue edema. Previously it was hypothesized that lowered transmembrane potential, leading to reduced activity of the sodium pump and elevation of intracellular sodium, would initiate and sustain the mitogenic process14-15. Our data do not support this theory. It is noteworthy though that most sodium in tissues is in the extracellular compartment, and major ion shifts are required for the above mentioned extreme changes. Also, there are often areas of edema in and around tumors that may be responsible for some of the reported elevations in sodium levels.
Proton has the highest magnetic resonance sensitivity, and is the most abundant nucleus in biological molecules. Therefore 1H NMRS bears the greatest potential for the detection of biochemical properties of cancer, and oncological proton NMRS has attracted a lot of interest in recent years42,43. High resolution NMRS of extracts, with unambiguously assigned signals, provides the means for pursuing such biochemical markers. Indeed, our 1H NMRS studies demonstrated that pancreatic malignant transformation is characterized by high levels of the amimo acid taurine. Taurine is the end product of methionine and cystine, and is unique among other amino acids in that it is a free amino acid in the cytosol, and is not a constituent of a protein. It plays a role in the absorption of fats, in membranes protection, possibly through detoxification, antioxidation and osmotic regulation mechanisms, and also as a neurotransmitter in the central nervous system46,47. An association between taurine and neoplastic diseases has been described in the past48. Learning the mechanisms of these changes may provide important data for basic cancer research. In case these high resolution features will be likewise detected by localized 1H NMRS, taurine may as well serve as a clinical diagnostic marker.
CONCLUSION
This paper presents the basic concepts of NMRS, and demonstrates its potential for studying acute pancreatic and pancreatic cancer. In acute pancreatitis a characteristic signal in the phosphorous (31P) spectra was found that may be used as a marker of the early phases of the disease. Gradual depletion of the high energy compounds paralleled the extension of the pathological lesions. Proton (1H) NMRS of pancreatic cancer revealed that the amino acid taurine was elevated compared to the normal pancreas. 31P spectra provided data on the mechanism of action of treatment with anti-metabolism agent.
Accepted for publication december, 1996.
Abbreviation used:
- 1. KAPLAN, O. & COHEN, J.S. - Metabolism of breast cancer cells as revealed by non-invasive magnetic resonance spectroscopy studies. Breast Cancer Res. Treat., 31: 285-299, 1994.
- 2. DALY, P. F. & COHEN, J.S. - Magnetic resonance spectroscopy of tumors and potential in vivo applications. Cancer Res., 49: 770-779, 1989.
- 3. KAPLAN, O.; VAN Zijl, P.C.M.; COHEN, J. S. - NMR spectroscopy of metabolism in cells and perfused organs. In: STUMPE, R. & SEELIG, S.(eds.), - In Vivo Magnetic Resonance Spectroscopy. Berlin, Springer, 1992. 28, p. 3-54.
- 4. GADIAN, D.G. - Nuclear Magnetic Resonance and its Application to Living Systems, New York, Oxford University Press, 1982. p. 1-170.
- 5. COHEN, S. J.; KNOP, R.H.; NAVON, G.; FOXALL, D. - Nuclear magnetic resonance in biology and medicine. Life Chem. Rep., 1: 368, 1983.
- 6. SHANKR-NARAYAN, K.; MORESS, E.; CHATAM, J.C.; BARKER, P.B. - 31P NMR of mammalian cells encapsulated in alginate gels utilizing a ncw phos-phate-free perfusion medium. NMR in Biomed., 3: 23-26, 1990.
- 7. S1NDELAR, W.F. & KURMAN, C.C. - Nitrosamine-induced pancreatic carcinogenesis in outbred and inbred Syrian hamsters. Carcinogenesis, 3: 1021-1026, 1982.
- 8. DISSIN, J.; MILLS, L.R.; MAINS, D.L., BLACK, O.; WEBSTER, P.D. - Experimental induction of pancreatic adenocarcinoma in rats. J. Natl. Cancer Inst., 55: 857-864, 1975.
- 9. SATAKA, K.; MUKAI, R.; KATO, Y.; SHIM, K.; UMEYAMA, K. - Experimental pancreatic carcinoma as a model of human pancreatic carcinoma. Clin. Oncol, 10: 27-34, 1984.
- 10. LOWRY, O.H.. & PASSONNEAU, J.V.A. - A Flexible System of Enzymatic Analysis, New-York, Academic Press, 1972. p. 12-124.
- 11. ASKENAZY, N.; KUSHNIR, T.; NAVON, G.; KAPLAN, O. - Differences in metabolite content between intact pancreases and their perchloric acid extracts. A 2D 1H/31P correlation NMR study. NMR in Biomed., 3: 220-225, 1990.
