Abstract
Background: Early evaluation of the severity of acute pancreatitis requires measurements of many variables. Clinical parameters as well as CT scan have traditionally been used as predictors of severity, and complications. None of them however can predict the outcome early and reliably. Inflammatory cytokines were shown to play an important role in the inflammatory cascade, which occurs early in the course of the disease. The aim of the present study is to evaluate the predictive value of plasma interleukin-6 (IL-6) and interleukin-1 (IL-1) levels in experimental pancreatitis in rats. Methods: Male wistar rats were anesthetized and pancreatitis was induced by intraparenchymal injection of 5% (group 2) and 10% (group 3) sodium taurocholate (TC), resulting in 2 distinct groups of severity. In sham controls (group 1), saline was injected into the pancreas in the same fashion. Blood samples were obtained before and 2, 4, 24, and 96 hours after the induction of pancreatitis and plasma amylase, lipase, LDH, IL-1 and IL-6 levels were measured. Mortality was recorded every 8 hours. Pancreatitis severity was also assessed by histopathology. Results: Four hours after pancreatitis induction, plasma amylase, lipase and LDH levels were markedly increased in the pancreatitis groups. In the sham control group, moderate increases were also observed. No consistent significant difference in amylase, lipase or LDH levels was observed between the groups. At 2 hours from pancreatitis induction, IL-6 levels increased mildly in-groups 1 and 2, and decreased to the baseline levels at 24 hours. In-group 3, the increase in IL-6 levels was significantly higher then in-groups 1 and 2 (p=0.029 and 0.036 respectively), and correlated well with pancreatitis severity as defined by pathology (p=0.01) and mortality rates (p=0.037). No difference in IL-1 levels was observed at 2,4 and 24 hours from induction. At 96 hours IL-1 levels were higher in group 3 then in groups 1 and 2 (p=0.037). Conclusion: IL-6 plasma levels correlated well with the severity of the disease as reflected by the mortality rates and pathological score. IL-6 levels may be a reliable predictor of severity and mortality in acute pancreatitis. This marker can be used as early as 2 hours and up to 24 hours from the beginning of the inflammatory process. IL-1 levels at 96 hours also correlated with pathology, but were not found to predict outcome at the early phases of the disease.
Pancreatitis; Inflammation; Cytokines; IL-1; IL-6; TNF
EVALUATION OF INFLAMMATORY CYTOKINES AS PROGNOSTIC MARKERS IN EXPERIMENTAL ACUTE PANCREATITIS IN RATS1 1. This study wa supported by Tel-Aviv University. 2. Department of Surgery "A". 3. Unit of Clinical Immunology. 4. Department of Pathology.
Haim Paran 2 1. This study wa supported by Tel-Aviv University. 2. Department of Surgery "A". 3. Unit of Clinical Immunology. 4. Department of Pathology.
Galit Sivak 2 1. This study wa supported by Tel-Aviv University. 2. Department of Surgery "A". 3. Unit of Clinical Immunology. 4. Department of Pathology.
Ami Mayo2 1. This study wa supported by Tel-Aviv University. 2. Department of Surgery "A". 3. Unit of Clinical Immunology. 4. Department of Pathology.
Uri Freund 2 1. This study wa supported by Tel-Aviv University. 2. Department of Surgery "A". 3. Unit of Clinical Immunology. 4. Department of Pathology.
Tamar Reshef3 1. This study wa supported by Tel-Aviv University. 2. Department of Surgery "A". 3. Unit of Clinical Immunology. 4. Department of Pathology.
Dvora Kidron4 1. This study wa supported by Tel-Aviv University. 2. Department of Surgery "A". 3. Unit of Clinical Immunology. 4. Department of Pathology.
