Abstracts
To describe the integration process of grafts of total human skin and keloid in hamster (Mesocricetus auratus) cheek pouch, whose sub-epithelium is naturally an "Immunologically Privileged Site". Fragments of human normal skin and keloid from the breast region of mulatto female patients were transplanted into the cheek pouch subepithelium in situ. Surgical procedure and grafted pouches for microscopic exam at several time points of the transplantation were standardized. The integration of grafted fragments of human skin and keloid was seen in late periods (84 days) since the microscopic assessment showed the presence of blood vases within the conjunctive tissue of grafted fragments. It was also possible to see among the grafted fragments the epithelium, the appearing of early cellular infiltrated, epithelial secretion of keratin, the presence of melanocytes, and delayed changes on the aspect of collagen fibers of conjunctive tissue. Pooled results allow to define hamster cheek pouch sub-epithelium as an experimental model to investigating heterologous graft physiology of human total skin and keloid with epithelium.
Mesocricetus; Transplantation, heterologous; Skin transplantation; Keloid
Descrever a integração dos enxertos de pele total humana e quelóide na bolsa jugal do hamster (Mesocricetus auratus), cujo subepitélio é, naturalmente, um "Local de Privilégio Imunológico". Foram transplantados fragmentos, de pele humana normal e de quelóide, obtidos da região mamária de pacientes pardas, no subepitélio da bolsa jugal in situ. O procedimento operatório, e de preparo das bolsas enxertadas para exame microscópico em vários períodos de transplante, foi padronizado. Verificou-se a integração dos fragmentos enxertados de pele humana e de quelóide em períodos tardios (84 dias), uma vez que a avaliação microscópica revelou a presença de vasos sangüíneos no tecido conjuntivo dos fragmentos enxertados. Foi também possível observar, nos fragmentos enxertados, o epitélio, o aparecimento de infiltrado celular incipiente, secreção epitelial de queratina, presença de melanócitos e alterações tardias no aspecto das fibras colágenas do tecido conjuntivo. Os resultados, em conjunto, permitem estabelecer o subepitélio da bolsa jugal do hamster como modelo experimental para investigação da fisiologia de tranplantes heterólogos de pele total humana e quelóide com epitélio.
Mesocricetus; Transplante heterólogo; Transplante de pele; Quelóide
EXPERIMENTAL MODELS
Hamster (Mesocricetus auratus) cheek pouch as an experimental model to investigate human skin and keloid heterologous graft1 1 This paper is a part of a Thesis presented to Federal University of São Paulo Paulista School of Medicine in 2002 by the Post-Graduation Program of Recovering Plastic Surgery (UNIFESP-EPM), to reach the Master Degree. The experimental work was performed in the Discipline of Immunology of the Parasitology, Microbiology, and Immunology Department of UNIFESP-EPM.
Bolsa jugal no hamster (Mesocricetus auratus) como modelo experimental de investigação de enxertos heterólogos de pele humana e quelóide
Bernardo HochmanI; Lydia Masako FerreiraII; Flaviane Cássia Vilas BôasIII; Mario MarianoIV
IMaster Degree in Plastic Surgery and Post-Graduation Student at Doctor level by the Post-Graduation Program of Recovering Plastic Surgery of UNIFESP-EPM
IITitular Professor and Head of the Discipline Plastic Surgery of the UNIFESP-EPM Surgery Department, and Coordinator of the Post-Graduation Program for Recovering Plastic Surgery of UNIFESP-EPM
IIIMedical Doctor of UNIFESP-EPM
IVTitular Professor of the Biomedical Sciences Institute of São Paulo University (USP) Immunology Department, Titular Professor of the Pathology Department of Veterinary Medical and Zootechnical School of USP, and Visitor Professor of the Discipline Immunology in the Parasitology, Microbiology, and Immunology Department of UNIFESP-EPM
Correspondence Correspondence to Dr. Bernardo Hochman UNIFESP-EPM, Plastic Surgery Division, Surgery Division Rua Napoleão de Barros, 715, 4° andar CEP 04024-900 São Paulo Tel: (11)55760418 FAX: (11) 55716579 sandra.dcir@epm.br, lydia.dcir@epm.br
ABSTRACT
To describe the integration process of grafts of total human skin and keloid in hamster (Mesocricetus auratus) cheek pouch, whose sub-epithelium is naturally an "Immunologically Privileged Site". Fragments of human normal skin and keloid from the breast region of mulatto female patients were transplanted into the cheek pouch subepithelium in situ. Surgical procedure and grafted pouches for microscopic exam at several time points of the transplantation were standardized. The integration of grafted fragments of human skin and keloid was seen in late periods (84 days) since the microscopic assessment showed the presence of blood vases within the conjunctive tissue of grafted fragments. It was also possible to see among the grafted fragments the epithelium, the appearing of early cellular infiltrated, epithelial secretion of keratin, the presence of melanocytes, and delayed changes on the aspect of collagen fibers of conjunctive tissue. Pooled results allow to define hamster cheek pouch sub-epithelium as an experimental model to investigating heterologous graft physiology of human total skin and keloid with epithelium.
