Abstracts
Many experimental surgerical procedures have been perfomed in the analyse of the phenomenon of brain trophism and plasticity, however undesirable intercorrence can occour leading to specific changes in the results that should be taken into attention. To study this issue we have promoted a transient cardiogenic interruption of the blood flow together with a transient occlusion of the bilateral common carotid arteries (2VO) in rats and analysed the state of activation of astrocyte and microglia by means of the glial fibrillary acidic protein (GFAP) and OX42 immunohistochemistry, respectively. Rats were submitted to incomplete global cerebral ischemia (IGCI) by occlusion of the bilateral carotid arteries for 30 minutes. During the IGCI surgical, some rats received a higher dose of the chloral hydrate anaesthesia which promoted a cardiogenic interruption of the blood flow (CIBF) for a period of 10 minutes followed by and prompt reperfusion. During that period, animals were submited to a cardiac massage and ventilated. Sham operation were made in control animals. Rats were killed and their brains processed 14 days after the surgery. The animals that have received a IGCI showed a slight astroglial and microglial reaction in all subfields of the hippocampal formation, however the animal submitted to CIBF showed a massive infiltration of the reactive astrocyte and microglia in CA1 subfield. This results demonstrated that a transient occlusion of the bilateral common carotid arteries leads to activation of glial cells in the hippocampus, however this response can be remarkable changed in animal developing a transient systemic hypoperfusion during surgery. Thus, an accurated monitoration of the hemodinamic condition of the animal has to be done in experimental models of brain ischemia and the results have to be analysed in view of this aspect.
Astrocyte; Microglia; Cerebral ischemia; Imunohistochemistry; Image analysis
Muitos procedimentos experimentais são desenvolvidos para analisar o fenômeno do trofismo e plasticidade cerebral. Entretanto, eventos indesejáveis durante os procedimentos cirúrgicos podem ocorrer promovendo mudanças específicas nos resultados que devem ser levadas em consideração. Para estudar possibilidade, interrupção cardiogênica transitória do fluxo sangüíneo junto com a oclussão bilateral transitória das artérias caróridas comum (2VO) foi realizada em ratos e o estado de ativação de astrócitos e microglia foi analisado através da imunohistoquímica da proteína fibrilar ácida glial (GFAP) and OX42, respectivamente. Os ratos foram submetidos à isquemia cerebral global incompleta (IGCI) pela oclusão bilateral das artérias caróditas por 20 minutos. Durante o procedimento cirurgico da IGCI, alguns ratos receberam uma alta dose de anestésico de hidrato de cloral que promoveu uma interrupção cardiogênica do fluxo sangüíneo (CIBF) por um período de 10 minutos. Durante este período os ratos foram submetidos a massagem cardíaca e ventilados. Uma operação simulada foi realizada nos ratos controles. Os ratos foram mortos 14 dias após a cirurgia e seus cérebros processados para a imunohistoquímica. Os animais que receberam uma IGCI apresentaram uma leve reação astroglial e microglial em todos os sub-campos da formação hipocampal, entretanto os animais submetidos à CIBF mostraram uma infiltração massiça de astrócito e microglia reativos no sub-campo CA1. Estes resultados demonstram que oclusão bilateral transitória das artérias carótidas comum ativam as células gliais no hipocampo, entretanto esta resposta pode ser mudada substancialmente nos animais desenvolvendo hipoperfusão sistêmica durante o procedimento cirúrgico. Então, monitoramente acurado das condições hemodinâmicas do animal deve ser feito em modelos de isquemia cerebral e os resultados devem ser analisados em vista deste aspecto.
Astrócito; Microglia; Isquemia cerebral; Imunohistoquímica; Analise de imagem
3 - ORIGINAL ARTICLE
GLIAL REACTION IN THE HIPPOCAMPUS AFTER GLOBAL CARDIOGENIC ISCHEMIA1 1 Article from the Laboratory of Neurotrophic Factor and Neuronal Plasticity, Department of Anatomy, University of São Paulo (USP), São Paulo, Brazil. 2 Master Student from Institute of Psicology, University of São Paulo (USP), São Paulo, Brazil. 3 Full Professor, Department of Anatomy, Institute of Biomedical Science, University of São Paulo, São Paulo, Brazil.
Emerson Fachin Martins2 1 Article from the Laboratory of Neurotrophic Factor and Neuronal Plasticity, Department of Anatomy, University of São Paulo (USP), São Paulo, Brazil. 2 Master Student from Institute of Psicology, University of São Paulo (USP), São Paulo, Brazil. 3 Full Professor, Department of Anatomy, Institute of Biomedical Science, University of São Paulo, São Paulo, Brazil.
Gerson Chadi3 1 Article from the Laboratory of Neurotrophic Factor and Neuronal Plasticity, Department of Anatomy, University of São Paulo (USP), São Paulo, Brazil. 2 Master Student from Institute of Psicology, University of São Paulo (USP), São Paulo, Brazil. 3 Full Professor, Department of Anatomy, Institute of Biomedical Science, University of São Paulo, São Paulo, Brazil.
Martins EF, Chadi G. Glial reaction in the hippocampus after global cardiogenic ischemia. Acta Cir Bras [serial online] 2001 Jan-Mar;16(1). Available from: URL: http://www.scielo.br/acb.