Paran H, Sivak G, Mayo A, Freund U, Reshef T, Kidron D. Evaluation of inflammatory cytokines as prognostic markers in experimental acute pancreatitis in rats. Acta Cir Bras [serial online] 2000 Apr-Jun;15(2). Available from: URL: http://www.scielo.br/acb
SUMMARY: Background: Early evaluation of the severity of acute pancreatitis requires measurements of many variables. Clinical parameters as well as CT scan have traditionally been used as predictors of severity, and complications. None of them however can predict the outcome early and reliably. Inflammatory cytokines were shown to play an important role in the inflammatory cascade, which occurs early in the course of the disease. The aim of the present study is to evaluate the predictive value of plasma interleukin-6 (IL-6) and interleukin-1 (IL-1) levels in experimental pancreatitis in rats. Methods: Male wistar rats were anesthetized and pancreatitis was induced by intraparenchymal injection of 5% (group 2) and 10% (group 3) sodium taurocholate (TC), resulting in 2 distinct groups of severity. In sham controls (group 1), saline was injected into the pancreas in the same fashion. Blood samples were obtained before and 2, 4, 24, and 96 hours after the induction of pancreatitis and plasma amylase, lipase, LDH, IL-1 and IL-6 levels were measured. Mortality was recorded every 8 hours. Pancreatitis severity was also assessed by histopathology. Results: Four hours after pancreatitis induction, plasma amylase, lipase and LDH levels were markedly increased in the pancreatitis groups. In the sham control group, moderate increases were also observed. No consistent significant difference in amylase, lipase or LDH levels was observed between the groups. At 2 hours from pancreatitis induction, IL-6 levels increased mildly in-groups 1 and 2, and decreased to the baseline levels at 24 hours. In-group 3, the increase in IL-6 levels was significantly higher then in-groups 1 and 2 (p=0.029 and 0.036 respectively), and correlated well with pancreatitis severity as defined by pathology (p=0.01) and mortality rates (p=0.037). No difference in IL-1 levels was observed at 2,4 and 24 hours from induction. At 96 hours IL-1 levels were higher in group 3 then in groups 1 and 2 (p=0.037). Conclusion: IL-6 plasma levels correlated well with the severity of the disease as reflected by the mortality rates and pathological score. IL-6 levels may be a reliable predictor of severity and mortality in acute pancreatitis. This marker can be used as early as 2 hours and up to 24 hours from the beginning of the inflammatory process. IL-1 levels at 96 hours also correlated with pathology, but were not found to predict outcome at the early phases of the disease.
SUBJECT HEADINGS: Pancreatitis. Inflammation. Cytokines. IL-1. IL-6. TNF.
INTRODUCTION
Acute pancreatitis may present as mild, self-limiting disorder, or a severe disease with sometimes fatal course. The pathological features range from mild edema, to hemorrhage and necrosis and no clear correlation was found between the clinical presentation and pathological damage (1). Although its pathophysiology has been extensively investigated, it is only partly understood. No specific treatment has been found so far to prevent the deleterious effects of the digestive enzymes on the pancreas and peri-pancreatic tissues. As a result its management has been empirical, mainly by suppressing the pancreatic secretions (2). Evaluation of the severity of the disease, as well as prediction of outcome are difficult, and are assessed according to prognostic clinical signs (3,4), and serial CT scans (5). Both methods are not sensitive at the early phase of the disease, and this can cause a delay in identifying the patients who need intensive care treatment. It has been demonstrated that inflammatory cytokine levels correlate well in several inflammatory as well as stressful conditions, and their use as early predictors of complications and outcome during severe acute pancreatitis has been suggested (6,7,8). In the present study the time course of the plasma levels of two inflammatory cytokines, and their correlation with the severity of the disease was evaluated, in order to assess their value as early prognostic markers of the severity of acute pancreatitis.
