Abstracts
The prospects for allotransplantation of pancreatic islets in man depend on the development of methods that provide sufficient quantities of pancreatic islets from a single donor, which are capable, when transplanted, of achieve the normalization of carbohydrate metabolism. Objective: Evaluate the efficacy of the isolation of Langerhans islets from dogs, by means of mechanical-enzymatic separation technique with stationary digestion using collagenase, and purification with a discontinuous dextran density gradient. Methods: The counting of islet numbers and evaluation of their sizes was accomplished by staining with diphenylthiocarbazone and using stereoscopic microscopes equipped with eyepiece reticule for the measurement of average diameters of stained islets. Results: The results disclosed that the average number of islets isolated was 81032.20 ± 24736.79 and the average number of islets isolated per kg of body weight was 6938.70 ± 1392.43. The average number of islets isolated per kg of body weight showed significant correlation with body weight and weight of the pancreas resected. Conclusion: The number of islets isolated, of a single donor, by mechanical-enzymatic separation, stationary collagenase digestion and discontinuous dextran density gradient purification can be sufficient to success of pancreatic islets transplant in dogs.
Islets of Langerhans; Transplantation; Diabetes Mellitus; Diabetes Mellitus, Experimental
A perspectiva do alotransplante de ilhotas pancreáticas no homem está na dependência do desenvolvimento de métodos que propiciem quantidades suficientes de ilhotas pancreáticas, originadas de doador único, capazes de, quando transplantadas, levarem à normalização do metabolismo dos hidratos de carbono. Objetivo: Avaliar, em cães, a eficácia do isolamento das ilhotas de Langerhans por meio da técnica de separação mecânica-enzimática, digestão estacionária com colagenase e purificação pelo gradiente de densidade descontínua de dextran. Métodos: A contagem do número e avaliação do tamanho das ilhotas foi realizada pela coloração com difeniltiocarbazona e exame em microscópio estereoscópico munido de retícula ocular para medição do diâmetro médio das ilhotas coradas. Resultados: O número médio de ilhotas isoladas foi de 81032,20 ± 24736.79 e o número médio de ilhotas/kg de peso corpóreo foi de 6938,70± 1392,43. O número médio de ilhotas/kg de peso corpóreo obtido mostrou correlação significativa com peso corpóreo e peso do pâncreas extirpado. Conclusão: Estes resultados sugerem que o número de ilhotas isoladas, de um único doador, por meio da separação mecânica-enzimática, digestão estacionária com colagenase e purificação pelo gradiente de densidade descontínua de dextran pode ser suficiente para o sucesso do transplante de ilhotas pancreáticas em cães.
Ilhotas de Langerhans; Transplante; Diabetes Mellitus; Diabetes Mellitus, Experimental
2 ORIGINAL ARTICLE
PANCREATIC ISLET ISOLATION BY MECHANICAL-ENZYMATIC SEPARATION, STATIONARY COLLAGENASE DIGESTION AND DEXTRAN DISCONTINUOUS DENSITY GRADIENT PURIFICATION. EXPERIMENTAL STUDY IN DOGS1 1 From Laboratory of Experimental Surgery of Faculty of Medicine of ABC, Santo André (SP), Brazil.
Jaques Waisberg2 2 Assistant Professor, Department of Surgery, Faculty of Medicine of ABC.
Heloísa Prado Soares3 3 Student of Medicine, Faculty of Medicine of ABC.
Luís Gustavo Altieri3 3 Student of Medicine, Faculty of Medicine of ABC.
Osíris Ramaciotti4 4 Head Professor, Operative Technique, Faculty of Medicine of ABC.
Manlio Basilio Speranzini5 5 Head Professor, Gastrointestinal Surgery, Faculty of Medicine of ABC.
Waisberg J, Soares HP, Altieri LG, Ramaciotti O, Speranzini MB. Pancreatic islet isolation by mechanical-enzymatic separation, stationary collagenase digestion and dextran discontinuous density gradient purification: experimental study in dogs. Acta Cir Bras [serial online] 2002 Mar-Apr;17(2). Available from URL: http://www.scielo.br/acb.
