Abstracts
The effects of chronic alcohol consumption during pregnancy were analysed in the gestation and offspring of alcoholized mice. Female C57BL/6J mice were placed overnight with stud males and the presence of a sperm plug in the next morning indicated the onset of gestation. Pregnant mice were distributed in two weight-matched groups. In the alcoholized group, the mice received a high protein liquid diet ad libitum containing 27.5% of ethanol-derived calories (5.28% v/v) from gestation day 5 to 19. The control group received the same volume of diet containing isocaloric amounts of maltose-dextrin substituted for ethanol. After postnatal day zero, the dams received food pellets and tap water ad libitum. On postnatal day 6 the pups were counted and weighed at variable intervals up to the 60th day of life. The majority of the pregnant dams that have received ethanol completed the gestational period, and the chronic consumption of alcohol did not interfere with the number of dams that gave birth. The alcoholized and control dams gained an equivalent weight and consumed an equivalent volume of diet throughout the gestation. The number of pups from alcohol diet dams was 46,26% smaller compared with the control group. There were less male than female pups in the offspring of alcoholized mice. Teratogeny like gastroschisis and limb malformation were present in the offspring of alcoholized dams. The body weight of the offspring of alcoholized mice increased from the 18th to the 36th postnatal day.
Ethanol; Mice; Fetal alcohol syndrome; Teratogens
Investigou-se os efeitos do consumo crônico de álcool na prole de camundongas alcoolizadas na prenhez. Camundongas da espécie C57BL/6J foram acasaladas. A presença de tampão vaginal de esperma indicava o início da prenhez. As camundongas prenhes foram distribuidas em 2 grupos: no grupo experimento, as camundongas receberam dieta líquida rica em proteinas, ad libitum, contendo 27,5% (5,28% v/v) de suas calorias derivadas do etanol, do 5º ao 19º dia de prenhez. O grupo controle recebeu o mesmo volume de dieta, contendo quantidades isocalóricas de dextrino-maltose em substituição ao etanol. Ao término do período de prenhez e pós-natal as mães receberam ração e água. Os filhotes foram pesados e contados em intervalos variáveis do 6º ao 60º dia de vida pós-natal. As mães alcoolizadas e as controles ganharam peso equivalente, e consumiram volumes equivalentes de dieta durante a prenhez. A maioria das camundongas do grupo experimento completou o período de prenhez, e o consumo crônico de álcool não alterou o número de animais prenhes que efetivamente chegaram a dar à luz. O número de filhotes do grupo de camundongas alcoolizadas foi significantemente menor que o grupo controle. O número reduzido de filhotes foi mais pronunciado entre os machos, além de ocorrer natimortalidade e teratogenia como gastrosquise e malformações de membros. A massa corpórea das proles das camundongas do grupo álcool etílico aumentou do 18º ao 36º dia de vida pós-natal.
Etanol; Camundongos; Síndrome alcoólica fetal; Teratogênios
Effects of ethanol on offspring of C57BL/6J mice alcoholized during gestation1 1 Summary of Master thesis approved at the Postgraduate Course in Perinatology of Teaching and Research Institute, Albert Einstein Hospital, São Paulo, SP. 2 M. D., Research Fellow of Department of Anatomy, Institute of Biomedical Sciences, University of São Paulo; Neonatologist, Master in Perinatology of Albert Einstein Hospital 3 Full Professor of Experimental Surgery, Federal University of São Paulo; General Coordinator of Post Graduate Courses, Teaching and Research Institute, Albert Einstein Hospital 4 Director of Neonatal Unit; Coordinator of Post Graduate Course in Perinatology, Teaching and Research Institute, Albert Einstein Hospital 5 Full Professor of Anatomy, Department of Anatomy, Biomedical Sciences Institute, University of São Paulo
Hermann Grinfeld2 1 Summary of Master thesis approved at the Postgraduate Course in Perinatology of Teaching and Research Institute, Albert Einstein Hospital, São Paulo, SP. 2 M. D., Research Fellow of Department of Anatomy, Institute of Biomedical Sciences, University of São Paulo; Neonatologist, Master in Perinatology of Albert Einstein Hospital 3 Full Professor of Experimental Surgery, Federal University of São Paulo; General Coordinator of Post Graduate Courses, Teaching and Research Institute, Albert Einstein Hospital 4 Director of Neonatal Unit; Coordinator of Post Graduate Course in Perinatology, Teaching and Research Institute, Albert Einstein Hospital 5 Full Professor of Anatomy, Department of Anatomy, Biomedical Sciences Institute, University of São Paulo