METHOD
Animal preparation: Male Wistar rats (250 -350 gr) were used. Animals were kept as outlined in the "Guide for the Care and use of Laboratory Animals" (NIH Publication #85-23, 1985). General anesthesia was induced by intraperitoneal injection of 60 mg/kg of Nembutal (Sanosi laboratories, Paris, France). After anesthesia, the femoral vein was cannulated with a 20G PTFE-catheter (Venflon, Helsingborg, Sweden) which was secured in place by a ligature. After each use, the catheter was flushed with 0.3ml of heparin solution (1000 units per 100ml of normal saline). Pancreatitis was induced by a model of intraparenchymal injections of sodium taurocholate as previously described (9,10). Briefly, the abdomen was shaved and a midline laparotomy incision was performed. The stomach, duodenum, pancreas and spleen were carefully brought out from the abdominal cavity through the abdominal incision. Pancreatitis was induced by multiple (between 7 and 10) intraparenchymal pancreatic infiltration (30 gauge needle) of 0.3 ml/100gr body weight, sodium taurocholate solution at 5% and 10% concentrations. In sham controls, saline was infiltrated into the pancreatic parenchyma in the same fashion. The injections were performed by carefully introducing the tip of the needle into several parts of the pancreas, under magnifying lenses, while avoiding damage to the blood vessels, and the pancreatic ducts. After the injection, the organs were carefully brought back into the peritoneal cavity, and the incision was closed with a continuous 3-0 silk suture.
The model is easy to perform, highly reproducible, and results in a homogenous progressive injury to the pancreas, with a dose dependent degree of acinar necrosis and a dose dependent moderate mortality rate (9,10).
After pancreatitis induction the animals were left to recover for one hour, and put back into unrestraining cages, 3 rats per cage, and allowed water ad libitum, for 24 hours. Food (standard rat chow) was then also allowed ad libitum.
The rats were divided into 3 groups:
Group 1 (n=16): animals were sham operated, and saline was injected into the pancreas, 0.3ml/100gr body weight.
Group 2 (n=20): pancreatitis was induced by 5% TC solution.
Group 3 (n=30): Pancreatitis was induced by 10% TC solution.
Pathological examination
All surviving animals were killed at 96 hours from pancreatitis induction. The pancreas was then harvested and trimmed of fat. Macroscopic appearance was evaluated and the gland was then fixed in 10% formaldehyde, processed in paraffin and stained with hematoxylin-eosin. Microscopic examination was performed by one pathologist (D.K.) in a blinded fashion. The injury was evaluated by recording the number of lobes affected by edema, partial necrosis, total necrosis and leukocyte infiltration. Vascular thrombi, bacterial colonies, fat necrosis and hemorrhages were also recorded [11].
Biochemical tests
Blood samples (0.7 ml) were obtained from the rats immediately before and 2 hours, 4 hours, 24 hours, and 96 hours after pancreatitis induction. The blood volume was replaced by normal saline (v/v). In order to avoid severe anemia, the animals in each study group were randomly divided in two subgroups, and blood samplings were obtained in alternate fashion, only three times from each animal during the study. The blood samples were immediately centrifuged and the plasma samples were kept at -70o until tested. The plasma was tested for amylase, lipase and LDH levels (Hitachi Analyzer 737) and for IL-1 and IL-6 levels (Quantikine M, R&D Systems Minneapolis, USA) in blinded fashion, by an experienced laboratory technician.
Statistical analysis
Results are expressed as mean ± standard deviation. One way analysis of variance (F test), non-paired T test, Chi Square test and Anova were used (SPSS, SPSS inc. software, USA). Significance was accepted when P<0.05.
RESULTS
Mortality rate
The mortality rate in the sham control group was 0%. In group 2 it was 30%, and in group 3, 80% (p<0.01) (fig. 1).
Enzyme levels
Two hours after pancreatitis induction, plasma amylase, lipase and LDH levels were markedly increased in the pancreatitis groups. In the sham control group, moderate increases were also observed (fig 2). No consistent significant difference in amylase, lipase or LDH levels was observed between the 3 groups at 2 or 4 hours. Twenty-four hours after pancreatitis induction, serum amylase and lipase decreased to almost base-line levels, while LDH levels remained mildly increased and returned to the base-line levels after 96 hours. No correlation was found between the enzyme level and the mortality rates.