- 12. KUSHNIR, T.; KAPLAN, O.; ASKENAZY, N.; NAVON, G. - Identification of a characteristic P-3 1 NMR signal of acute pancreatitis with the aid of 1H/3 IP correlated 2D measurements of intact pancreas. Magn. Reson. Med., 10: 119-124, 1989.
- 13. NORDBACK, I.H.; CLEMENS, J.A.; CHAKO, V.P.; OLSON, J.L.; CAMERON J.L. - Changes in high-energy phosphate metabolism and celi morphology in four models of acute experimental pancreatitis. Ann. Surg., 213: 341-349, 1991.
- 14. CONE, CD. Jr. - EIectroosmotic interactions accompanying mitosis initiation in sarcoma cells in vitro. Trans. N. Y. Acad. Sei, 31: 404-427, 1969.
- 15. CAMERON, I.L.; SMITH, N.K.R.; POOL, T.B.; SPARKS, R.L. - Intracellular concentration of sodium and other elements as related to mitogenesis and oncogenesis in vivo. Cancer Res., 40: 1493-1500, 1980.
- 16. DEMETRAKOPOULOS, G.E.; L1NN, B. AMOS, H. - Starvation, deoxysugars, ouabain, and ATP metabolism in normal and malignant cells. Biochim, Biophys. Acta, 6: 65-74, 1982.
- 17. PUTZOLU, S. & TADDEI, I. - New perspectives in anticancer therapy: a review on lonidamine. Drugs of Today 25: 195-209, 1989.
- 18. BEN-HORIN, H.; VIVI, M.; VIVI, A.; NAVON, G.; KAPLAN, O. - Mechanism of action of the anti-neoplastic agent lonidamine - 31P and 13C NMR studies. Cancer Res., 55: 2814-2821, 1995.
- 19. REMY, C; ARUS, C; ZIEDLER, A.; LAI, E.S.; MORENO, A.; LE-FUR, Y.; DECORPS, M. - In vivo, ex vivo, and in vitro one-and two-dimensional nuclear magnetic resonance spectroscopy of an intracerebral glioma in rat brain: assignment of resonances. J. Neurochem., 62: 166-179, 1994.
- 20. NEEMAN, M. & DEGANI, H. - Metabolic studies of estrogen and tamoxifen treated human breast cancer cells by nuclear magnetic resonance spectroscopy. Cancer Res., 49: 589-594, 1989.
- 21. GALONS, J.P.; FANTINI, J.; VION-DURY, J.; COZZONE, P.J.; CANIONI, P. - Effect of VIP on the glycogen metabolism of human colon adenocarcinoma cells studied by 13C nuclear magnetic resonance spectroscopy. Int. J. Cancer, 45: 168-173, 1990.
- 22. KAPLAN, O.; JAROSZEWSKI, J.; FAUSTINO, P,J.; ZUGMAIER, G.; ENNIS, B.W.; LIPPMAN, M.C.; COHEN, J.S. - Toxicity and effects of epidermal growth factor on glucose metabolism of MDA-468 human breast cancer cells. .. Biol. Chem., 265: 13641-13649, 1990.
- 23. LENZEN, S. - Insulin secretion by isolated perfused rat and mouse pancreas. Am. J. Physioi, 236: E391-E400, 1979.
- 24. PEGG, D.E.; KLEMPNAUER, J.; DIAPER, M.P.; TAY- LOR, M.J. - Assessment of hypothermic preservation of the pancreas in the rat by a normothermic perfusion assay. .. Surg. Res., 33: 194-200, 1982.
- 25. REAVEN, E.; CURRY, D.; MOORE, J.; REAVEN, G. - Effect of age and environmental factors on insulin release from the perfused pancreas of the rat... Clin. Invest, 71: 345-350, 1983.
- 26. LONGNECKER, O.S. -. Pathology and pathogenesis of discases of the pancreas. Am. J. Pathol, 107: 103-126, 1982.
- 27. STEER, M.L. - Workshop on experimental pancreatitis. Dig. Dis. Sci, 30: 575-580, 1985.
- 28. AMERICAN CANCER SOCIETY. Cancer facts and figures 1991. Atlanta: American Cancer Society, 1991.
- 29. WARSKAW, A.L. & CASTILLO, CF. - Pancreatic carcinoma. N. Engl. J. Med., 326: 455-465, 1992.
- 30. DALY, P.F. & COHEN, J.S. - Magnetic resonance spectroscopy of tumors and its potential in vivo applications: a review. Cancer Res., 49: 110-119. 1989.
- 31. KAPLAN, O. & COHEN, J.S. - Metabolism of breast cancer cells as revealed by non-invasive magnetic resonance spectroscopy studies. Breast Cancer Res. Treat., 31: 285-299, 1994.