Saul Goldenberg3 1 Summary of Master thesis approved at the Postgraduate Course in Perinatology of Teaching and Research Institute, Albert Einstein Hospital, São Paulo, SP. 2 M. D., Research Fellow of Department of Anatomy, Institute of Biomedical Sciences, University of São Paulo; Neonatologist, Master in Perinatology of Albert Einstein Hospital 3 Full Professor of Experimental Surgery, Federal University of São Paulo; General Coordinator of Post Graduate Courses, Teaching and Research Institute, Albert Einstein Hospital 4 Director of Neonatal Unit; Coordinator of Post Graduate Course in Perinatology, Teaching and Research Institute, Albert Einstein Hospital 5 Full Professor of Anatomy, Department of Anatomy, Biomedical Sciences Institute, University of São Paulo
Conceição Aparecida de Mattos Segre4 1 Summary of Master thesis approved at the Postgraduate Course in Perinatology of Teaching and Research Institute, Albert Einstein Hospital, São Paulo, SP. 2 M. D., Research Fellow of Department of Anatomy, Institute of Biomedical Sciences, University of São Paulo; Neonatologist, Master in Perinatology of Albert Einstein Hospital 3 Full Professor of Experimental Surgery, Federal University of São Paulo; General Coordinator of Post Graduate Courses, Teaching and Research Institute, Albert Einstein Hospital 4 Director of Neonatal Unit; Coordinator of Post Graduate Course in Perinatology, Teaching and Research Institute, Albert Einstein Hospital 5 Full Professor of Anatomy, Department of Anatomy, Biomedical Sciences Institute, University of São Paulo
Gerson Chadi5 1 Summary of Master thesis approved at the Postgraduate Course in Perinatology of Teaching and Research Institute, Albert Einstein Hospital, São Paulo, SP. 2 M. D., Research Fellow of Department of Anatomy, Institute of Biomedical Sciences, University of São Paulo; Neonatologist, Master in Perinatology of Albert Einstein Hospital 3 Full Professor of Experimental Surgery, Federal University of São Paulo; General Coordinator of Post Graduate Courses, Teaching and Research Institute, Albert Einstein Hospital 4 Director of Neonatal Unit; Coordinator of Post Graduate Course in Perinatology, Teaching and Research Institute, Albert Einstein Hospital 5 Full Professor of Anatomy, Department of Anatomy, Biomedical Sciences Institute, University of São Paulo
Grinfeld H, Goldenberg S, Segre CAM, Chadi G. Effects of ethanol on offspring of C57BL/6J mice alcoholized during gestation. Acta Cir Bras [serial online] 1999 Jul-Sept;14(3). Available from: URL: http://www.scielo.br/acb .
SUMMARY: The effects of chronic alcohol consumption during pregnancy were analysed in the gestation and offspring of alcoholized mice. Female C57BL/6J mice were placed overnight with stud males and the presence of a sperm plug in the next morning indicated the onset of gestation. Pregnant mice were distributed in two weight-matched groups. In the alcoholized group, the mice received a high protein liquid diet ad libitum containing 27.5% of ethanol-derived calories (5.28% v/v) from gestation day 5 to 19. The control group received the same volume of diet containing isocaloric amounts of maltose-dextrin substituted for ethanol. After postnatal day zero, the dams received food pellets and tap water ad libitum. On postnatal day 6 the pups were counted and weighed at variable intervals up to the 60th day of life. The majority of the pregnant dams that have received ethanol completed the gestational period, and the chronic consumption of alcohol did not interfere with the number of dams that gave birth. The alcoholized and control dams gained an equivalent weight and consumed an equivalent volume of diet throughout the gestation. The number of pups from alcohol diet dams was 46,26% smaller compared with the control group. There were less male than female pups in the offspring of alcoholized mice. Teratogeny like gastroschisis and limb malformation were present in the offspring of alcoholized dams. The body weight of the offspring of alcoholized mice increased from the 18th to the 36th postnatal day.
