Abstracts
PURPOSE: To evaluate the feasibility of gastric ischemic conditioning by videolaparoscopy in rats, as well as its functional effect. METHODS: Twenty EPM-1 Wistar male rats were submitted to gastric devascularization through videolaparoscopy with intra-abdominal pressure of 4mmHg pneumoperitoneum through the placement of three ports into the abdominal cavity. The animals were distributed in two groups of 10 animals each according to their sacrifice day, 7 (G7) and 14 (G14) days for evaluation of gastric wall perfusion by laser-Doppler flowmetry before the devascularization, 10 minutes after and 7 or 14 days according to the group they belonged to. RESULTS: The flowmetry after the devascularization showed better progressive results of the gastric perfusion mainly in G14. CONCLUSIONS: The gastric ischemic conditioning model is feasible by videolaparoscopy. The ischemic conditioning allows the re-establishment of the blood perfusion on the gastric wall.
Video assisted surgery; Stomach; Ischemic conditioning; Rats
OBJETIVO: Avaliar a exeqüibilidade do condicionamento isquêmico por videolaparoscopia e o seu efeito funcional, na parede gástrica de ratos. MÉTODOS: Vinte ratos machos, EPM-1 Wistar foram submetidos a desvascularização gástrica proximal, por videolaparoscopia, com pressão intra-abdominal de pneumoperitônio de 4mmHg através da colocação de três trocartes na parede abdominal. Os animais foram distribuídos em dois grupos de 10 animais cada de acordo com a data de sacrifício, ou seja, 7 (G7) e 14 (G14) dias para avaliação da perfusão da parede gástrica com fluxometria por laser-Doppler antes da desvascularização, 10 minutos após e 7 ou 14 dias de acordo com o grupo a que pertenciam. RESULTADOS: A fluxometria revelou melhora progressiva da perfusão gástrica principalmente no G14, após a desvascularização. CONCLUSÕES: O modelo de condicionamento isquêmico do estômago é exeqüível por videolaparoscopia. O condicionamento isquêmico permite o restabelecimento da perfusão sangüínea na parede gástrica.
Cirurgia vídeo-assistida; Estômago; Condicionamento Isquêmico; Ratos
ORIGINAL ARTICLE
Videolaparoscopic model for the gastric ischemic conditioning in rats1 1 . Experimental model developed and studied for a doctoral thesis within the postgraduate program in Operative Technique and Experimental Surgery at the Department of Surgery, Federal University of São Paulo - Escola Paulista de Medicina (UNIFESP-EPM), São Paulo, Brazil.
Luís Fernando Paes LemeI; Edna Frasson de Souza MonteroII; José Carlos Del GrandeIII; Alessandro de RinaldisIV; Djalma José FagundesV
IDoctor; PhD from the postgraduate program in Operative Technique and Experimental Surgery, UNIFESP-EPM
IIAffiliated Professor, Department of Surgery, UNIFESP-EPM
IIIAdjunct Professor, Discipline of Surgical Gastroenterology, UNIFESP-EPM
IVCollaborating doctor, Discipline of Surgical Gastroenterology, UNIFESP-EPM
VCoordinator of the postgraduate program in Surgery and Experimentation, UNIFESP-EPM
Correspondence Correspondence to Luís Fernando Paes Leme Rua Biobedas, 148/ Apto. 133 04302-010 São Paulo SP - Brazil paeslemeana@uol.com.br
ABSTRACT
PURPOSE: To evaluate the feasibility of gastric ischemic conditioning by videolaparoscopy in rats, as well as its functional effect.
METHODS: Twenty EPM-1 Wistar male rats were submitted to gastric devascularization through videolaparoscopy with intra-abdominal pressure of 4mmHg pneumoperitoneum through the placement of three ports into the abdominal cavity. The animals were distributed in two groups of 10 animals each according to their sacrifice day, 7 (G7) and 14 (G14) days for evaluation of gastric wall perfusion by laser-Doppler flowmetry before the devascularization, 10 minutes after and 7 or 14 days according to the group they belonged to.
RESULTS: The flowmetry after the devascularization showed better progressive results of the gastric perfusion mainly in G14.