- 32. NEEMAN, M. & DEGANI, H. - Early estrogen induced metabolic changes and their inhibition by actinomycin D and cyeloheximide in human breast cancer cell: 31P and 13C NMR studies. Proc. Natl. Acad. Sci., USA 86: 5585-5589, 1989.
- 33. KAPLAN, O.; NANON, G.; LYON, R.C.; FAUSTINO, P.J.; STRAKA, E.J.; COHEN, J. S. - Effects of 2-deoxyglucose on dmg- sensitive and drug- resistant breast cancer cells: Toxicity and magnetic resonance spectroscopy studies of metabolism. Cancer Res., 50: 544-551, 1990.
- 34. GLAHOLM1, J.; LEACH, M.O.; COLL1NS, D.J.; MANSI, I; SHARP, J.C.; MADDEN, A.; SMITH, I.E.; MCCREADY, V.R. - In vivo 31P magnetic resonance spectroscopy for monitoring treatment response in breast cancer. Lancet, 1: 1326-1327, 1989.
- 35. NG, T.C.; MAJORS, A.W.; VIJAYKUMAR, S.; BALDW1N, N.J.; KARAL1S, I.; MEANEY, T.F.; SHIN, K.H.; THOMAS, F.J. - Therapeutic response of breast carcinoma monitored by 31P MRS in situ. Magn. Reson. Med, 10: 125-134, 1989.
- 36. MERCHANT, T.E.; GIERKE, L.W.; MENESES, P.; GLONEK, T. - 3 IP magnetic resonance spectroscopy profiles of neoplastic human breast tissues. Cancer Res., 48: 5112-5118, 1988.
- 37. Negendank, W. G., Crowly, M. G., Ryan, J. P., Keller, N. A., and Evelhoch, J. L. - Bone and soft tissue lesions: diagnosis with combined H-I MR imaging andP-31 MR spectroscopy. Radiology, 173: 181-188, 1989.
- 38. SU, B., KAPPLER, F., SZWERGOLD, B.S., BROWN, T.R. - Identification of a putative tumor marker in breast and colon cancer. Cancer Res., 53: 1751-1754, 1993.
- 39. MOUNTFORD, CE., & TATTERSALL, M.H.N. - Proton magnetic resonance spectroscopy and tumour detection. Cancer Surv., 6: 285-314, 1987.
- 40. FOSSEL, E.T.; CARR, J.M.; McDONAGH, J. - Detection of malignant tumors. Water-suppressed proton nuclear magnetic resonance speetroscopy of plasma. N. Engl. J. Med, 315: 1369-1376, 1986.
- 41. SHULMAN, R.G. - NMR - another cancer-test disappointment. N, Engl. J. Med., 322: 1002-1003, 1990.
- 42. MURAT, C; ESCLASSAN, J.; DAUMAS, M.; LEVRAT, J.H.; PALEVODY, C; VTNCESNSINI, P.D.; HOLLANDE, E. - Enhanced membrane phospholipid metabolism in human pancreatic adenocarcinoma cell lines detected by low-resolution 1H NMR speetroscopy. Pancreas, 4: 145-152, 1989.
- 43. SMIT1I, I.C.P. & CHMURNY, G.N. - NMR spectroscopy and research: thc present and the future. An. Approach, 62(15): 853A, 1990.
- 44. MOUNTFORS, CE.; HOLMES, K.T.; MACINNON, W.B.; WRIGHT, L.C. Tumour development and evolution as monitored by 1H MRS. In: DE CERTAINES, J. D. Magnetic Resonance Spectroscopy of Biofluids - A New Tool in Clinical Biology, London, World Scientific Publishing Co. Pte Ltd, 1989. p. 103-118.
- 45. LEAN, CL.; MACKINNON, W.B.; MOUNTFORD, CE. Fucose in 1H COSY spectra of plasma membrane fragments shed from human colorectal cells. Magn. Reson. Med., 20: 306-311, 1991.
- 46. GEGGEL, H.S.; AMENT, M.E.; HECKENLIVELY, J.R.; MARTIN, D.A.; KOPPLE, J.D. - Nutritional requirement for taurine in patients receiving long-term parenteral nutrition. N. Engl. J. Med., 312: 142-146, 1985.
- 47. WRIGHT, CE.; TALLAN, H.H.; YONG, Y.L. - Taurine: biological update. Ann. Rev. Biochim., 55: 427-453, 1986.
- 48. ROBERTS, E. & FRANKEL, S. - Free amino acids in normal and neoplastic tissues of mice as studied by paper chromatograohy. Cancer Res., 9: 645-648, 1949
Publication Dates
-
Publication in this collection
01 Dec 2010 -
Date of issue
Mar 1997