SUBJECT HEADINGS: Ethanol. Mice. Fetal alcohol syndrome. Teratogens.
INTRODUCTION
Prenatal exposure to ethanol has been associated with a variety of abnormalities in the fetus and in offspring: prenatal death, growth retardation, malformations and behavioral deficits. In 1968 Lemoine and co-workers described for the first time the teratogenic effects of alcohol in children of alcoholized mothers, while Jones and Smith, in 1973, identified the fetal alcohol syndrome (FAS). Since then researchers were eager to develop an animal model in order to reproduce the teratogenic abnormalities described.
Becker and co-workers identified, in 1994, the periods of gestation of mice when exposure to ethanol was more deleterious to fetal development, describing the biochemical changes observed in pregnant mice and their fetuses. Wainwright and co-workers, in 1996, described the reduction of the number of pups of the B6D2F mice when administering liquid diet with 27,5 % of calories derived from ethanol during the pregnancy of these animals.
The critical period of prenatal exposure to ethanol in animals is variable. Therefore, if an organ or tissue has its embrionic development in the highest dynamics during this exposure, the chances of ocurrence of teratogenic defects can increase. As a consequence, excessive and chronic consumption of alcohol during pregnancy may cause growth retardation, structural defects in the majority of organs, and neurologic impairment11.
The mechanisms of the teratogenic action due to ethanol in the embrionic phase of fetal development is not yet well clarified, and this issue has been currently subject of intense investigation27.
Randall and co-workers (1991) administered one single dose of ethanol, via gavage, in C57BL/6J pregnant mice on the 10th day of pregnancy. Middaugh and Boggan, in 1991, gave ethanol via a liquid diet ad libitum to pregnant mice from the 5th to the 17th day of pregnancy, and showed that teratogenic defects in central nervous, locomotor, cardiovascular and urinary systems of the offspring do not depend on the way and time of ethanol administration but they are closely influenced by the concentration of administered ethanol.
METHOD
Pathogen free female C57BL6J isogenic mice 10 weeks of age (20-25 g) obtained from the Central Animal Colony of the Biomedical Sciences Institute (University of São Paulo) were used in this study. For breeding, each nulliparous female mouse was caged with an individual stud male in the evening. The following morning, females were examined for vaginal plugs. The presence of a plug was considered indicative of conception and that day was designated as embryonic day (E) 0. Pregnant females were divided into two weight-matched groups: an ethanol consuming dams (n=16) which were given liquid diet ad libitum containing 27.5% ethanol-derived calories (5,28% v/v; alcohol diet; BioServ, N.J.) and pair-fed dams (n=12) which received the same volume of liquid diet containing isocaloric amounts of maltose-dextrin substituted for ethanol (control diet; BioServ, N.J.). Thus, four female mice of the control diet group had two pair-fed dams of the ethanol-diet group.
Administration of the diet began on E5 and continued until E19. The ethanol was gradually introduced as follows: a mixture of 1/3 ethanol and 2/3 control diets in the first day, 2/3 ethanol and 1/3 control diets in the second day and 3/3 ethanol diet in the third day. On E18 the ethanol was inversely withdrawn as introduced with the onset of gestation. Diets were replenished between 8:00 and 9:00 a.m. with 30 to 50 ml of diet, everyday. All dams were housed individually under constant conditions of temperature, humidity and lighting (12 h light-dark cycle). Pair-fed dams were fed the same volume of liquid diet as that consumed ad libitum by the weight-matched ethanol-fed dams on the respective embryonic day.
The volume of the liquid diet consumed by each mouse from both groups was recorded daily, and the body weight of the dams was verified every second day. The length of gestation was carefully recorded in both groups.
The neonates were examined and their number counted on postnatal day 6 (P6). The neonates were not manipulated before P6 to avoid maternal aggression against them, which could interfere with the number of live offspring. The body weight of the offspring was regularly recorded from P6 to P60.