Key words: Mesocricetus. Transplantation, heterologous. Skin transplantation. Keloid.
RESUMO
Descrever a integração dos enxertos de pele total humana e quelóide na bolsa jugal do hamster (Mesocricetus auratus), cujo subepitélio é, naturalmente, um "Local de Privilégio Imunológico". Foram transplantados fragmentos, de pele humana normal e de quelóide, obtidos da região mamária de pacientes pardas, no subepitélio da bolsa jugal in situ. O procedimento operatório, e de preparo das bolsas enxertadas para exame microscópico em vários períodos de transplante, foi padronizado. Verificou-se a integração dos fragmentos enxertados de pele humana e de quelóide em períodos tardios (84 dias), uma vez que a avaliação microscópica revelou a presença de vasos sangüíneos no tecido conjuntivo dos fragmentos enxertados. Foi também possível observar, nos fragmentos enxertados, o epitélio, o aparecimento de infiltrado celular incipiente, secreção epitelial de queratina, presença de melanócitos e alterações tardias no aspecto das fibras colágenas do tecido conjuntivo. Os resultados, em conjunto, permitem estabelecer o subepitélio da bolsa jugal do hamster como modelo experimental para investigação da fisiologia de tranplantes heterólogos de pele total humana e quelóide com epitélio.
Descritores:Mesocricetus. Transplante heterólogo. Transplante de pele. Quelóide.
Introduction
In 1963, Shepro, Kula, Halkett1 expressed the enthusiasm of the scientific community of that time, when they referred to the hamster cheek pouch sub-epithelium as a model for transplantation studies:"'Atypical', 'privileged', 'unique' are but a few of the many terms utilized by investigators in their description of the hamster cheek pouch as a site for the transplantation of normal and abnormal tissues." These comments where based on the known, but up to now not completely understood, phenomenon of the integration of both homo- and heterologous grafts in the hamster cheek pouch.
Cheek pouches are sac-like diverticles or invaginations of highly bilaterally distensible cheek mucosas, whose main functions are the storage and transportation of food. The cheek wall is constituted by epithelial cells layers which are supported by loose and naturally immunodeficient areolar conjunctive tissue, which is considered an "Immunological Privileged Site".
An Immunological Privileged Site would be mainly a result of the absence or deficiency of a suitable afferent anatomical pathway to drain antigenic material to a lymphatic regional station which would recognize it and would start an immune response.2 In the hamster cheek pouch sub-epithelium these would be regionally the surface cervical lymphonodes.1,3
Existing literature is scarce and not enough detailed about total or keloid human skin transplantation in the hamster cheek pouch, which is not approached neither standardized as an experimental model for studying these tissues.4
The ultra-structure of the pouch wall epithelium is similar to the human skin, reason why it is also known as "skin without follicles and glands".5 It presents a basal, spiny, granular and corneous layer which is similar to the human gum epidermis or epithelium, or hard palate.6,7
In terms of keloid, since Homo sapiens is the only known species in which this lesion is developed, this is a major limitation for obtaining an experimental model in vivo, which could be consensually accepted.8
Proposition
The present study has as its primary objective to analyze this animal, which presents an immunological privileged site, as an experimental model for studying the integration of skin heterologous grafts and keloid.