ABSTRACT: Many experimental surgerical procedures have been perfomed in the analyse of the phenomenon of brain trophism and plasticity, however undesirable intercorrence can occour leading to specific changes in the results that should be taken into attention. To study this issue we have promoted a transient cardiogenic interruption of the blood flow together with a transient occlusion of the bilateral common carotid arteries (2VO) in rats and analysed the state of activation of astrocyte and microglia by means of the glial fibrillary acidic protein (GFAP) and OX42 immunohistochemistry, respectively. Rats were submitted to incomplete global cerebral ischemia (IGCI) by occlusion of the bilateral carotid arteries for 30 minutes. During the IGCI surgical, some rats received a higher dose of the chloral hydrate anaesthesia which promoted a cardiogenic interruption of the blood flow (CIBF) for a period of 10 minutes followed by and prompt reperfusion. During that period, animals were submited to a cardiac massage and ventilated. Sham operation were made in control animals. Rats were killed and their brains processed 14 days after the surgery. The animals that have received a IGCI showed a slight astroglial and microglial reaction in all subfields of the hippocampal formation, however the animal submitted to CIBF showed a massive infiltration of the reactive astrocyte and microglia in CA1 subfield. This results demonstrated that a transient occlusion of the bilateral common carotid arteries leads to activation of glial cells in the hippocampus, however this response can be remarkable changed in animal developing a transient systemic hypoperfusion during surgery. Thus, an accurated monitoration of the hemodinamic condition of the animal has to be done in experimental models of brain ischemia and the results have to be analysed in view of this aspect.
SUBJECT HEADINGS: Astrocyte. Microglia. Cerebral ischemia. Imunohistochemistry. Image analysis.
INTRODUCTION
In the last 10 years the neuroscience field of research has been improved substantially with the development of the new techniques, which have been employed in many experiments involving the study of neuronal trophism and plasticity. Many neurological sequeles after experimental models of neurotrauma and ischemia have been observed to be improved by neuronal trophic and plastic responses1, 2, 3, 4, 5.
It has been showing that the paracrine trophic responses promoted by activated glial cells such as reactive astrocyte and microglia are substantially important in the mechanisms leading neurons to support injury as well as to develop subsequently neuronal plasticity6, 7. It was shown by SANTIAGO RAMON & CAJAL, DEL-RIO HORTEGA and many other recent investigators that the modulation in the function of reactive glial cells trigger an ideal environment for a proper neuronal function8. Astrocyte and microglia readly react to neuronal lesion as well as to the breakdown of the neuronal homestasis9, 10, 11, 12. Long lasting glial reaction has also been described depending on the type and magnitude of the lesion13, 14. Actived glial cells in an injured brain region can tigger secretion of many molecules which regulate the local inflamation. The later trophic and plastic neuronal events are dependent on the magnitude and the fashion of earlier glial reaction6, 15, 16, 10, 17, 18.
Specific subfields of the hippocampal formation such as CA1 region have been showen to be particularly vunerable to ischemic damage19. Four vessel occlusion (4VO) model followed by reperfusion leads to a specific degeneration of de CA1 subfield of the hippocampal formation three days after the insult20. Furtheremore, the transient bilateral occlusion of common carotid arteries (2VO) model of brain ischemia in addition with systemic hypotension also leads to injury in specific brain region21. It has been described that glial reaction following ischemic damage also interferes with the neuronal maintenance or degeneration22, 23.
Many experimental manipulations like microneurosurgeries, stereotaxical injection, microdialisis have been perfomed in rodents as well as primates in order to analyse the phenomenum of brain trophism and plasticity24, 25. However, it becomes necessary to demonstrate how undesirable events, i. e. systemic hypotension during neurosurgerical experimental procedures can promote specific lesions in the brain.
To study this issue we have promoted a 2VO of transient ischemia with or without cardiogenic interruption of blood flow in rats and analysed the activation of astrocyte and microglia by means of well defined markers for these glial cells such as the immunohistochemistry of the glial fibrillary acidic protein (GFAP) and OX42, respectively. The degree of the changes was quantified by means of microdensitometric image analysis.
METHODS
Incomplete global cerebral ischemia (IGCI)
Adult male Wistar rats [body weight (b.w.) 240-280 g] from the Institute of Biomedical Science (São Paulo, Brazil) were used in the present study. The rats were kept under controlled temperature and humidity conditions with a standardized light and dark cycle (lights on at 0700 h and off at 1900 h) and with free access to food pellets and tap water. Under chloral hydrate anesthesia (Merck, Germany, 0.42 mg/g, b.w.), animals were placed in a microsurgical apparatus and by means of a neck midline incision, the two common carotid arteries were exposed and wound with threads without damaging the vessels and the vagus nerves. Bilateral incomplete cerebral ischemia (ICI) was promoted by looping the threads wound around the common carotid arteries for 30 minutes21, 26. After that a complete reperfusion was promptly promoted.