ABSTRACT: The prospects for allotransplantation of pancreatic islets in man depend on the development of methods that provide sufficient quantities of pancreatic islets from a single donor, which are capable, when transplanted, of achieve the normalization of carbohydrate metabolism. Objective: Evaluate the efficacy of the isolation of Langerhans islets from dogs, by means of mechanical-enzymatic separation technique with stationary digestion using collagenase, and purification with a discontinuous dextran density gradient. Methods: The counting of islet numbers and evaluation of their sizes was accomplished by staining with diphenylthiocarbazone and using stereoscopic microscopes equipped with eyepiece reticule for the measurement of average diameters of stained islets. Results: The results disclosed that the average number of islets isolated was 81032.20 ± 24736.79 and the average number of islets isolated per kg of body weight was 6938.70 ± 1392.43. The average number of islets isolated per kg of body weight showed significant correlation with body weight and weight of the pancreas resected. Conclusion: The number of islets isolated, of a single donor, by mechanical-enzymatic separation, stationary collagenase digestion and discontinuous dextran density gradient purification can be sufficient to success of pancreatic islets transplant in dogs.
KEY WORDS: Islets of Langerhans. Transplantation. Diabetes Mellitus. Diabetes Mellitus, Experimental.
INTRODUCTION
The transplantation of Langerhans islets has been studied as an alternative to the vascularized transplantation of the pancreas, as this presents potential advantages. It avoids large-magnitude procedures and their possible complications, the tissue to be transplanted can be furnished by multiple donors, and the Langerhans islets can be stored and frozen until they are utilized 1. The pancreatic islets are susceptible to immunomodulation by culturing them in appropriate media and by other in vitro techniques to diminish their immunogenicity 2. In addition, grafting of the pancreatic islets can be achieved at sites with portal vein drainage, without the risk of pancreatic fistula or vascular thrombosis occurring, as in vascularized transplantations of the organ (3). The disadvantages of Langerhans islet transplantation are the difficulty of isolating human islets and their immunogenicity, which requires appreciable quantities of immunosuppressor drugs to prevent their rejection 1.
The more compact pancreas of the large mammals yields a low number of isolated, functionally viable pancreatic islets due to the firm connections existing between the islets and the pancreatic exocrine tissue surrounding 4. Although in 1990 RICORDI et al 5 reported having obtained significant quantities of pancreatic islets from large mammals by utilizing automated separation equipment, the yield of islets from the present preparation methods is extremely variable 6,7.
Thus, it still remains necessary to find reproducible techniques that are capable of increasing the yield from pancreatic islet cell isolation, as one of the main problems limiting pancreatic islet cell transplantation programs in humans is the variability in yield from cell isolation 8,9.
The objective of this study was to study in dogs the efficacy of the isolation of Langerhans islets by means of the technique of mechanical-enzymatic separation, stationary digestion using collagenase, and purification with discontinuous dextran density gradient.
METHODS
Ten male young adult mongrel dogs with body weight upon admission in the range of 8 to 12 kg were utilized. A veterinary physician kept the animals in accordance with the standards of the Animal Protection Society and under supervision. The operative interventions were performed in the laboratories of experimental surgery.
The anesthetic utilized was sodium pentobarbital, at a dosage of 25 mg/kg of body weight, diluted in distilled water and injected via peripheral venous puncture into the radial vein of one of the front paws of the animal. The dogs were maintained under positive pressure respiration via pulmonary ventilation controlled with a pressure respirator (Model 600, K. Takaoka, São Paulo), duly regulated to supply a respiratory frequency of 20 movements/minute.