CONCLUSIONS: The gastric ischemic conditioning model is feasible by videolaparoscopy. The ischemic conditioning allows the re-establishment of the blood perfusion on the gastric wall.
Key words: Video assisted surgery. Stomach. Ischemic conditioning. Rats.
Introduction
Urschel et al.1,2 experimentally provoked ischemia in the proximal region of the stomach by means of ligature of the left gastric artery, via laparoscopic access. They observed a fall in the gastric blood flow immediately after the vascular ligation, followed by its reestablishment. This phenomenon was named ischemic conditioning of the stomach. Alfabet et al.3 evaluated laser-Doppler flowmetry in the gastric wall of rats at various times subsequent to ligature of the left gastric vessels. They showed that the blood flow returned to base levels after 10 days.
In utilizing the stomach as a substitute for the esophagus, such ischemic conditioning could be done as an operative procedure preceding esophagectomy. In this manner, adaptation of the vascularization of the segment to be transposed would occur, thereby preventing the complications resulting from ischemia in the region of the anastomosis.
Abdominal staging for cancer of the esophagus can be performed both via open surgery and via video laparoscopy. However, there has been increasing utilization of video laparoscopy, both as a diagnostic operative procedure and as a therapeutic approach, since its advent at the end of the 1980s. The greater utilization of this minimally invasive procedure, in substitution for conventional techniques, has been due to the fact that it promotes reduced morbidity and mortality during operations4.
On the one hand, video laparoscopy is a good method for the abdominal staging of cancer of the esophagus and promotes reduced operative morbidity. On the other hand, acute gastric ischemia is an important causal factor in dehiscence of the anastomosis. Therefore, to follow on with research topics within video laparoscopy at the Discipline of Operative Technique and Experimental Surgery of UNIFESP-EPM, it was decided to verify the feasibility of the video laparoscopic model for ischemic conditioning of the stomach in rats.
Methods
This work was assessed and approved by the research ethics committee of Hospital São Paulo and UNIFESP-EPM, protocol number 1196/00.
Twenty-three male rats (Rattus norvegicus albinus) of the EPM-1 Wistar strain were utilized, with an average weight of 358 grams and aged between 4 and 5 months, coming from the UNIFESP-EPM Development Center for Experimental Models for Medicine and Biology. The animals were kept in the sectoral vivarium of the Discipline of Operative Technique and Experimental Surgery of UNIFESP-EPM, for a period of seven days for their adaptation, receiving water and appropriate feed ad libitum. In the preoperative period, the animals were submitted to fasting, not receiving solid foods for 12 hours, while continuing to receive water ad libitum.
All the animals were submitted to induced pneumoperitoneum and gastric devascularization, since each animal was its own control. For the evaluation of the gastric blood perfusion, the animals were divided into two groups of 10 animals each, according to their day of sacrificing, i.e. 7 days (G7) or 14 days (G14) after the devascularization. Measurements were made of the blood flow in the gastric serosa, with oximetry in the animal's tail, at the following times: Ti - just after inducing pneumoperitoneum; T10, T7d and T14d - 10 minutes, 7 days and 14 days after the gastric devascularization, respectively.
Atropine sulfate at a dose of 0.044 mg/kg by subcutaneous route was utilized as a pre-anesthesia medication. The anesthesia administered was a compound containing 10 mg of xylazine per kg of body weight and 60 mg of cetamine per kg of body weight, applied by intramuscular route into the side of the animal's right rear paw. To perform the euthanasia, 19.1% potassium chloride was utilized, by endovenous route, after performing the reoperation, with the animals still under the effect of anesthesia.
Gastric devascularization
The anesthetized animals were placed on a plastic board in horizontal dorsal decubitus, and were fixed by the paws using adhesive tape. The board was, in its turn, placed on top of a heated bed that was regulated to 37 C.