Cardiogenic interruption of the blood flow (CIBF)
Twenty minutes after IGCI some rats received a higher dose chloral hydrate anesthesia (Merck, Germany, 0.65 mg/g b.w.) which promoted a cardiogenic interruption of the blood flow for a period of 10 minutes5. During that period animals were submited to a cardiac massage and ventilation. Reperfusion occurs immediately after the reanimation. Thus, the total period of ICI in this groups was also 30 minutes.
Immunohistochemical procedures
Fourteen days after the global ischemia, the animals were deeply anesthetized and sacrificed by a transcardiac perfusion with 70 ml isotonic saline at room temperature followed by 350 ml of fixation fluid (4ºC) over a period of 6 minutes. The fixative consisted of paraformaldehyde in 0.1 M phosphate buffer, pH 6.9. The brains were removed, kept in the fixative solution at 4ºC for 90 minutes, rinsed in 20% sucrose (Synth, São Paulo, Brazil) dissolved in 0.1 M phosphate buffered saline (PBS), pH 7.4, for 48 h, frozen in dry ice-cooled (-40ºC) isopentane (Sigma) and stored at 70ºC until use.
Adjacent serial 60 mm thick coronal brain sections were obtained with a cryostat (Leica, CM 3000, Germany) at rostrocaudal levels 2.30 mm to 5.80 mm according to the atlas of PAXINOS AND WATSON27. The sections were sampled systematically during sectioning. Ten series in a rostrocaudal order including every ten section were used for immunohistochemistry.
Immunoreactivity was detected by the avidin-biotin peroxidase technique28. Floating sections were washed 2 x 10 minutes in 0.1M PBS, pH 7.4. Sections from series one and two were used to label astrocyte and microglia, respectively. The series of sections were incubated for 48 h at 4ºC under shaking with a rabbit polyclonal antiserum against glial fibrillary acidic protein (GFAP, Dakoparts, Denmark) diluted 1:1200 or with a mouse monoclonal antiserum against OX 42 (Harlan, USA) (Chadi et al., 1993; Cerutti and Chadi, 2000). The antibodies were diluted in PBS containing 0.5% Triton X-100 (Sigma) and 1% BSA (Sigma). After that, the series of the sections were washed again in PBS (2 x 10 minutes) and incubated with biotinylated either goat anti-rabbit or horse anti-mouse immunoglobulins, both diluted 1:250 (Vector, USA) for 2 hours. The sections were washed again in PBS and incubated with an avidin-biotin peroxidase complex (both diluted 1:125, Vectastain, Vector, for 90 minutes). Immunoreactivity was visualized using 3-3-diaminobenzidine tetrahydrocloride (DAB, Sigma) as a chromogen and H2O2 (0.05%, v/v, Sigma) for 8 minutes. The GFAP and OX 42 immunostained sections were counterstained with cresyl violet to allow interalia the visualization of the glial cell nuclei and the neuronal cell bodies.
Semiquantitative microdensitometric analysis of the GFAP and OX42 imunoreactivities
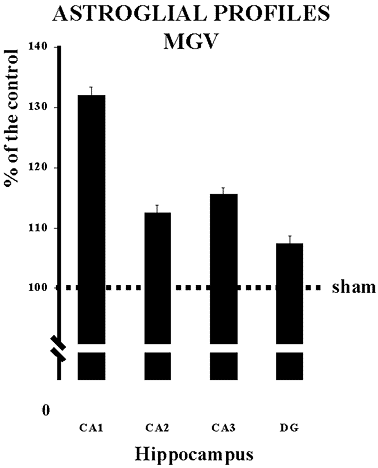
The microdensitometric analysis was made in 3 sections on both right and left sides of the hippocampus by means of an IBAS image analyser (Zeiss-Kontron). The subfields CA1, CA2, CA3 of the piramidal cell layers and denteate gyrus (DG) were specifically analysed. The image analysis procedures have been described previously29, 30. Briefly, the image was acquired by a television camera from the microscope (x 63.5 objective). After shading correction, a discrimination procedure was performed according to the following: the mean grey value (MGV) and s.e.m. of grey matter in the area of the hippocampus devoided of specific labeling (background, bg) was measured. Grey values darker than MGV 3 s.e.m. were considered as belonging to specific labeling and thus discriminated. The specific (sp) MGV was then defined as the difference between the bg MGV and the MGV of the discriminated profiles. In the present analysis, this parameter reflects the amount per cell of GFAP or OX42 immunoreactivities plus the cresyl violet staning. It must be remembered that in the absence of a standart curve, MGV only gives semiquantitative evaluations of the intensity of the immunoreactivity.
RESULTS
Analysis of the GFAP immunoreactivity in the sham operated rat
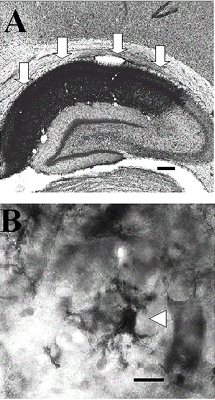
We found GFAP immunoreactive astroglial profiles homogeneously distributed throughout the cerebral cortical layers of the neocortex of sham operated rats. In the hippocampal formation, astroglial profiles were also found in all layers of the CA1, CA2 and CA3 subfields as well as in the DG (Fig.1A). The GFAP immunoreactive profiles showed moderate amount of GFAP immunoreactvity in the cytoplasm (Fig.1B). It was possible to see thin GFAP immunoreactive processes projecting from the citoplasm of the labeled astrocytes (Fig.1B).