Statistical analysis:
The study of the results was performed on the Statview software (version 4.0) of MacIntosh, at the Laboratory of Neurotrophic Factors and Neuronal Plasticity, Department of Anatomy, Biomedical Sciences Institute, University of São Paulo. According to the nature of the variables, we used the t Test, parametric, to compare the variations in number of offspring in mice of control and alcohol groups. In all tests we used the level of 5% (alfa=0,05) to reject the hypothesis of null, so that values of p<0,05 were considered significant.
RESULTS
The consumption of hiperproteic liquid diet by all mice utilized in the present study (control and ethanol groups) is summarized in Figure 1. We observed that the amount of diet offered for both groups was very similar during the whole pregnancy (Fig. 1A). However, the consumption of diet by the non-pregnant mice was uneven in all periods of pregnancy, remarkably on the final third (Fig. 1B). At this point, the alcoholized group consumed lesser amounts of diet than the control group. On the 17th day of pregnancy (E17) the non-pregnant alcoholized dams consumed 21,19% less diet than the control group of dams (Fig. 1B). Consumption of diet with or without ethanol by the pregnant mice was very similar throughout gestation (Fig. 1A.; 1C.) . On the 19th day of pregnancy, control group mice consumed 8,18% more diet than on the 6th day, and ethanol group mice consumed 13,78% more diet than on the 6th day. However, no difference was observed in the volume of liquid diet consumed by both groups.
. Figure shows the consumption of the hiperproteic protein liquid diet by pregnant mice of control and alcohol groups. The vaginal plug was observed in all dams. The consumption was daily recorded from embryonic day (E6) to E19. The consumption of all pregnant dams of the experiment by control (control diet) and alcohol (alcohol diet) groups is shown in Fig. A. The consumption by the dams that did not show any offspring (non-pregnant dams) and those that have delivered at least one neonate (pregnant dams) are shown in Fig. B and C, respectively. *p<0,005, according to t test.
The body weight of the dams from control and ethanol groups is shown in Figure 2. We have not observed any difference in the body weight of control and alcohol diet groups throughout gestation (Fig. 2A). The weight gain of the alcoholized and control non-pregnant mice was 25,56% and 15,73% respectively, from 5th to 17th day of pregnancy. On the other hand, this weight gain by the alcoholized and control pregnant dams was 31,71% and 34,21%, respectively (Fig. 2A-C).
Figure shows the weight gain of pregnant mice from control and alcohol groups. The vaginal plug was observed in all dams. The numbers represent the mean ± s.e.m. of the body weight recorded every second day, from embryonic day 5 (E5) to E17. The weights of all pregnant dams from both control (control diet) and alcohol (alcohol diet) groups is shown in Fig. A (n = 3 - 12). The weights of the dams that did not show any offspring (non-pregnant dams) and by those who have delivered at least one neonate (pregnant dams) are shown in Fig. B and C, respectively.
The average of days of pregnancy of the dams of both groups who gave birth was 19,22 days and 19,18 days, respectively (Fig. 3). The consumption of ethanol by pregnant dams did not interfere the effectiveness of pregnancy (Fig. 4). Therefore, 75% of the pregnant mice of both groups went to term, giving birth to one live pup at least, while 25% of the dams of both groups did not present any offspring at term (Fig. 4).
Figure shows the average and s.e.m. of days of gestation of pregnant dams from control and alcohol groups. n = 9 11.
Figure shows the effectiveness of gestation of all dams of the experiment. In all dams the vaginal plugs were found. Effective dams delivered at least one neonatal and non effective dams have not completed the gestation. n = 12 16 (control diet group and alcohol diet group, respectively).
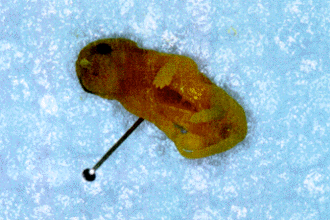
The offspring was examined, counted and weighed only on the 6th postnatal day. Stillborns were immediately removed and fixed in formalin solution (4%) and examined 7 days later. Some neonates presented eye, limbs (Fig. 5) and kidney malformations, while one showed gastroschisis (Fig. 6). The average number of live pups from mice of the control group on the 6th postnatal day was 6,5 (Fig. 7). This number was statistically significant, 46,15% smaller in the offspring of the alcoholized mice (Fig. 7).