Under antiseptic conditions, after trichotomy of the anterior abdominal wall, pneumoperitoneum was induced. The closed technique for inducing pneumoperitoneum was utilized, or in other words, the abdominal wall was punctured in the umbilical region using a Veress needle, and CO2 was insufflated at a rate of 0.5 liter per minute, with the utilization of an electronic insufflator (Walz®), until the predetermined intra-abdominal pressure of 4 mmHg was attained. The Veress needle was adapted so as to allow pneumoperitoneum to be induced and, subsequently, the passage of an optic fiber of 2 mm in diameter.
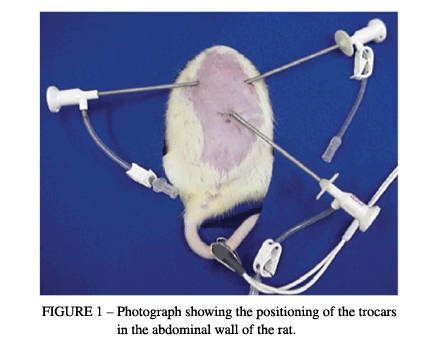
While the state of pneumoperitoneum at a pressure of 4 mmHg continued, two other punctures were made, with the placement of trocars of 2 mm in diameter in the right hypochondrium, 0.5 cm below the right costal margin and 1.5 cm from the xiphoid appendix, and in the left hypochondrium, 0.5 cm below the left costal margin and 1.5 cm from the xiphoid appendix (Figure 1).
The video-surgery instruments utilized in this experiment for access to the intraperitoneal cavity consisted of graspers, scissors, 5-mm clip applicators and 2-mm optic fibers. After positioning the trocars, the oxygen saturation was measured by utilizing an oximeter (Takaoka®). The sensor of the oximeter was connected to the animal's tail.
Then, utilizing the left trocar, an endoscopic transducer of 2 mm in diameter (E-Transonic Systems Inc) was introduced for laser-Doppler fluxometry (BLF-21DU - Transonic Systems Inc), and three measurements in the gastric fundus were made.
After making the measurements of the arterial O2 saturation and the blood flow in the gastric serosa, the devascularization of the gastric fundus was begun. Scissors introduced through the left trocar and graspers introduced through the right trocar were utilized to dissect the gastrohepatic ligament, approach the cavity behind the omentum and identify the left gastric arterial and venous trunks. The 2-mm trocar was replaced by a 5-mm trocar that allowed the introduction of an automatic clip applicator for ligating the left gastric artery and vein, and also the short gastric vessels, by means of the placement of vascular clips (Figures 2 and 3).
Ten minutes after performing this procedure, measurements were again made of the arterial O2 saturation (oximetry) and the blood flow in the serosa of the gastric fundus. A careful review of the cavity was then undertaken, and the graspers and trocars were then removed. After deflating the peritoneal cavity, the ports were closed using monofilament 5-0 polyamine thread. The animals were then kept in individual cages with water and feed appropriate for the species, for 7 or 14 days, according to the group they belonged to.
Reoperation
After the period determined for each group, the animals were again anesthetized and submitted to induced pneumoperitoneum at a pressure of 4 mmHg, according to the technique already described. After being left in a state of pneumoperitoneum for 10 minutes, the blood flow in the gastric serosa was measured through a trocar placed in the left hypochondrium, at three locations in the region of the gastric fundus.
Statistics
For the analysis of the results, parametric and non-parametric tests were utilized, taking into consideration the nature of the variables or the variability of the measurements made. The following tests were applied:
1 Student t test or Mann-Whitney test for comparing the 7-day and 14-day groups, in relation to the values of the variables studied.
2 Variance analysis by Friedman ranks, with the objective of comparing the initial, 10-minute and euthanasia times in relation to the flowmetry values, for each group separately.
For all the tests, the level for rejection of the nullity hypothesis was set at 0.05 or 5%, and the significant values were signaled using an asterisk.