Analysis of the GFAP immunoreactivity in the IGCI and CIBF rats
The GFAP immunoreactivity was not changed in the cerebral cortical layers of the neocortex of IGCI rats. Homogeneous distribution was also observed in all subfields (CA1, CA2, CA3 and DG) of the hippocampal formation of the ICGI rats, however a slight astroglial reaction characterized by a small increase in the number and in the size of this glial cell was found in these regions of ICGI rats (Fig. 1C).
The GFAP immunoreactive profiles was also homogeneously distributed throughout the cerebral cortical layers of the neocortex of the CIBF rats and they were very similar those found in that region of sham operated rats. However, in all subregions of the hippocampal formation of the CIBF rats the GFAP immunoreactive profiles had a cytoplasm which accumulated large amount of GFAP immunoreactive (Fig.2B). Massive increases in the number of the GFAP immunoreactive astroglial profiles with enlarged cytoplasmic processes were found in the hippocampal formation of the CIBF rats (Fig.2B). Those findings were more prominent in the CA1 subfield of the hippocampal formation of the CIBF rats where a massive infliltration of reactive GFAP immunoreactive astrocytes was seen (Fig. 2).
The microdensitometric analysis of the GFAP immunoreactivity demonstrated that the cerebral cardiogenic ischemia increases the spMGV of the GFAP immunoreactive astroglial profiles by 12.52% in the region CA2, 15.53% in the CA3 and 7.43% in the DG 14 days after the ischemic insult compared to the corespondent regions of the sham operated (Fig.3). However, the major increase of 32% was observed in CA1 subfield of the hippocampal formation of the CIBF rats (Fig.3).
Analysis of the OX42 immunoreactivity in the sham operated rats
We found the presence of the OX42 immunoreactive microglial profiles homogeneously distributed throughout the cerebral cortical layers of the neocortex and all subfields of the hippocampal formation of the sham operated rats. The OX42 immunoreactive profiles showed small cytoplasm which accumulated low amount of OX42 immunoreactivity (Fig.4B). It was observed several delicate OX42 immunoreactive processes projecting from the cytoplasm (Fig.4B).
Analysis of the OX42 immunoreactivity in the IGCI and CIBF rats
OX42 immunoreactive microglial profiles was seen homogeneously distributed throughout the cerebral cortical layers in the neocortex and throughout the hippocampal formation of the IGCI rats, however a slight microglial reaction characterized by an increased number of profiles could to be observed in those regions (Fig. 4C).
The OX42 immunoreactivity in the cerebral cortical layer of the neocortex of the CIBF rats was similar to that found in the IGCI. However, an increased number of OX42 immunoreactive profiles showing enlarged cytoplasm and higher amount of OX42 immunoreactivity was found in all subregions of the hippocampal formation of the CIBF rats (Fig. 5A). Many OX 42 immunoreactive profiles had round shape and short process in the haippocampal formation of the CIBF (Fig. 5B). A massive OX42 immunoreactivity was observed in the CA1 subfield of the hyppocampus of the CIBF rat (Fig. 5A). The analysis of the cresyl violet stained neuronal profiles in the subregions of the hippocampal formation showed no changes in the pyramidal cell layer of the CIBF rats 14 days after surgery, however a massive disappearance of the pyramidal neurons of the CA1 region was found after CIBF (Fig. 2A and 5A).
The microdensitometric analysis of the OX 42 immunoreactivity demonstrated that the cerebral cardiogenic ischemia increases the spMGV of the OX 42 immunoreactive microglial profiles by 9.38% in the CA2, 9.09% in the CA3 and 2.59% in the DG 14 days after the ischemic insult compared to the corespondent regions of the sham operated rats (Fig. 6). However, the major increase of 22,21% was observed in CA1 subfield of the hippocampal formation of the CIBF rats (Fig. 6).
DISCUSSION
In this study an incomplete global cerebral ischemia was performed by means of a transient occlusion of the bilateral commum carotid arteries in a well characterized experimental model of brain hypoperfusion called 2-VO21. The effects of the transient 2-VO performed here on the forebrain astroglial and microglial activation as well as the disappearance of pyramidal neurons of CA1 region of the hippocampal formation were remarkably potentiated by a temporary cardiogenic interruption of the systemic blood flow. Using the advantage of immunohistochemistry to specifically label glial cells combined with quantitative microdensitometric image analysis we have described here the degree of glial activation in the most vulnerable brain regions to ischemia i.e. subfields of the hyppocampal formation. The disappearance of neurons stained by cresyl violet was also analysed following the transient global ischemia.
Following experimental transient brain ischemia with reperfusion (IGCI procedure), morphological and neurochemical changes take place in degenerative and survival neurons as well as in the close by glial cells31, 32, 33, 34, 35, 36, 37, 38, 39. The degree of the changes can varie depending on the resistance of a particular neuronal cell population40 as well as on the locally inflammatory-mediated responses41.
In an experimental point of view, it has also to be emphasized that regarding the effects of a transient global ischemia, the hemodinamic conditions of the laboratory animals during surgery may interfere substantially with the analysis of the results.