Picture of two siblings of an alcoholized dam, one (left) apparently with no external malformations, and the other showing general reduction in size and anterior limb malformation.
Figure shows the number of neonates on postnatal day 6 (P6) of dams that have consumed hyperproteic liquid alcohol and control diets. mean and s.e.m , n = 9 12 mothers. *** p<0,001 according to t test.
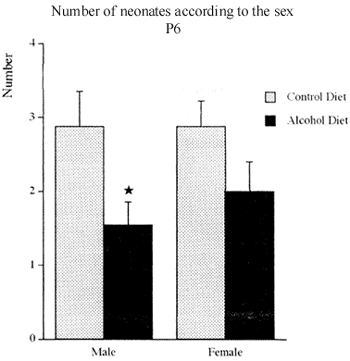
The offspring of the alcoholized group had less male than female pups. The reduction was statistically significant, 46,26%, comparing with the offspring of the control group (Fig. 8).
Figure shows the number of male and female neonates on postnatal day 6 (P6) of dams that have consumed hyperproteic liquid alcohol and control diets. Mean I s.e.m, n = 9 12 mothers. * p<0,05 according to t test.
The data on the body weight of the offspring are shown in the figure 9. From the 18th postnatal day, offspring of alcoholized mice showed increased weight gain after postnatal day 12 to 36, comparing with the weight gain of the offspring of the control group. However, statistically significant difference was observed only on the 36th postnatal day (18,27%).
Figure shows the time course of the body weight of the offspring of the dams which have consumed hyperproteic liquid diet containing ethanol (alcohol) or isocaloric amount of maltose dextrin in substitution for ethanol (control). The body weight was recorded regularly on postnatal days 6, 12, 18, 26, 36, 46 and 60. ** p<0,01, according to t test.
Also, shows the body weights of the offspring obtained on 6th, 12th, 18th, 26th, 36th, 46th and 60th postnatal day. On the 36th day, p<0,01, according to test.
DISCUSSION
There are several studies published in the literature that pointed out the effects of ethanol in offspring of alcoholized mice, in order to make clear the association between alcoholism during pregnancy and the teratogenic defects found in the fetus. Female mice of C57BL/6J strain were chosen in this study because of small size, low cost and easy handling. The comparison between findings in offspring of humans and mice exposed to ethanol in utero are fairly congruent, confirming the choice for this species in laboratory studies of the FAS. Other species of animals were used to study the effects of ethanol administered during pregnancy. DiBattista (1989) used golden hamsters to observe the voluntary consumption of ethanol during pregnancy and lactation. Cartwright and Smith (1995) utilized chick embryos, administering one single dosis of ethanol to show the fenotipic malformations of the teratogeny of ethanol. Furthermore, ethanol has been described to be teratogenic to fish, dogs, pigs and monkeys28,16,12,10.
In this study, concentration of ethanol in the diet corresponded to 27,5% of the calories. This is the concentration under which most of the investigations about gestational alcoholism showed the teratogenic effects of ethanol in offspring of rodents5. The concentration of ethanol utilized in this study was chosen due to the fact that the majority of researchers used between 10% and 35% of the caloric content in the form of ethanol24,23.
The alcoholic diet was introduced gradually from the 5th day of pregnancy on in order to assure the continuity of pregnancy, with less ocurrence of reabsorbed embryos or interruption of pregnancy. Ethanol can be given orally ad libitum23, via intragastric gavage15, or with an intraperitoneal injection32. Some researchers used inhalatory administration of ethanol in their experimental design29.
In the present study, the consumption of liquid diet by the female mice was equivalent in both alcohol and control groups throughout pregnancy. However, the pregnant mice that did not end gestation showed differences in consumption on the third end of pregnancy. On 17th day of gestation the mice of the control group consumed a significant higher amount of diet compared to alcohol group. These results are in accordance with DiBattista (1989), who observed differences in consumption of the alcohol and control diets, calling also the attention for the fact that there is less ingestion of caloric substances by the end of pregnancy of rats and hamster.