In all the animals operated, laparotomy was performed through a medial incision. Once the abdominal cavity was exposed, the main pancreatic duct was isolated immediately before its penetration in the duodenal wall. By dissection, the ascending and descending branches of the pancreatic duct were isolated from the surrounding parenchyma. Next, with the aid of a 2.5x magnifying glass, the ascending and descending branches were opened using and catheterized separately using polyvinyl catheters of diameter 3 Fr directed towards the pancreas. Next, the main pancreatic duct was tied and sectioned beside the duodenal wall. Via the catheters placed in the ascending and descending branches of the main pancreatic duct, a slow infusion of Hanks balance saline solution (HBSS) equilibrated to pH 7.4 using sodium bicarbonate, was made. To this, 0.4% collagenase V (Sigma Chemical, USA) was added at 1200 U/mg and 4ºC, in sufficient volume to obtain adequate interlobular distention of the pancreatic gland.
Next, total resection of the pancreas was performed, with the preservation of the duodenum in accordance with the technique described by COBB & MERRELL 10, keeping the recurrent duodenal artery. The main pancreatic vascular trunks (gastroduodenal vessels and branches of the upper mesenteric vessels) were tied only at the end of resection of the gland, so as to reduce the ischemia period during this procedure time. At the end of the total pancreatectomy, the animals, still under anesthesia, were sacrificed by means of an intravenous injection of 25% potassium chloride.
After obstruction of the main vascular connections, the gland was removed, weighed and immediately, the catheters placed in the ascending and descending branches of the main pancreatic duct were connected to a circulating perfusion apparatus (Intravenol, USA). The pancreas was then submitted to perfusion using HBSS at 4ºC with 5 mg/ml of collagenase V (1200 U/mg) at a volume twice the weight of the pancreas, with a flow of approximately 20 ml/minute and perfusion pressure maintained between 200 and 300 mm Hg. The perfusion liquid was gradually heated up to 37ºC and the perfusion was maintained for 10-12 minutes until the gland became mucoid.
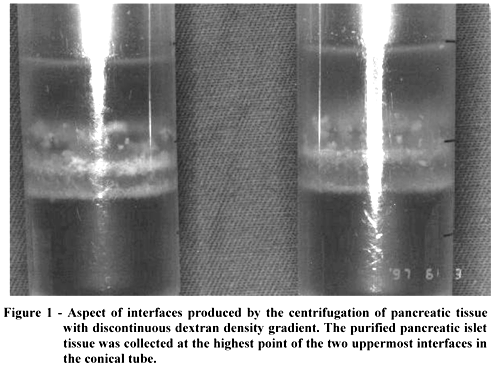
The pancreatic tissue was then cooled by immersion in HBSS at 4ºC. the large ducts and vascular structures were dissected from the pancreatic parenchyma. Next, the pancreatic tissue was fragmented using scissors until each fragment was around 5 mm in diameter. The fragments were washed in HBSS at 4ºC and decanted three times. The pancreatic tissue was then aspirated into a syringe with capacity of 50 ml and of a diameter 14G, without a needle and filtered via a stainless steel screen with a pore mesh of 400m in diameter. The tissue held on the steel screen was suspended in HBSS at 4ºC, placed in a mechanical mixer at 150 oscillations per minute, in a water bath at 37ºC for 8 minutes and filtered. The filtration product was centrifuged in a refrigerated laboratory centrifuge at 200 g for 1 minute at a temperature between 5 and 10ºC, washed in HBSS at 4ºC and decanted twice. The pancreatic tissue recovered was submitted to separation of the islets of the exocrine tissue with discontinuous dextran density gradient (Dextran T 70, Pharmacia Biotech DB, Sweden). For this, the pancreatic tissue was divided into aliquots of 2 ml, which were placed in conical tubes (Nunc, Copenhagen, Denmark) of capacity 50 ml, and suspended in 12 ml of 32% dextran of molecular weight 70,000 Dalton (density 1094/ml) in HBSS. Next, 4 ml of 32% dextran in HBSS were added to each conical tube below the pancreatic tissue, and 4 ml of each of the following dextran solutions above the pancreatic tissue suspension: 27% (density 1085), 23% (density 1075) and 11% (density 1041). The conical tubes were centrifuged at 40g for four minutes and at 500 g for 12 minutes at a temperature between 5 and 10ºC in a refrigerated laboratory centrifuge. With the aid of graduated pipettes of capacity 10 ml, the purified pancreatic islet tissue was collected at the highest point of the two uppermost interfaces of the discontinuous dextran gradients, i.e. at the density interfaces 1041/1075 and 1075/1095 (Fig.1). The tissue recovered was washed in HBSS at 4ºC, centrifuged at 200 g at a temperature between 5 and 10ºC for one minute and suspended in 30 ml of medium 199 (Adolfo Lutz Institute, São Paulo) at 4ºC.