The 2-VO model of brain schemia in rats employed in this study has been extensively used in order to analyse the mechanisms triggering neuronal death or maintenance21.
Another model of transient global brain ischemia called 4-VO has been also performed in rats when higher degree of lesion is desier42. In this model, a permanent occlusion of the vertebral arteries is followed by a transient occlusion of the common carotid arteries, bilaterally. It has also to be mentioned that the high level of mortality (80%)19 accompanied 4-VO procedure may sometimes make it difficult to elaborate more complex biochemical and behavioral experiments.
It has to be considered that anesthetic agents favorably effect outcome from brain ischemia43 even though this may not be the case of chloral hydrate employed in the present work which could not prevent further damage in the CA1 neurons of the hyppocampal formation after cardiogenic ischemia.
In the case of more severe ischemia followed by reperfusion, it is well known that neurons of neocortical layers 3, 5 and 6, small to medium striatal neurons and hippocampal pyramidal neurons of the CA1 and CA4 regions are more susceptible to schemic damaged20, 26.
Ischemia produced by bilateral carotid artery occlusion as performed in the present analysis is able to increase the concentration of the extracellular amino acids glutamate, aspartate, GABA and taurune which in turn is modulated by the stimulation of adenosine A1 receptors44. Furthermore, a permanent occlusion of both common carotid arteries reduces the muscanirric acetylcoholine receptor binding in the frontal cortex and hyppocampus 12 weeks after surgery with learning impairment showed by the hypoperfusioned rats45. The heat shock protein 70 that is associated with several celular processes, including DNA replication and transport of proteins across membrans, is expressed in the CA1, CA3 and CA4 pyramidal neurons of the hippocampus following a transient forebrain ischemia promoted by 4-VO model46.
It has been described that prior the death of CA1 neurons i.e. 24 hours post ischemia (four vessel occlusion), subfields of the hippocampus show calpain mediated and spectrum breakdown products, an increased silver staining and a decreased neurophysiological response to afferent stimulation47.
Lipid peroxidation takes place in brain regions where iron is deposited late after transient forebrain ischemia34. Because an accumulation of calcium is implicated in excitotoxic cell death, many studies have attempted to correlate the vulnerability of neurons with the presence or absence of the calcium binding proteins parvalbumin and calbindin, because of their calcium-buffering abilities40.
Other fact to be considered is that the different model/intensity of brain schemia regimes may lead to a differential pore-like opening of the blood-brain barrier48 which in turn may also be correlated with the selective neuronal death by changing the clearance and/or diffusion of neurotrophic and neurotoxic substances at the ischemic site49.
A massive diminution of the pyramidal neurons stained by cresyl violet of the CA1 region of the hyppocampus together with a remarkable astroglial and microglial activation in this region of the CIBF rats observed in the present study demonstrated that the intensity of the effects promoted by the 2-VO model of ischemia may be potentiated by systemic hypoperfusion. The reduction of the mean arterial blood pressure to 40 mmHg by hypovolenic hypotension50 has been associated with a 15 minutes occlusion of both common carotid arteries to perform a experimental bilateral incomplete cerebral ischemia21.
The reaction of glial cells, i.e. the astrocytic response, has commonly been described following an injury of the central nervous system14. Animals submitted to cerebral ischemia models have showed astroglial and microglial activation in selective vulnerable brain regions51, 49, 52. Following a global cerebral ischemia, the insult of CA1 subfield of the hippocampal formation leads to a local infiltration of microglia and astrocyte51, 49.
In the present analysis a higher degree of astroglial and microglial reaction was found in the CA1 subfield of the ischemic rat submitted an additional cardiogenic hypoperfusion of the blood flow, which can be correlated with the degree of CA1 lesion, since a major disappearance of CA1 neurons was in the CIBF rats.
The upregulation of the synthesis of basic fibroblast growth factor (bFGF) by reactive astrocytes, a neurotrophic factor with actions on hippocampal neurons53 was described in the ischemic hippocampus following ischemia54, 55. On the other hand, reactive astrocytes can synthesize increased amount of endotelin (ET) 1 and 3 in the CA1 damaged region after ischemia as an increased binding of ET is seen in activated microglial aggregation on damaged pyramidal cell layer of this region52. These observation may help to explain the massive glial activation in the CA1 subfield after transient global ischemia potentiated by cardiogenic hypoperfusion.
CONCLUSION
The present study demonstrated that an adequate monitoration of the hemodinamic conditions of animal is required to experiments involving brain ischemia. Furthermore, activation of microglia and astrocytes, labeled by immunohistochemistry is a good parameter to analyse the degree of brain ischemia.
ACKNOWLEDGMENTS
FAPESP (98/13122-5; 98/17030-4), CNPq. We wish to thanks Ms Patricia Rodrigues de Campos for excellent technical assistance.
Martins EF, Chadi G. Reação glial no hipocampo após isquemia global cardiogênica. Acta Cir Bras [serial online] 2001 Jan-Mar;16(1). Available from: URL: http://www.scielo.br/acb.