In our study, the mice of both groups showed equivalent weight gain during pregnancy, remarkably the ones who ended gestation with live neonates. The weights of the ethanol and control groups of mice was very similar by the end of gestation, reinforcing the fact that the nutritional factor did not interfere with the results. Nevertheless, ethanol did not modify the continuity of pregnancy, and when the mice were mated with males they were receiving diet with no alcohol at all.
In the studied sample, the number of animals was adequate, allowing the distribution in two groups: control and experiment. Although the possibility of reabsorbed embryos or miscarriage was always present due to the alcoholization of the mice, the effectiveness of the pregancies in both groups was 75%, according to what is described in the literature3,5. The same can be said about the time of gestation, that in both groups was 19 to 20 days, with no statistical differences.
The offspring was counted only on 6th day after birth, to avoid motherss agression against the pups. The decreased number of pups of the alcoholized group of mice is in accordance with what was also found by Wainwright and co-workers (1996). In this study, the authors used a liquid diet based on concentrated fatty acids and safflower oil, instead of the hiperproteic diet used by us.
The decreased number of male pups of the ethanol group, comparing with the control group, was an experimental finding of our study not yet published in the literature.
Some of the pups of the alcohol group had weights below average, with high mortality rate up to the 7th postnatal day, a finding that has also been described by Boggan and collaborators (1996). Likewise, the data of weight gain up to the 21th postnatal day is also found in a previous publication5. In order to avoid excessive manipulation, the data of weight gain of the offspring of both groups represent the average of weights obtained in the offspring of each mouse. Therefore, it is possible that the decreased weight of some pups could be compensated by the increased weight of other members of the offspring. In fact, we observed that during milking, some pups of the offspring of the ethanol group showed similar weight gain, data also found in some pups of the offspring of control groups. It is possible that the decreased number of offspring of the ethanol group caused an increased offer of milk for the surviving pups by their mothers, who in turn may have contributed to the increased weight gain in the immediate postmilking period, observed in the present study. The actual weight gain of the pups of the alcoholized group on the 36th postnatal day is in disagreement with the paper published by Boggan and collaborators (1996), who observed an increased weight gain of 7,14% on the 15th postnatal day of male pups in the alcoholized group.
The choice of the period of alcoholization during pregnancy and the study of the effects in offspring was based in the study of Wainwright and co-workers (1996), who showed reduction in the number of live offspring due to chronic use of ethanol, as well as decreased number of effective pregnancies and teratogeny, and decreased weight in the adult animal.
In our study we observed reduction of live pups and the presence of teratogeny. Webster and collaborators (1986) pointed out that the model of chronic and daily exposure of ethanol during the pregnancy of mice is more adequate to study FAS, comparing with the acute exposure. However, teratogeny in central nervous system, locomotor system e urinary tract can also occur with a single high dose of ethanol24.
The majority of the malformations found in some pups of the ethanol group are described in the literature, specially those referred as abnormalities of the central nervous system, extremities and urinary tract7. In the present study, microcephaly, microphtalmy and reduction in size of paws were also found, as described in the literature in other experimental models of maternal-fetal intoxication with ethanol18. The presence of a stillborn with gastroschisis was not yet descibed in mice, but in rats2.
As a continuity of the present work, the brains of the offspring of alcoholized and control mice are being processed by the immunohystochemical method in order to analyse the cellular effects of the exposure to ethanol.
CONCLUSIONS
Ethanol given during gestation of C57BL6/J mice may cause:
1. decreased number of pups in the offspring, specially those of male gender;
2. teratogeny, like limb and eye malformation, and gastroschisis;
3. increased weight of the offspring in the postnatal period, from the 18th to the 36th day.
ACKNOWLEDGEMENTS
We are grateful to Dr. Carrie H. Randall, University of South Carolina, Charleston, USA, for the valuable advices given during the experimental procedures and in the preparation of the manuscript.
This work was supported by grants from FAPESP, São Paulo (95/9060-6) and CNPq, Brasília, Brasil.
Grinfeld H, Goldenberg S, Segre CAM, Chadi G. Efeitos do etanol na prole de camundongas C57BL/6J alcoolizadas na prenhez. Acta Cir Bras [serial online] 1999 Jul-Sept;14(3). Available from: URL: http://www.scielo.br/acb .