Duplicate samples of 50 mL obtained from the final preparation of purified pancreatic islets were stained using diphenylthiocarbazone (Imperial Chemical, England) staining solution and submitted to examination under the stereoscopic microscope at a magnification of 3.2x and furnished with an optical reticule for measuring the diameters of the stained islets. The staining solution was prepared using the method of BONNEIVE-NIELSEN et al 11, to act on pH physiology. For this, 10 mg of ditizone were completely dissolved in 3 ml of pure ethanol with 50 mL of concentrated ammonium hydroxide. The working solution (pH 7.8) was made up at the time of the examination, diluting the stock solution 1:100 in HBSS. For each prepared pancreas volume, nine volumes of the ditizone solution were added, mixed and incubated for 10 minutes at ambient temperature with occasional agitation. The count of pancreatic islet diameters was estimated from the sample average and the dilution of the islet tissue utilized in the sampling. Only unfragmented islets of diameter greater than 60 mm were counted.
The following statistical models were utilized: arithmetic average and standard deviation, variance analysis, and correlation with the respective correlation coefficient. In all the tests, the significance level of 95% was adopted (p< 0.05).
RESULTS
For the animals, the average body weight was 11.47 ± 1.44 kg. The average weight of the pancreas was 28.04 ± 3.83 grams, the average total number of islets isolated was 81032.20 ± 24736.79 islets and the average number of islets isolated per kg of body weight was 6938.70 ± 1392.43 islets (Table 1). All animals, except one, reached more than 5000.00 islets per kg of body weight.
The statistical analysis revealed that there was significant correlation between the body weight and the weight of the pancreas with the total number of islets isolated of each animal and the number of isolated islets per kg of body weight (p< 0,05) (Table 2).
DISCUSSION
A viable mass of transplanted islets is essential for functional evolution, both on an experimental and a clinical basis. One of the main reasons for the failure of a clinical transplantation of islets aimed at inducing insulin independence is an inadequate mass of purified islets from a single donor. The quantification of the number and volume of islets between the different laboratories is still imprecise 12,13,14.
It has been reported 2,14 that receptor dogs implanted with more than 4400 islets per kg of body weight resulted in a 100% success rate in autotransplantation. The long-term survival can be predicted in accordance with the total number of islets autotransplanted and functioning. Other authors have concluded that in dogs, the quantity of approximately 5000 islets per kg of body weight of the receptor is the minimum limit for inducing prolonged normoglycemia 6,11. In the present study, the average number of islets isolated per kg of body weight was 6938.70 ± 1392.43 islets. In all of animals, but one, was reached more than 5000 islets per kg of body weight and in only one dog it was obtained less than 5000 but upper than 4400 islets per kg of body weight.
During the isolation process, the two main causes of lesions in the islets are due to enzyme action during the collagenase digestion process and anaerobic lesions during the purification and washing of the cells 6. The mechanical manipulation represented by centrifugation and transfers, and the exposure to hyper-osmolar chemical substances during the purification process of the Langerhans islet preparation, may damage and fragment the islets, altering their viability and functionality in the ectopic transplantation site 2,7,14.
The majority of for canine pancreatic islet preparation protocols for transplantation include: 1- pancreatectomy with maintenance of the main nutrient vessels of the pancreas, until the end of the resection of the gland; 2- in situ interlobular separation of the gland, using nutrient solution and collagenase; 3- stationary digestion of the gland, using nutrient solution and collagenase in circulation apparatus; 4- fragmentation of the pancreatic tissue via utilization of discontinuous dextran density gradients, Ficoll or albumin, and separation of debris and non-parenchymatous structures; 5- partial isolation of pancreatic islet tissue 12. These principles were combined in our study, which permitted a reduction in the tissue volume for transplantation to be obtained.