RESUMO: Muitos procedimentos experimentais são desenvolvidos para analisar o fenômeno do trofismo e plasticidade cerebral. Entretanto, eventos indesejáveis durante os procedimentos cirúrgicos podem ocorrer promovendo mudanças específicas nos resultados que devem ser levadas em consideração. Para estudar possibilidade, interrupção cardiogênica transitória do fluxo sangüíneo junto com a oclussão bilateral transitória das artérias caróridas comum (2VO) foi realizada em ratos e o estado de ativação de astrócitos e microglia foi analisado através da imunohistoquímica da proteína fibrilar ácida glial (GFAP) and OX42, respectivamente. Os ratos foram submetidos à isquemia cerebral global incompleta (IGCI) pela oclusão bilateral das artérias caróditas por 20 minutos. Durante o procedimento cirurgico da IGCI, alguns ratos receberam uma alta dose de anestésico de hidrato de cloral que promoveu uma interrupção cardiogênica do fluxo sangüíneo (CIBF) por um período de 10 minutos. Durante este período os ratos foram submetidos a massagem cardíaca e ventilados. Uma operação simulada foi realizada nos ratos controles. Os ratos foram mortos 14 dias após a cirurgia e seus cérebros processados para a imunohistoquímica. Os animais que receberam uma IGCI apresentaram uma leve reação astroglial e microglial em todos os sub-campos da formação hipocampal, entretanto os animais submetidos à CIBF mostraram uma infiltração massiça de astrócito e microglia reativos no sub-campo CA1. Estes resultados demonstram que oclusão bilateral transitória das artérias carótidas comum ativam as células gliais no hipocampo, entretanto esta resposta pode ser mudada substancialmente nos animais desenvolvendo hipoperfusão sistêmica durante o procedimento cirúrgico. Então, monitoramente acurado das condições hemodinâmicas do animal deve ser feito em modelos de isquemia cerebral e os resultados devem ser analisados em vista deste aspecto.
DESCRITORES: Astrócito. Microglia. Isquemia cerebral. Imunohistoquímica. Analise de imagem.
Address for correspondence:
G Chadi
Departamento de Anatomia, Instituto de Ciências Biomédicas
Universidade de São Paulo
Av. Prof. Lineu Prestes, 2415
Cidade Universitária
São Paulo - Brasil
05508-900
Phone: (55) (11)3818-7384
FAX: (55) (11)3818-7366
e-mail: gerchadi@usp.br
Data do recebimento: 14/10/2000
Data da revisão: 23/11/2000
Data da aprovação: 02/01/2001
- 1. Clemens JA, Ho PPK, Panetta JA. LY178002 reduces rat brain damage after transient global forebrain ischemia. Stroke 1991;22:1048-52.
- 2. Clemens JA, Smalstig EB, Bhagwandin B, Panetta JA. Preservation of a functionally intact neuronal network after global ischemia. Neurosc Lett 1994;170:244-6.
- 3. Green EJ, Dietrich WD, Van Dijk F, Busto R, Markgraf CG, McCabe PM, Ginsberg MD, Schneiderman N. Protective effects of brain hypothermia on behaviour and histopathology following global cerebral ischemia in rats. Brain Res 1992;580:197-204.
- 4. Gunaydin B, Babacan A. Cerebral hypoperfusion after cardiac surgery and anesthetic strategies: a comparative study with high dose fentanyl and barbiturate anesthesia. Ann Thorac Cardiovasc Surg 1998;4:12-7.
- 5. Krajewski S, Krajewski M, Ellerby LM, Welsh K, Xie Z, Deveraux QL, Salvesen GS, Bredesen DE, Rosenthal RE, Fiskum G, Reed JC. Release of caspase-9 from mitochondria during neuronal apoptosis and cerebral ischemia. Proc Natl Acad Sci 1999;96:5752-7.
- 6. Baumann N, Baron, Van EA, Jacque C, Zalc B. Glial biology and disorders. Curr Opin Neurol Neurosurg 1993;6:27-33.
- 7. McMillian MK, Thai L, Hong J-S, O'Callaghan JP, Pennypacker KR. Brain injury in a dish: a model for reactive gliosis. Trends Neurosci 1994;17:138-42.
- 8. Barnett NL, Pow DV, Robinson SR. Inhibition of Muller cell glutamine synthetase rapidly impairs the retinal response to light. Glia 1993;30: 64-73.
- 9. Cerutti SM, Chadi G. S100 immunoreactivity is increased in reative astrocytes of the visual pathways following a mechanical lesion of the rat occipital cortex. Cell Biol Int 2000;24:35-49.
- 10. Giulian D, Vaca K. Inflammatory glia mediate delayed neuronal damage after ischemia in the central nervous system. Stroke 1993;I84-90.
- 11. Glenn JA, Sonceau JB, Wynder HJ, Thomas WE. Histochemical evidence for microglia-like macrophages in the rat trigeminal ganglion. J Anat 1993;475-81.
- 12. Stichel CC, Muller HW. Extensive and long-lasting changes of glial cells following transection of the postcommissural fornix in the adult rat. Glia 1994;10:89-100.
- 13. Gomide VC, Chadi G. The trophic factors S-100beta and basic fibroblast growth factor are increased in the forebrain reactive astrocytes of adult callosotomized rat. Brain Res 1999;835:162-74.