RESUMO: Investigou-se os efeitos do consumo crônico de álcool na prole de camundongas alcoolizadas na prenhez. Camundongas da espécie C57BL/6J foram acasaladas. A presença de tampão vaginal de esperma indicava o início da prenhez. As camundongas prenhes foram distribuidas em 2 grupos: no grupo experimento, as camundongas receberam dieta líquida rica em proteinas, ad libitum, contendo 27,5% (5,28% v/v) de suas calorias derivadas do etanol, do 5º ao 19º dia de prenhez. O grupo controle recebeu o mesmo volume de dieta, contendo quantidades isocalóricas de dextrino-maltose em substituição ao etanol. Ao término do período de prenhez e pós-natal as mães receberam ração e água. Os filhotes foram pesados e contados em intervalos variáveis do 6º ao 60º dia de vida pós-natal. As mães alcoolizadas e as controles ganharam peso equivalente, e consumiram volumes equivalentes de dieta durante a prenhez. A maioria das camundongas do grupo experimento completou o período de prenhez, e o consumo crônico de álcool não alterou o número de animais prenhes que efetivamente chegaram a dar à luz. O número de filhotes do grupo de camundongas alcoolizadas foi significantemente menor que o grupo controle. O número reduzido de filhotes foi mais pronunciado entre os machos, além de ocorrer natimortalidade e teratogenia como gastrosquise e malformações de membros. A massa corpórea das proles das camundongas do grupo álcool etílico aumentou do 18º ao 36º dia de vida pós-natal.
DESCRITORES: Etanol. Camundongos. Síndrome alcoólica fetal. Teratogênios.
Address for correspondence:
Hermann Grinfeld
Av. Prof. Lineu Prestes, 2415 Depto. Anatomia
05508-900 São Paulo SP
Tel: 55-11-818 7384/Fax: 55-11-813 0845
email: grinfeld@einstein.br . .
Data do recebimento: 08/06/99
Data da revisão: 01/07/99
Data da aprovação:02/08/99
- 1. Ashwell KW, Zhang LL. Optic nerve hypoplasia in an acute exposure model of fetal alcohol syndrome. Neurotox Teratol 1994;16:161-7.
- 2. Beauchemin RR Jr, Gartner LP, Provenza DV. Alcohol induced cardiac malformations in the rat. Anat Anz 1984;155:17-28.
- 3. Becker HC, Randall CL, Salo AL, Saulnier JL, Weathersby RT. Animal research. Alcohol Health Res World 1994;18:10-6.
- 4. Becker HC. The effects of alcohol on fetal and postnatal development in mice. Report to the National Institute on Alcohol Abuse and Alcoholism, 1992.
- 5. Boggan WO, Xu W, Shepherd CL, Middaugh LD. Effects of prenatal ethanol exposure on dopamine systems in C57BL/6J mice. Neurotox Teratol 1996;18:41-8.
- 6. Cartwright MM, Smith SM. Increased cell death and neural crest cell numbers in ethanol-exposed embryos: partial basis for the fetal alcohol syndrome phenotype. Alcohol Clin Exp Res 1995;19:378-86.
- 7. Chadi G, Grinfeld H, Gomide VC, Scabello RT, Silva CM, Coracini KF, Fernandes CJCS. Efeitos da exposiçăo prenatal ao álcool. Plasticidade do sistema nervoso central. Arq Cient 1997;2(Parte 1):47-9.
- 8. Chadi G, Fernandes CJCS, Da Silva CM, Coracini, KF, Scabello RT, Gomide VC, Grinfeld H. Interruption of chronic alcohol consumption with the onset of pregnancy does not prevent microcephaly in offspring of Wistar rats. Alcohol Clin Exp Res 1997;21:48A.
- 9. Chernoff GF. The fetal alcohol syndrome in mice: an animal model. Teratology 1977;15:223-30.
- 10. Clarren SK, Bowden DM. Measures of alcohol damage in utero in the pigtailed macaque (Macaca nemestrina). Ciba Found Symp 1984;105:157-60.
- 11. Clarren SK, Smith DW. The fetal alcohol syndrome. N Engl J Med 1978;298:1063-7.