Method description
Surgical technique
Surgeries in hamsters are performed under general intraperitoneal anesthesia. An association of 2-(2,6 xylidine)-5,6-dihydro-4H-1,3-tiazine hydrochloride (Rompun®) in the dose 0.1 mL/kg with ketamine hydrochloride (Francotar®) in the dose 0.075 mL/kg is recommended. Cheek pouches have to be washed from the oral cavity through water jets applied with a plastic syringe without needle, in order to remove any food or sawdust remainders, which may be present in them. Each pouch is out reversed by an eversion maneuver with 2 Adson-Brown's forceps, which put it distended and fixed with 13 x 4.5 sized needles, on the coated styropor surface of the operating field (FIGURE 1).
Fragments to be grafted are obtained after resection, with curved scissors iris, of the skin or keloid remaining adipose tissue from the surgical suite. These fragments may be extracted from any part of the epithelium surface by using a 2 mm diameter circular punch. Once all the skin or keloid fragments are extracted, they are reserved within a polystyrene reservoir with ice, immerged and protected inside a plastic container with 0.9% physiologic solution, until the moment of being grafted (FIGURE 2).
A 5 mm incision is made on a proximal avascularized area related to the animal mouth, from the first wall or epithelial layer of the pouch wall, in order to not touching the pouch retractor muscle fibers, inserted into its proximal third, with the aim of avoiding a possible contact with lymphatic vases.9 With delicate dissection scissors, a rhombic divulsion in tunnel is performed on the conjunctive tissue in the opposite direction of the animal mouth, to the most distal pouch site. With a long and thin pincer, the skin or keloid fragment is inserted between two layers of the pouch epithelium (intrawall position), at its most distal site. Once positioned, the grafted fragment is slightly compressed between the two epithelial layers of the pouch wall, with one of the index fingers. This maneuver aims to allow the fragment naturally fixed by the adjacent areolar conjunctive tissue (FIGURE 3). Epithelium incision borders are united by simple bi-digital coaptation of the wound borders. Then, the pouch is invaginated into the oral cavity.10
Operative procedures in animals may be performed without following, as a mandatory measure, the rigor of conventional standards for antisepsis and asepsis.11 Al least procedure latex gloves should be worn to protect the staff.12
Vase lesions at the dissection must be avoided to protect any possible antigenic contact through blood. After being invaginated, the pouch wall accommodates the grafts and protects them from external traumas. Thus, the use of post-operative protective dressing is not needed and several animals are allowed in each cage.
Caution must be used to not perform an excessive divulsion of the pouches sub-epithelium. Pouch muscle contraction the animal applies to empty the pouch content may make easy the grafted fragment extrusion.
Pouches preparation technique for histological exam .
The preparation of the grafted fragment to be sent to histological exam has to be standardized to direct the laboratory technician to find the graft. Sometimes, the graft is not easily visible after dehydration and formaldehyde treatment.