Histopathology
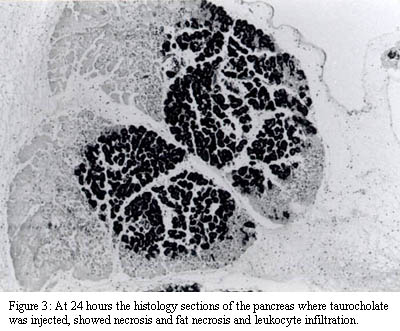
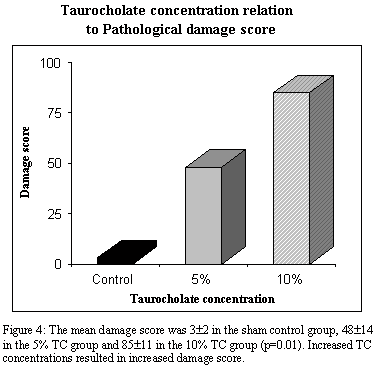
The histology sections of the rats with TC induced pancreatitis showed necrosis and leukocyte infiltration of the pancreatic lobes to various degrees, (fig.3), as well as fat necrosis, hemorrhages and vascular thrombi of the peripancreatic tissue. Increased TC concentrations resulted in increased damage score (Fig.4). The mean damage score was 3± 2 in the sham control group, 48± 14 in the 5% TC group and 85± 11 in the 10% TC group (p=0.01). Bacterial colonies were common in-group 3 where necrosis was prominent.
IL-6 levels
At two hours from pancreatitis induction, IL-6 levels increased mildly in-groups 1 and 2, (from 3.9± 4.5 to 42.4± 33.5 pgr/l, p=0.006 and from 4.1± 4.7 to 46.4± 28.5 pgr/l, p=0.002, respectively). The level remained significantly elevated up to 24 hours (9.7± 2.7pgr/l, p=0.001 and 27.5± 37.4 pgr/l, p=0.03, respectively), returning to base-line levels at 96 hours. In-group 3 the increase in IL-6 levels was significantly higher then in groups 1 and 2 (130.4± 30 pgr/l, P=0.029 and 0.036 respectively)(fig.5). The significant increase in IL-6 from base-line levels in-group 3, was sustained up to 24 hours (p=0.038, 0.002, 0.007 at 2,4,and 24 hours respectively) and then also decreased to baseline levels at 96 hours from pancreatitis induction. The increased IL-6 levels correlated well with disease severity as defined by the mortality rates (p=0.037) and the pathological score (p=0.01). The IL-6 levels also corrected with mortality rates regardless of taurocholate concentration used. The predictive value of Il-6 levels, lower then 40pgr/l at 4 hours, was 92%.
IL-1 levels
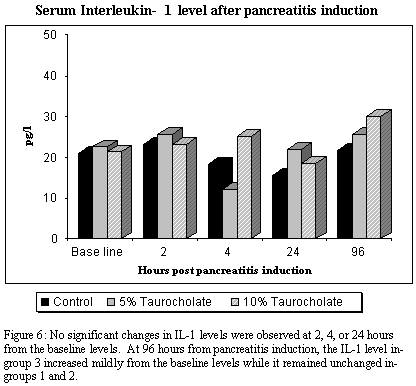
No significant changes in IL-1 levels were observed at 2, 4, or 24 hours from the baseline levels. At 96 hours from pancreatitis induction, the IL-1 levels in-group 3 increased from baseline levels of 21.5± 4.4 to 30.1± 4.7 pg/l (p=0.03), while it remained unchanged in groups 1 and 2, (p=0.037 between the groups) (fig.6). This late increase in IL-1 correlated with the pathological severity score but no did not correlate with mortality rates since death occurred earlier, and blood levels were obtained only from those animals alive at this stage.
DISCUSSION
Acute pancreatitis remains a serious pathological condition with significant mortality rate, depending on the severity of the disease. The main cause of morbidity and mortality are sepsis and multi-organ failure. It has been shown that the systemic manifestation of the disease are mediated by inflammatory cytokines (12,13) Recent clinical trials with specific drugs aimed at blocking these inflammatory mediators, showed disappointing results (13,14), in spite of promising results in experimental studies (15,16,17,18). This discrepancy could be explained by the difficulty in selecting the severe cases, i.e. those suitable for these treatment modalities, early in the course of the disease.