- 12. Dexter JD, Tumbleson ME, Decker JD, Middleton CC. Comparison of the offspring of three serial pregnancies during voluntary alcohol consumption in Sinclair (S-1) miniature swine. Neurobehav Toxicol Teratol 1983;5:229-33.
- 13. DiBattista D. Voluntary ethanol consumption during pregnancy and lactation in the golden hamster. Phisiol Behav 1989;46:771-3.
- 14. Driscoll CD, Streissguth A, Riley EP. Prenatal alcohol exposure: comparability of effects in humans and animal models. Neurotox Teratol 1990;12:231-7.
- 15. Dumas RM, Rabe A. Augmented memory loss in aging mice after embryonic exposure to alcohol. Neurotox Teratol 1994;16:605-12.
- 16. Elli FW, Pick JR. An animal model of the fetal alcohol syndrome in beagles. Alcohol Clin Exp Res 1980;4:123-7.
- 17. Gillian DM, Kotch LE. Alcohol-related birth defects in long and short sleep mice: postnatal litter mortality. Alcoholism 1990;7:483-7.
- 18. Giknis MLA, Damjanov I, Rubin E. The differential transplacental effects of ethanol in four mouse strains. Neurobehav Toxicol, 1980;2:235-6.
- 19. Jones KL, Smith DW. Recognition of the fetal alcohol syndrome in early infancy. Lancet 1973;2:999-1001.
- 20. Kronick JB. Teratogenic effects of ethyl alcohol administered to pregnant mice. Am J Obst Gynecol 1976;124:676-7.
- 21. Lemoine P, Harrouseau H, Borteyru JP, Menuet JC. Les enfants de parents alcooliques: anomalies observées, a propos de 127 cas. Quest Med 1968; 21:476-82.
- 22. Middaugh LD, Boggan WO. Postnatal growth deficits in prenatal ethanol-exposed mice: characteristics and critical periods. Alcohol Clin Exp Res 1991;15:919-26.
- 23. Middaugh LD, Boggan WO. Perinatal maternal ethanol effects on pregnant mice and on offspring viability and growth: influences of exposure time and weaning diet. Alcohol Clin Exp Res 1995;19:1351-8.
- 24. Randall CL, Anton RF. Aspirin reduces alcohol-induced prenatal mortality and malformations in mice. Alcohol Clin Exp Res 1984;8:513-5.
- 25. Randall CL, Anton RF, Becker HC, Hale RH, Ekblad U. Aspirin dose-dependently reduces alcohol-induced birth defects and prostaglandin E levels in mice. Teratology 1991;44:521-9.
- 26. Siegel S. Estadística no parametrica. México: Trillas; 1975.
- 27. Schenker S, Becker HC, Randall CL, Phillips DK, Baskin GS, Henderson GI. Fetal alcohol syndrome : current status of pathogenesis. Alcohol Clin Exp Res 1990;14:635-47.
- 28. Stockard CR. The influence of alcohol and other anaesthetics on embryonic development. Am J Anat 1910;10:369-73.
- 29. Ukita K, Fukui Y, Shiota K. Effects of prenatal alcohol exposure: influence of an ADH inhibitor and a chronic inhalation study. Reprod Toxicol 1993;7:273-81.
- 30. Wainwright PE, Huang YS, Lévesque S, Mutsaers L, McCutcheon D, Balcaen P, Hammond J. Effects of dietary gama linoleic acid and prenatal ethanol on mouse brain and behaviour. Pharmacol Biochem Behav 1996;53:843-51.
- 31. Webster WS. Alcohol as a teratogen: a teratological perspective of the fetal alcohol syndrome. In: Crow KE, Batt RD. Human metabolism of alcohol: pharmacokinetics, medicolegal aspects and general interests. Boca Raton: FL-CRC Press; 1989. p.133-55.
- 32. Webster WS, Walsh DA, Lipson AH, McEwen SE. Some teratogenic properties of ethanol and acetaldehyde in C57BL/6J mice: implications for the study of fetal alcohol syndrome. Teratology 1980;27:227-30.
Publication Dates
-
Publication in this collection
28 Oct 1999 -
Date of issue
Sept 1999
History
-
Accepted
02 Aug 1999 -
Reviewed
01 July 1999 -
Received
08 June 1999