- 14. Stromberg I, Bjorklund H, Dahl D, Jonsson G, Sundstrom E, Olson L. Astrocyte responses to dopaminergic denervations by 6-hydroxydopamine and 1-methyl-4-phenyl-12,3,6-tetrahydropyridine as evidenced by glial fibrillary acidic protein immunohistochemistry. Brain Res Bull 1986;17:225-36.
- 15. Chadi G, Tinner B, Agnati LF, Fuxe K. Basic fibroblast growth factor (bFGF, FGF-2) immunoreactivity exists in the noradrenaline, adrenaline and 5-HT nerve cells of the rat brain. Neurosci Lett 1993;160:171-6.
- 16. Clemens JA, Stephenson DT, Smalstig EB, Roberts EF, Johnstone EM, Sharp JD, Little SP, Kramer RM. Reactive glial express cytosolic phospholipase A2 after transient global forebrain ischemia in the rat. Stroke 1996;27:527-35.
- 17. Giulian D, Vaca K, Corpuz M. Brain glia release factors with opposing actions upon neuronal survival. J Neurosci 1993;13:29-37.
- 18. Martin DL. Synthesis and release of neuroactive substances by glial cells. Glia 1992;5:81-94.
- 19. Herguido MJ, Carceller F, Roda JM, Avendano C. Hippocampal cell loss in transient global cerebral ischemia in rats: a critical assessment. Neuroscience 1999;93:71-80.
- 20. Pulsinelli WA. Selective neuronal vulnerability: morphological and molecular characteristics. In: Molecular Mechanisms of ischemic brain damage. New York: Elsevier; 1985. p 29-37.
- 21. Németh G, Cintra A, Herb J.M, Ding A, Goldstein M, Agnati LF, Hoyer S, Fuxe K. Changes in striatal dopamine neurohistochemistry and biochemistry after incomplete transient cerebral ischemia in the rat. Exp Brain Res 1991;86:545-54.
- 22. May P, Clemens J, Panetta J, Smalsting E, Stephenson D, Fuson K. Induction of sulfated glycoprotein-2 (clusterin) and glial fibrillary acidic protein (GFAP) RNA expression following transient global ischemia is differentially attenuated by LY231617. Mol Brain Res 1996;42:145-8.
- 23. Torp R, Lekieffre D, Levy LM, Haug FM, Danbolt NC, Meldrum BS, Ottersen OP. Reduced postischemic expression of glial glutamate transporter, GLT1, in the rat hippocampus. Exp Brain Res 1995;103:51-8.
- 24. Chadi G, Cao Y, Pettersson RF, Fuxe K. Temporal and spatial increase of astroglial basic fibroblast growth factor synthesis after 6-hydroxydopamine-induced degeneration of the nigrostriatal dopamine neurons. Neuroscience 1994;61:891-910.
- 25. Chadi G, Castelucci P, Gomide VC. Experimental microneurosurgery of the central and peripheral nervous system in the study of the neuronal and glial trophism and plasticity. Acta Cir Bra 1998;13:8-17.
- 26. Smith ML, Auer RN, Siesjo BK. The density and distribution of ischemic brain injury in the rat following 2-10 min of forebrain ischemia. Acta Neuropathol (Berl) 1984;64:319-32.
- 27. Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Sidney: Academic Press; 1986.
- 28. Hsu M, Buzsaki G. Vulnerability of mossy fiber targets in the rat hippocampus to forebrain ischemia. J Neurosci 1993;13:3964-79.
- 29. Agnati LF, Fuxe K, Zoli M, Zini I, Harfstrand A. Morphometrical and microdensitometrical studies on phenylethanolamine-n-methyltransferase and neuropeptide Y- immunoreactive neurons in the rostral medulla oblongata of the adult and old male rat. Neuroscience 1988;26:461-78.
- 30. Zoli M, Agnati LF, Fuxe K, Zini I, Merlo PE, Grimaldi R, Harfstrand A, Goldstein M, Wikstrom AC, Gustafsson JA. Morphometrical and microdensitometrical studies on phenylethanolamine-N- methyltransferase- and neuropeptide Y-immunoreactive nerve terminals and on glucocorticoid receptor-immunoreactive nerve cell nuclei in the paraventricular hypothalamic nucleus in adult and old male rats. Neuroscience 1988;26:479-92.
- 31. Ferrand-Drake M, Wieloch T. The time course of DNA fragmentation in the choroid plexus and the CA1 region following transient global ischemia in the rat brain: the effect of intra-ischemic hypothermia. Neuroscience 1999;93:537-49.
- 32. Hsu SM, Raine L, Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem 1981;29:577-80.
- 33. Kirino T. Delayed neuronal death in the gerbil hippocampys following ischemia. Brain Res 1982;239:57-69.
- 34. Kondo Y, Asanuma M, Nishibayashi S, Iwata E, Ogawa N. Late-onset lipid peroxidation and neuronal cell death following transient forebrain ischemia in rat. Brain Brain Res 1997;772:37-44.
- 35. McGahan L, Hakim A, Robertson GS. Hippocampal Myc and p53 expression following transient global ischemia. Brain Res Mol Brain Res 1998;56:133-45.