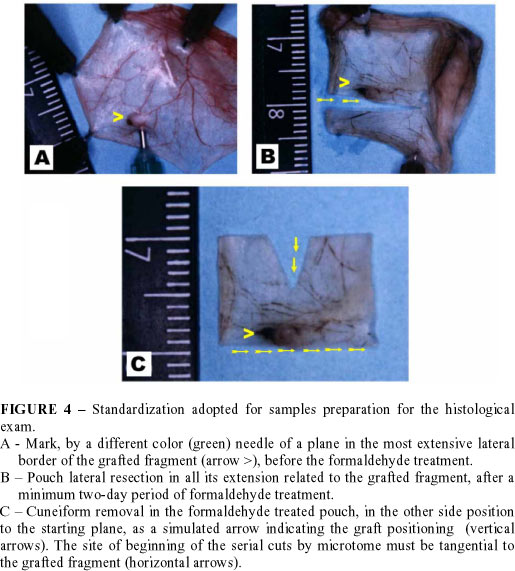
Resected cheek pouches containing the grafted fragment are disposed on a small piece of polystyrene and fixed with 13 x 4.5 (brown colored) needles. To make easy the location of the formaldehyde treated graft, its most extensive border is marked with a different needle (30 x 8 or green color, for example), transfixing the pouch in a lateral positioning to the graft, and it is agreed to opt by the lateral border which is the most distal to the pouch retractor muscle. Afterwards, samples disposed this way, including the polystyrene piece, are immerged into flasks with 12% formaldehyde. These flasks should be wrapped with aluminum foil to prevent the fixing photodegradation. After 48 hours of formaldehyde fixing the sector of the pouch, which contains the graft, is cut in a rectangular shape. One of the larger sides of this rectangular segment is sectioned tangentially to the grafted fragment, at the level of the plane marked by the hole of the different needle left (30 x 8 or green colored). In the other great side of the rectangle, with straight iris scissors, a small cuneiform triangle is cut. This triangular cut is to simulate for the histology technician a kind of arrow pointed to the border where the graft is located and, from where the microtome cuts have to begin. The most extensive lateral border of the grafted fragment is marked aiming that histological cuts may enfold both epithelium and dermis (FIGURE 4).13 Pieces should be sent to the histology laboratory for being processed, in tubes with 12% formaldehyde solution and protected from light.
Perspectives
Hamster cheek pouch epithelium may be used, a priori, for grafting tissues from different organs. Indications of the present study focuses heterologous grafts of both normal skin and keloid in the pouch in situ, and they are extensive to studies of heterologous grafts of hypertrophic scares, neoplasias, and other cutaneous diseases.
Pouch epithelium shows histological similarity with human skin (FIGURE 5).
In terms of keloid, the pouch presents tissue mast cells with a secretion pattern similar to the human one. This is an interesting characteristic to be studied for keloid, since this lesion presents an increased amount of mast cells and histamina (FIGURE 6).14
In the hamster cheek pouch there is not need of de-epithelization of the skin or keloid fragments to be grafted. The purpose of this procedure, which is a routine in the keloid grafts in athymic mice has as its objective that the tissue fragments are not externally grafted on the animals skin.15,16 This tactic aims that the grafts be more protected from losts caused by external traumas, besides increasing the contact surface with the receptor bed to make easier the integration of the grafted tissue. However, this tactic makes impossible the study of the grafted tissue epithelium. Therefore, the study of the cutaneous graft epithelium has an accurate indication in the hamster cheek pouch as an experimental model.
When possible, the pouch in situ grafting is preferable, due to its greater operational easiness and practicity. It is indicated in cases where there is interest of analyzing an epithelium with complete architecture, in studies whose observation time is inferior to the period in which the efective compression would start by the keratin encystment (approximately from the 6th week).4,13
However, it is possible to indicate heterologous grafts in situ in researches where the presence of the epithelium or even its compression by the keratin encystment is not relevant to the study objective, or in cases of dermis or only conjunctive tissue graft. In addition to these examples of direct grafting, it is also possible to include in situ epidermis and centrifugated dermis cells grafting,17 under the form of soluble filtrated as tumoral cells,18 or from cells culture.
Another peculiarity allowed by this experimental model, is that pouches sub-epithelia are, from an immunologic point of view, independent from each other. Local events, occurring by the integration of a homo- or heterologous graft in a pouch, would not have influence on the other side pouch.19 It would be possible grafting, in the same animal, fragment of skin in a pouch, and keloid in the other one.
The hamster presents, in its cheek pouch, a site of easy approach, with a minimum trauma, and its dissection is made easier because the epithelial walls of the pouch are translucent. So, hamster cheek pouch is, indeed, a suitable experimental model for investigating the integration process of skin or keloid heterologous grafts.