The key factors in the separation process of the pancreatic islets are the obtaining of adequate interlobular separation of the connective tissue stroma, the accomplishment of a delicate non-traumatic dissociation of the islets and the utilization of the discontinuous density gradient for the purification of the mass7, 8,13.
All these technical aspects in the preparation and implantation of the pancreatic islet transplants point out the need for a greater initial volume of islets to compensate for the loss of viable islets in the course of the various stages of the process 13. Although the digestion by collagenase is necessary, there is a balance between the need for tissue dispersal and the preservation of the islet mass, whose optimization appears to be obtained after 20 minutes of enzymatic digestion (8,13,14) as was utilized in this study.
The islets of rodents can easily be distinguished from the exocrine tissue, whereas the islets in large mammals are frequently difficult to distinguish from degranulated acinic cell conglomerates and collapsed ducts. The utilization of histochemical or functional methods for identifying pancreatic islets that did not consume too much time. For this, we utilized diphenylthiocarbazone (ditizone), a substance with the property of forming bindings with zinc, which in its turn forms part of the insulin molecule. This was utilized to stain islets in vitro in the various stages of the transplantation preparation process. The staining with ditizone was stressed when the islets were adhering with the duct tissue, and when the acinic cell was degranulated 15.
Another critical question is in relation to the fragmentation of the islets, which could lead to an overestimate of the results from counting the islets. This possibility was minimized by the exclusion of all the endocrine particles smaller than 60 mm in the count that was made of pancreatic islet cells stained by ditizone and observed with the stereoscopic microscope. Careful examination of the particles revealed that some of them became fragments of large islets. It is probable that the digestion, the division of the pancreatic tissue, and the agitation of the tissue preparation for the transplantation caused fragmentation of the islets and some losses 3.
CONCLUSIONS
In dogs, separation of Langerhans islets from the exocrine pancreatic tissue by means of the mechanical-enzymatic technique, with stationary digestion using collagenase and discontinuous dextran density gradient could obtain sufficient numbers of islets and represents an efficacious method for obtaining a critical mass of endocrine pancreatic tissue for Langerhans islet transplantation experiments.
Waisberg J, Soares HP, Altieri LG, Ramaciotti O, Speranzini MB. Isolamento das ilhotas pancreáticas pela separação mecânica-enzimática digestão estacionária com colagenase e purificação com gradiente de densidade descontínua de dextran: estudo experimental em cães. Acta Cir Bras [serial online] 2002 Mar-Abr;17(2). Disponível em URL: http://www.scielo.br/acb.
RESUMO: A perspectiva do alotransplante de ilhotas pancreáticas no homem está na dependência do desenvolvimento de métodos que propiciem quantidades suficientes de ilhotas pancreáticas, originadas de doador único, capazes de, quando transplantadas, levarem à normalização do metabolismo dos hidratos de carbono. Objetivo: Avaliar, em cães, a eficácia do isolamento das ilhotas de Langerhans por meio da técnica de separação mecânica-enzimática, digestão estacionária com colagenase e purificação pelo gradiente de densidade descontínua de dextran. Métodos: A contagem do número e avaliação do tamanho das ilhotas foi realizada pela coloração com difeniltiocarbazona e exame em microscópio estereoscópico munido de retícula ocular para medição do diâmetro médio das ilhotas coradas. Resultados: O número médio de ilhotas isoladas foi de 81032,20 ± 24736.79 e o número médio de ilhotas/kg de peso corpóreo foi de 6938,70± 1392,43. O número médio de ilhotas/kg de peso corpóreo obtido mostrou correlação significativa com peso corpóreo e peso do pâncreas extirpado. Conclusão: Estes resultados sugerem que o número de ilhotas isoladas, de um único doador, por meio da separação mecânica-enzimática, digestão estacionária com colagenase e purificação pelo gradiente de densidade descontínua de dextran pode ser suficiente para o sucesso do transplante de ilhotas pancreáticas em cães.