Clinical parameters as well as CT scan have traditionally been used as predictors of severity, and complications (3,4,5). None of them however can predict the outcome early and reliably. The ideal prognostic marker should be elevated within a few hours from the beginning of the inflammatory process and remain elevated at constant levels for at least 24 hours in order to allow early recognition of the severity of the disease.
Early mediators of systemic inflammatory response such as TNF and PAF are very short lived in the blood and thus unsuitable as practical markers of severity (13,19,20) since the aim of the study was to define a marker for clinical applications. IL-1 and IL-6 are generated within the pancreas and blood levels have been associated with the degree of inflammation and pancreatic damage (21,22), but their specific role in the course of the disease remains Unknown. Early works have shown that IL-6 and IL-1 levels correlated well with the severity of the disease, but only a few studies addressed their use as practical prognostic markers (23,24,25). In the present study, we evaluated the time course of IL-1 and IL-6 plasma levels, using commercially available kits, in an experimental model of pancreatitis where the severity of the disease is dose dependent. Plasma IL-6 levels were elevated at 2 hours from pancreatitis induction and remained elevated up to 24 hours. High levels of this cytokine were indicative of the severity of the disease as defined by pathological score, and mortality rates, during the first 24 hours of the disease. These findings were highly reproducible on repetition of the assay.
Significant increases in IL-1 levels were observed only at 96 hours, in the group with the highest pathological severity score but did not correlate with mortality. An early increase in IL-1 levels could have occurred early in the course of this model of pancreatitis and could have been missed since the first blood sample was obtained 2 hours from induction. The increase detected at 96 hours could reflect a late release of IL-1 in the course of the pancreatic injury such as the development of sepsis secondary to late colonization of the necrotic pancreas with bacteria, as shown in the pathological specimens.
In conclusion, in the present study, elevated IL-6, but not IL-1 levels proved to be an early and reliable marker of severity in this model of experimental pancreatitis. It's value, as a predictor of the outcome in acute pancreatitis in the clinical setting remains to be evaluated in clinical trials.
ACKNOWLEDGEMENTS
This study was supported in part by the Shreiber Grant (#960330) from the Tel-Aviv University.
Address for correspondence:
Haim Paran M. D.
Dept. of Surgery "A"
Meir Hospital, Kfar-Sava
Israel
Data do recebimento: 17/03/2000
Data da revisão: 10/04/2000
Data da aprovação: 15/05/2000
- 1 - Becker V. Pathological anatomy and pathogenesis of acute pancreatitis. World J Surg 1981;5:303-13.
- 2 - Steinberg W, Tenner S. Acute pancreatitis. N Engl J Med 1994;330:1198-210.
- 3 - Ranson JHC, Rifkind KM, Roses DF, Fink SD, Eng K, Spencer FC. Prognostic signs and the role of operative management in acute pancreatitis. Surg Gynecol Obstet 1974;139:69-81.
- 4 - Blamey SL, Imrie CW, O'Neil J, Gilmour WH, Carter DC. Prognostic factors in acute pancreatitis. Gut 1984;25:1340-6.
- 5 - Ranson JHC, Balthazar E, Caccavali R, Cooper M. Computed tomography and the prediction of pancreatic abscess in acute pancreatitis. Ann Surg 1985;201:656-63.
- 6 - Mckay C, Gallagher G, Baxter JN, Imrie CW. Systemic complications in acute pancreatitis are associatedwith increased monocyte cytokine release. Gut 1994;35:A575.
- 7 - De Beaux AC, Goldie AS, Ross JA, Carter DC, Fearon KC. Serum concentrations of inflammatory mediators related to organ failure in patients with acute pancreatitis. Br J Surg 1996;83(3):349-53.
- 8 - Oezcueruemez-Porsch M, Kunz D, Hardt PD, Fadgyas T, Kress O, Schulz HU, Schnell-Kretschmer H, Temme H, Westphal S, Luley C, Kloer HU. Diagnostic relevance of interleukin pattern, acute-phase proteins, and procalcitonin in early phase of post-ERCP pancreatitis. Dig Dis Sci 1998;43(8):1763-69.