Results
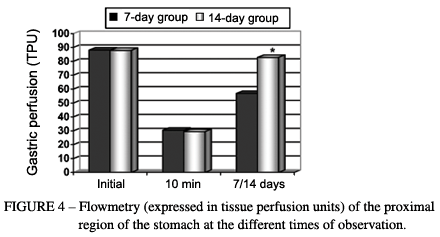
For the 7-day and 14-day groups, respectively, the tissue blood flow evaluated in the serosa of the proximal region of the gastric wall presented base values of 88 ± 6 and 88 ± 5 TPU (tissue perfusion units). Just after the ligation of the short gastric vessels and the left gastric artery and vein (10 minutes afterwards), there was a notable drop in the flow, to levels of 30 ± 4 and 29 ± 4 TPU, which progressively returned to the initial levels, reaching 57 ± 4 and 83 ± 6 TPU at 7 and 14 days after the operation, respectively. Variance analysis by Friedman ranks showed that the initial values were greater than those after the ligature of the gastric vessels (p < 0.05; critical X2 = 5.99; and calculated X2 = 20*). The Mann-Whitney test showed that the values at 14 days after the operation were greater than those at 7 days (p < 0.05; critical U = 23; and calculated U = 0*) (Figure 4). With regard to the oximetry, no differences between the groups were observed (Figure 5).
Discussion
Esophageal anastomoses present greater risk of dehiscence than do anastomoses performed in other segments of the digestive tract. Ligature of the short gastric, left gastric and gastro-omental arteries is usually necessary in order to mobilize the stomach to the cervical region for esophagogastric reconstruction in patients who have been submitted to esophagectomy. This devascularization leads to ischemia in the gastric fundus and is, consequently, held responsible for the high rate of dehiscence of esophagogastric anastomoses5,6,7. Nonetheless, there are authors who believe that this proximal gastric devascularization does not interfere in the irrigation of the stomach, because of the presence of the rich anastomotic network of the mucosal and submucosal layers8,9,10.
Some authors11,12,13 have reported a fall in gastric blood perfusion during the construction of the gastric tube, especially after ligature of the left gastric artery, although there was no correlation with the occurrence of fistulae in the anastomoses. On the other hand, Abo et al.14 found a direct correlation between the occurrence of fistulae in the anastomoses and reduced blood perfusion in the gastric tube, in a clinical study that utilized laser-Doppler flowmetry as the evaluation method.
With the aim of achieving better perfusion of the gastric fundus, Urschel et al.1,2 proposed the ligature of the left gastric artery to promote better vascularization in the region, prior to transposition of the stomach in experimental studies using rats. This proposal was based on the principle utilized in plastic surgery, for late-stage transposition of flaps, and has been named ischemic conditioning of the stomach. This better perfusion may be due not only to new vascularization that would occur as a response to continual tissue ischemia, but also to the alteration in the blood flow of the microcirculation promoted by denervation15 and the vascularization resulting from the scarring process itself16.
In our laboratory, Alfabet et al.3 made a study of the functioning of the gastric wall at different postoperative times and showed a recovery of tissue perfusion from the seventh day onwards and a return to base levels on the tenth day. The animal model chosen was the rat, which has been proposed by several authors who have shown this to be an experimentation animal that is suitable for the study of ischemic conditioning, and also for accomplishing research on video-surgery17,18.
In the present work, the hypothesis that ischemic conditioning can be induced by the ligature of the left gastric vessels and short gastric vessels in rats, via video laparoscopy, has been verified. It has been shown that the reestablishment of perfusion of the gastric wall, by means of ischemic conditioning of the stomach, takes place in a way similar to how it occurs in open surgery. Thus, if the studies on ischemic conditioning of the stomach are confirmed and reach the stage of extrapolation to patients with esophageal cancer, ligature of the left gastric vessels and short gastric vessels could be performed during video laparoscopy for the diagnosis and staging of the tumor. Moreover, ligature of the short gastric vessels has been added to the model put forward by Urschel et al.1,2, thereby bringing the experimental model closer to the human model, given that this step is essential for the mobilization of the stomach and also for the construction of the gastric tube. Because the ischemic conditioning of the stomach that was obtained is similar to what is presented in the literature, video-surgery as an operative technique for performing ischemic conditioning is feasible and viable, both in the experimental environment and in clinical practice.
References
Received: June 12, 2004
Review: July 21, 2004
Accepted: August 30, 2004
Conflict of interest: none
Financial source: none
How to cite this article:
Leme LFP, Montero EFS, Del Grande JC, Rinaldis A, Fagundes DJ. Videolaparoscopic model for the gastric ischemic conditioning in rats. Acta Cir Bras [serial online] 2004 Sept-Oct;19(5). Available from URL: http://www.scielo.br/acb [also in CD-ROM].