DESCRITORES: Ilhotas de Langerhans. Transplante. Diabetes Mellitus. Diabetes Mellitus, Experimental.
Conflito de interesses: nenhum
Fontes de financiamento: nenhuma
Address for correspondence:
Jaques Waisberg
Rua das Figueiras, 550/134
Santo André - SP
09080-300
Tel: (11)444-2461 Fax: (11)444-2160
Data do recebimento: 06/01/2002
Data da revisão: 23/01/2002
Data da aprovação: 13/02/200
- 1 - Wahoff DC, Papalois BE, Najarian JS, Kendall DM, Farney AC, Leone JP, Jessrun J, Dunn DL, Robertson RP, Sutherland DER. Autologus islet transplantation to prevent diabetes after pancreatic resection. Ann Surg 1995; 222:562-79.
- 2 - Kaufman DB, Morel P, Field MJ, Munn SR, Sutherland DER. Purified canine islet autograft. Transplantation 1990; 50:385-91.
- 3 - Alderson D, Kneteman NM, Olack BJ, Scharp D. Isolation and quantification of canine tissue for transplantation. Transplantation 1987; 4:579-81.
- 4 - Lakey JR, Cavanagh TJ, Zieger MA, Wright M. Evaluation of a purified enzyme blend for the recovery and function of a canine pancreatic islets. Cell Transplant 1998; 7:365-72.
- 5 - Ricordi C, Lacy PE, Finke EH, Olack BJ, Sharp DW. Automated method for isolation of human pancreatic islets. Diabetes 1988; 37:413-20.
- 6- Iwashita C, Asano T, Kenmochi T, Jingu K, Uematsu T, Nakagohri T, Hasegawa M, Maruyama M, Miyauchi H, Isono K. Combined method of mechanical chopper and automated two-step digestion technique for islet isolation from canine pancreas. Transplant Proc 1996; 28:337-8.
- 7 - Van der Vliet JA, Kaufman DB, Meloche RM, Heise JW, Field MJ, Heil JE, Najarian JS, Sutherland DER. A simple method of canine pancreatic islet isolation and intrahepatic transplantation. J Surg Res 1989; 46:129-34.
- 8 - Van der Burg MPM, Guicherit OR, Frölich M, Scherft JP, Bruijn JA, Gooszen HG. Impact of donor-related variables on islet isolation outcome in dogs. Diabetologia 1994; 37:111-4.
- 9 - Van der Burg MPM, Van Suylichem PTR, Guicherit OR, Frölich M, Lemkes HHPJ, Gooszen HG. Glycemic control mechanisms after canine islet autografting. Transplant Proc 1995; 27:3187-8.
- 10 - Cobb LF, Merrell RC. Total pancreatectomy in dogs. J Surg Res 1984; 37:234-40.
- 11 - Bonnevie-Nelson V, Skovgaard LT, Lernmark Ä. b-Cell function relative to islet volume and hormone content in the isolated perfused mouse pancreas. Endocrinology 1983; 112:1049-56.
- 12 - Horaguchi A, Merrell RC. Preparation of viable islet cells from dogs by a new method. Diabetes 1981; 30:455-8.
- 13 - Warnock GL, Rajotte RV. Critical mass of purified islets that induce normoglycemia after implantation into dogs. Diabetes 1988; 37:467-70.
- 14 Warnock GL, Dabbs KD, Evans MG, Cattral MS, Kneteman NM, Rajotte RV. Critical mass of islets that function after implantation in a large mammalian. Horm Metab Res 1990; 25(suppl):156-61.
- 15 - Latif Z, Noel J, Alejandro R. A simple method of staining fresh and cultured islets. Transplantation 1988; 45:827-30.
Publication Dates
-
Publication in this collection
05 June 2002 -
Date of issue
Apr 2002
History
-
Accepted
13 Feb 2000 -
Reviewed
23 Jan 2002 -
Received
06 Jan 2002