- 9 - Hadas N, Orda R, Orda S, Bawnik JB, Wiznitzer T. Experimental pancreatitis in rats caused by intraparenchymal injection of sodium taurocholate. Isr J Med Sci 1983;19:194-7.
- 10 - Paran H, Klausner J, Siegal A, Graff E, Freund U, Kaplan O. Effect of the somatostatin analogue octreotide on experimental pancreatitis in rats. J Surg Res 1996;62:201-6.
- 11 - Schmidt J, Rattner DW, Lewandrowski K, et al. A better model of acute pancreatitis for evaluating therapy. Ann Surg 1992;215:44-56.
- 12 - Gross V, Leser HG, Heinisch A, Scholmerish J. Inflammatory mediators and cytokines: new aspects of the pathophysiology and assessment of severity of acute pancreatitis. Hepato Gastroenterol 1993;40:522-30.
- 13 - Johnson CD. Platelet-activating factor and platelet-activating factor antagonists in acute pancreatitis. Dig Surg 1999;16(2):93-101.
- 14 - Brivet FG, Emilie D, Galanaud P. Pro and anti-inflammatory cytokines during acute severe pancreatitis: an early and sustained response, although unpredictable of death. Parisian study group on acute pancreatitis. Crit Care Med 1999 Apr;27(4):749-55.
- 15 - Hirano T. Cytokine suppressive agent improves survival rate in rats with acute pancreatitis of closed duodenal loop. J Surg Res 1999 Feb;81(2):224-9.
- 16 - Marton J, Farkas G, Takacs T, Nagy Z, Szasz Z, Varga J, Jarmay K, Balogh A, Lonovics J. Beneficial effects of pentoxifylline treatment of experimental acute pancreatitis in rats. Res Exp Med (Berl) 1998;197(5):293-9.
- 17 - Yang J, Denham W, Tracey KJ, Wang H, Kramer AA, Salhab KF, Norman J. The physiologic consequences of macrophage pacification during severe acute pancreatitis. Shock 1998;10(3):169-75.
- 18 - Hughes CB, Grewal HP, Gaber LW, Kotb M, El-din AB, Mann L, Gaber AO. Anti-TNFalpha therapy improves survival and ameliorates the pathophysiologic sequelae in acute pancreatitis in the rat. Am J Surg 1996;171(2):274-80.
- 19- Grewal HP, Kotb M, Mohey El Din, Ohman M, Salem A, Gaber L, Gaber AO. Induction of tumor necrosis factor in acute pancreatitis and its subsequent reduction after hepatic passage. Surgery 1994;115(2):213-21.
- 20 - Dolan S, Campbell G, Rowlands BJ. Biphasic tumor necrosis factor release in experimental acute pancreatitis. Gut 1994;35:A575.
- 21 - Norman J, Franz M, Riker A, et al. Rapid elevation of pro-inflammatory cytokines during acute pancreatitis and their origination within the pancreas. Surg Forum 1994;45:145-60.
- 22 - Norman J, Fink G, Franz M, Carter G. Systemic cytokine gene expression induced by pancreatitis. Gastroenterology 1995;108(Suppl A):1226.
- 23 - Inagaki T, Hoshino M, Hayakawa T, Ohara H, Yamada T, Yamada H, Iida M, Nakazawa T, Ogasawara T, Uchida A, Hasegawa C, Miyaji M, Takeuchi T. Interleukin 6 is a useful marker for early prediction of the severity of acute pancreatitis. Pancreas 1997;14(1):1-8.
- 24 - Heath DL, Cruickshank DH, Gudgeon M, et al. Role of Interleukin-6 in mediating the acute phase protein response and potential as an early means of severity assessment in acute pancreatitis. Pancreas 1993;66:41-5.
- 25 - Moscovitz H, Shofer F, Mignott H, Behrman A, Kilpatrick L. Plasma cytokine determinations in emergency department patients as a predictor of bacteremia and infectious diseases severity. Crit Care Med 1994;22(7):1102-7.
Publication Dates
-
Publication in this collection
08 June 2000 -
Date of issue
June 2000
History
-
Accepted
15 May 2000 -
Reviewed
10 Apr 2000 -
Received
17 Mar 2000