Conflito de interesse: nenhum
Fonte de financiamento: CAPES
Hochman B, Ferreira LM, Vilas Bôas FC, Mariano M. Hamster (Mesocricetus auratus) cheek pouch as an experimental model to investigate human skin and keloid heterologous graft. Acta Cir Bras [serial online] 2004 Vol 19 Special Edition. Available on URL: http://www.scielo.br/acb.
- 1. Shepro D, Kula N, Halkett JAE - The role of the cheek pouch in effecting transplantation immunity in the hamster. J Exp Med. 1963;117: 749-54.
- 2. Head JR, Billingham RE - Immunologically privileged sites in transplantation immunology and oncology. Perspec Biol Med. 1985;29(1):115-31.
- 3. Arruda MSP, Montenegro MR - The hamster cheek pouch: an immunologically privileged site suitable to the study of granulomatous infections. Rev Inst Med Trop São Paulo. 1995;7(4):303-9.
- 4. Kreider JW, Haft HM, Roode PB - Growth of human skin on the hamster. J Invest Dermatol. 1971;57(1):66-71.
- 5. Duling BR - The preparation and use of the hamster cheek pouch for studies of the microcirculation. Microvasc Res. 1973;5:423-9.
- 6. White FH, Gohari K - Cellular and nuclear volumetric alterations during differentiation of normal hamster cheek pouch epithelium. Arch Dermatol Res. 1982;273:307-18.
- 7. Whittle S, Swartzendruber DC, Kremer M, Squier CA, Wertz W - Lipids of hamster cheek pouch epithelium. Lipids. 1997;32(9):961-4.
- 8. Placik OJ, Lewis Vl - Immunologic Associations of keloids. Surg Gynecol Obstet. 1992;175:185-93.
- 9. Goldenberg DM, Steinborn W - Reduced lymphatic drainage from hamster cheek pouch. Proc Soc Exp Bio Med. 1970;135:724-6.
- 10. Hochman B, Ferreira LM, Bôas FCV, Mariano M - Investigação do transplante heterólogo de quelóide na bolsa jugal do hamster (Mesocricetus auratus). Acta Cir Bras. 2003;18(4):266-71.
- 11. Benhaim P, Anthony JP, Ferreira L, Borsanyi JP, Mathes SJ - Use of combination of low-dose cyclosporine and RS-61443 in a rat hindlimb model of composite tissue allotransplantation. Transplantation. 1996;61(4):527-32.
- 12. Lemon HM, Lutz BR, Pope R, Parsons L, Handler AH, Patt DI - Survival and growth of human tissues transplanted to hamster cheek pouch. Science. 1952;115:461-4.
- 13. Hochman B,Ferreira LM, Bôas FCV, Mariano M - Integração do enxerto heterólogo de pele humana no subepitélio da bolsa jugal do hamster (Mesocricetus auratus). Acta Cir Bras. 2003;18(5):415-30
- 14. Hakanson R, Sjoberg NO - Direct histochemical demonstration of histamine in cutaneous mast cells: urticaria pigmentosa and keloids. Experientia. 1969;25:854-5.
- 15. Shetlar MR, Shetlar CL, Hendricks L, Kischer CW - The use of athymic nude mice for the study of human keloids. Proc Soc Exp Biol Med. 1985;179: 549-52.
- 16. Estrem SA, Domayer M, Bardach J, Cram AE - Implantation of human keloid into athymic mice. Laryngoscope. 1987;97: 1214-8.
- 17. Wolf J, Harrison RG - The angiogenic stimulus of epidermis and epidermal components on the microvasculature of the hamster cheek pouch. Bibl Anat. 1973;12:490-6.
- 18. Wolf JE, Hubler WR. -Tumor angiogenic factor and human skin tumors. Arch Dermatol.1975;111:321-7.
- 19. Cohen SN - Comparision of autologous and heterologous normal skin grafts in the hamster cheek pouch. Proc Soc Exp Bio Med. 1961;106:677-80.
Publication Dates
-
Publication in this collection
17 Mar 2005 -
Date of issue
Dec 2004