*Color figures available in www.scielo.br/acb.
- 1. Urschel JD, Antkowiak JG, Delacure MD, Takita H. Ischemic conditioning (delay phenomenon) improves esophagogastric anastomotic wound healing in the rat. J Surg Oncol 1997; 66: 254 6.
- 2. Urschel JD. Ischemic conditioning of the rat stomach: implications for esophageal replacement with stomach. J Cardiocasc Surg 1995; 36: 191 3.
- 3. Alfabet C, Montero EFS, Paes Leme LF, Higashi VS, Sallum Fo CFC, Fagundes DJ, Gomes PO. Progressive gastric perfusion in rats: Role of ischemic conditioning. Microsurgery 2003; 23(5):513-6.
- 4. Soper NJ, Burnt LM, Kerbe K. Laparoscopic general surgery. N Engl J Med 1994; 330: 409 19.
- 5. Urschel JD. Esophagogastrostomy anastomotic leaks complicating esophagectomy: a review. Am J Surg 1996; 169: 634 40.
- 6. Balieux J, Adham M, Roche E, Meziat-Burdin A, Poupart M, Ducerf C, Carcinoma of the oesophagus. Anastomotic leaks after manual sutures incidence and treatment. Int Surg 1998; 83: 277 9.
- 7. Pinotti HW. Acesso extrapleural ao esôfago por frenolaparotomia. Ver Ass Med Brasil 1976; 22: 57 60.
- 8. Thomas DM, Langford Rm, Russell RCG, Le Quesne LP. The anatomical basis for gastric mobilization in total oesophagectomy. Br J Surg 1979; 66:20333.
- 9. Guerra A, Rodrigues H. Anatomia cirúrgica das artérias gástricas: seus territórios de predominância de distribuição e suas anastomoses. Rev Col Bras Cir 1982; 9:5560.
- 10. Schwember G, Aguila P, Miranda IB. Vascularización gástrica em preparacion de tubo gástrico para un reemplazo esofágico. Rev Chil Cir 1990; 42:3147.
- 11. Schilling MK, Redaelli C, Maurer C, Friess H, Büchler MW. Gastric microcirculatory changes during gastric tube formation: assessment with Laser-Doppler flowmetry. J Surg Res 1996; 62:1259.
- 12. Pierie J-PEN, DeGraaf PW, Poen H, e col. Impaired healing of cervical oesophagofastrostomies can be predicted by estimation of gastric serosal blood perfusion by laser Doppler flowmetry. Eur J Surg 1994; 160:599603.
- 13. Schilling MK, Mettler D, Redaelli C, Buchler MW. Circulatory and anatomic differences among experimental gastric tubes as esophageal replacement. World J Surg 1997; 21:9927.
- 14. Abo S, Kitamura M, Hashimoto M e col. Analysis of results of surgery performed over a 20-year period on 500 patients with cancer of the thoracic esophagus. Surg Today 1996; 26:7782.
- 15. Cutting CB, Robson MC, Koss N. Denervation supersensitivity and delay phenomenon. Plast Reconstr Surg 1978; 61:8817.
- 16. Jonsson K, Hunt TK, Brennan SS, Mathes SJ. Tissue oxygen measurements in delayed skin flaps: a reconsideration of the mechanisms of the delay phenomenon. Plast Reconstr Surg 1988; 82:32835.
- 17. Berguer R, Gutt C N. Laparoscopic colon surgery in a rat model. A preliminary report. Surg Endosc 1994; 8:11957.
- 18. Goldenberg A, Lobo EJ, Marcondes W, Louzada M, Barbosa CDL. Proposição de laparoscopia em ratos. Acta Cir Bras 1997; 12:21920.
Publication Dates
-
Publication in this collection
17 Nov 2004 -
Date of issue
Oct 2004
History
-
Reviewed
21 July 2004 -
Received
12 June 2004 -
Accepted
30 Aug 2004