- 36. McGahan L, Hakim AM, Nakabeppu Y, Robertson GS. Ischemia-induced CA1 neuronal death is preceded by elevated FosB and Jun expression and reduced NGFI-A and JunB levels. Brain Res Mol Brain Res 1998;56:146-61.
- 37. Nitsch C, Scotti AL, Monard D, Heim C, Sontag K-H. The glia-derived protease nexin 1 persists for over 1 year in rat brain areas selectively lesioned by transient global ischaemia. Eur J Neurosci 1993;5:292-7.
- 38. Schmidt-Kastner R, Bedard A, Hakim A. Transient expression of GAP-43 within the hippocampus after global brain ischemia in rat. Cell Tissue Res 1997;288:225-38.
- 39. Sugimura T, Sako K, Tohyama Y, Yonemasu Y. Consecutive in vivo measurement of nitric oxide in transient forebrain ischemic rat under normothermia and hypothermia. Brain Res 1998;808:313-6.
- 40. Freund TF, Buzsáki G, Leon A, Baimbridge KG, Somogyi P. Relationship of neuronal vulnerability and calcium binding protein immunoreactivity in ischemia. Exp Brain Res 1990;83:55-66.
- 41. Bell MD, Lopez GR, Lawson L, Hughes D, Fraser I, Gordon S, Perry VH. Upregulation of the macrophage scavenger receptor in response to different forms of injury in the CNS. J Neurocytol 1994;23:605-13.
- 42. Pulsinelli WA, Brierley JB. A new model of bilateral hemispheric ischemia in the unanesthetized rat. Stroke 1979;10:267-72.
- 43. Miura Y, Grocott HP, Bart RD, Pearlstein RD, Dexter F, Warner DS. Differential effects of anesthetic agents on outcome from near-complete but not incomplete global ischemia in the rat. Anesthesiology 1998;89:391-400.
- 44. Goda H, Ooboshi H, Nakane H, Ibayashi S, Sadoshima S, Fujishima M. Modulation of ischemia-evoked release of excitatory and inhibitory amino acids by adenosine A1 receptor agonist. Eur J Pharmacol 1998;357:149-55.
- 45. Tanaka K-I, Wada N, Hori K, Asanuma M, Nomura M, Ogawa N. Chronic cerebral hypoperfusion disrupts discriminative behavior in acquired-learning rats. J Neurosci Methods 1998;84:63-8.
- 46. Nishi S, Taki W, Uemura Y, Higashi T, Kikuchi H, Kudoh H, Satoh M, Nagata K. Ischemic tolerance due to the induction of HSP70 in a rat ischemic recirculation model. Brain Res 1993;615:281-8.
- 47. Bartus RT, Dean RL, Mennerick S, Eveleth D, Lynch G. Temporal ordering of pathogenic events following transient global ischemia. Brain Res 1998;790:1-13.
- 48. Preston E, Foster DO. Evidence for pore-like opening of the blood-brain barrier following forebrain ischemia in rats. Brain Res 1997;761:4-10.
- 49. Schmidt-Kastner R, Szymas J, Hossmann K. Immunohistochemical study of glial reaction and serum-protein extravasation in relation to neuronal damage in rat hippocampus after ischemia. Neuroscience 1990;38:527-40.
- 50. Ekolof B, Siesjo B. The effect of bilateral carotid artery ligation upon the blood flow and the energy state of the rat brain. Acta Physiol Sacand 1972;86:155-65.
- 51. Ordy JM, Wengenack TM, Bialobok P, Coleman PD, Rodier P, Baggs RB, Dunlap WP, Kates B. Selective vulnerability and early progression of hippocampal CA1 pyramidal cell degeneration and GFAP-positive astrocyte reactivity in the rat four-vessel occlusion model of transient global ischemia. Exp Neurol 1993;119:128-39.
- 52. Yamashita K, Niwa M, Kataoka Y, Shigematsu K, Himeno A, Tsutsumi K, Nakano-Nakshima M, Sakurai-Yamashita Y, Shibata S, Taniyama K. Microglia with an endothelin ETB receptor aggregate in rat hippocampus CA1 subfields following transient forebrain ischemia. J Neurochem 1994;63:1042-51.
- 53. Walicke PA. Basic and acidic fibroblast growth factors have trophic effects on neurons from multiple CNS regions. J Neurosci 1988;8:2618-27.
- 54. Lin TN, Te J, Lee M, Sun GY, Hsu CY. Induction of basic fibroblast growth factor (bFGF) expression following focal cerebral ischemia. Brain Res Mol Brain Res 1997;49:255-65.
- 55. Takami K, Kiyota Y, Iwane M, Miyamoto M, Tsukuda R, Igarashi K, Shino A, Wanaka A, Shiosaka S, Tohyama M. Upregulation of fibroblast growth factor-receptor messenger RNA expression in rat brain following transient forebrain ischemia. Exp Brain Res 1993;97:185-94.
Publication Dates
-
Publication in this collection
11 Sept 2003 -
Date of issue
Mar 2001
History
-
Accepted
02 Jan 2001 -
Reviewed
23 Nov 2000 -
Received
14 Oct 2000