Abstracts
The purpose of this study is to report the use of guinea pigs as experimental model to study the resitance of the tissue expander capsule. Two groups were studied. In both groups a round 20 cc tissue expander, attached to a multiperforated catheter was inserted. The pocket housing was standardized. The skin over the expanded area was tattooed demarcating the area and arc, which were measured before and after each expansion. Every 4 days, a volume corresponding to 10% of the expander's total capacity was injected. Animals of the control group received 3 cc of saline through the catheter at the same time of expansion. Animals of the experimental group received 3 cc of the HBGF-1 diluted with saline. The intraluminal pressure of the expander was measured before and after the injection. After its total filling, the animals were sacrificed and 5 cc more were injected into each expander. The pressure was measured after each 1 cc injected. The capsule was examined histologically by immunofluorescence, trichrome and hematoxylin-eosin stains with the purpose of quantifying collagen and fibroblasts. The experimental model to study the resistance of the tissue expander's capsule showed to be feasible in guinea pigs.
Guinea pigs; Capsular contracture; Tissue expander; HBGF-1
O objetivo deste estudo é relatar o uso de cobaias como modelo experimental para estudar a resistência da cápsula de expansores. Dois grupos foram estudados. Em ambos os grupos um expansor Redondo de 20 ml com um cateter multiperforado fixadoforam inseridos nos animais. A loja foi padronizada. A pele a ser expandida foi tatuada demarcando uma area e um arco que foram medidos a cada expansão. A cada 4 dias. Um volume correspondendo a 10 % da capacidade total do expansor foi injetado. Os animais do grupo controle receberam 3 ml de soro fisiológico pelo catéter de irrigação no mesmo momento da expansão. Os animais do grupo experimental receberam 3 ml de HBGF-1 diluido com soro fisiológico. A pressão intraluminal do expansor foi medida antes e após a injeção. Após o prenchimento total do expansor, os animais foram sacrificados e mais 5 ml foram injetados dentro de cada expansor. A pressão foi medida após cada 1 ml injetado. A cápsula foi examinada histologicamente por immunofluorescência, tricromo e hematoxilina-eosina com a finalidade de quantificar colágeno e fibroblastos. O modelo experimental para estudar a resistência da cápsula do expansor de pele mostrou-se factível em cobaias.
Cobaia; Contratura capsular; Expansor de pele; HBGF-1
EXPERIMENTAL MODELS
Guinea pigs as experimental model to evaluate the resistance of the tissue expander capsule1 1 Study developed at Federal University of São Paulo UNIFESP/EPM.
Cobaias como modelo experimental para avaliar a resistência da cápsula do tecido expandido
Fábio Xerfan NahasI; Luis O. VasconezII; Lydia Masako FerreiraIII
IPhD, Ínterim Professor, Plastic Surgery, Surgery Department of the Federal University of São Paulo UNIFESP/EPM
IIHead of the Plastic Surgery Division of Alabama University - Birmingham
IIIMD, PhD, Titular and Head of the Plastic Surgery, Surgery Department of the Federal University of São Paulo UNIFESP/EPM
Correspondence Correspondence to Fabio Xerfan Nahas UNIFESP-EPM, Plastic Surgery Division, Surgery Division Rua Napoleão de Barros, 715, 4º andar CEP 04024-900 São Paulo SP Tel: (11) 5576.4118 Fax: (11) 5571.6579 fabionahas@uol.com.br
ABSTRACT
The purpose of this study is to report the use of guinea pigs as experimental model to study the resitance of the tissue expander capsule. Two groups were studied. In both groups a round 20 cc tissue expander, attached to a multiperforated catheter was inserted. The pocket housing was standardized. The skin over the expanded area was tattooed demarcating the area and arc, which were measured before and after each expansion. Every 4 days, a volume corresponding to 10% of the expander's total capacity was injected. Animals of the control group received 3 cc of saline through the catheter at the same time of expansion. Animals of the experimental group received 3 cc of the HBGF-1 diluted with saline. The intraluminal pressure of the expander was measured before and after the injection. After its total filling, the animals were sacrificed and 5 cc more were injected into each expander. The pressure was measured after each 1 cc injected. The capsule was examined histologically by immunofluorescence, trichrome and hematoxylin-eosin stains with the purpose of quantifying collagen and fibroblasts. The experimental model to study the resistance of the tissue expander's capsule showed to be feasible in guinea pigs.
Key words: Guinea pigs. Capsular contracture. Tissue expander. HBGF-1.
RESUMO
O objetivo deste estudo é relatar o uso de cobaias como modelo experimental para estudar a resistência da cápsula de expansores. Dois grupos foram estudados. Em ambos os grupos um expansor Redondo de 20 ml com um cateter multiperforado fixadoforam inseridos nos animais. A loja foi padronizada. A pele a ser expandida foi tatuada demarcando uma area e um arco que foram medidos a cada expansão. A cada 4 dias. Um volume correspondendo a 10 % da capacidade total do expansor foi injetado. Os animais do grupo controle receberam 3 ml de soro fisiológico pelo catéter de irrigação no mesmo momento da expansão. Os animais do grupo experimental receberam 3 ml de HBGF-1 diluido com soro fisiológico. A pressão intraluminal do expansor foi medida antes e após a injeção. Após o prenchimento total do expansor, os animais foram sacrificados e mais 5 ml foram injetados dentro de cada expansor. A pressão foi medida após cada 1 ml injetado. A cápsula foi examinada histologicamente por immunofluorescência, tricromo e hematoxilina-eosina com a finalidade de quantificar colágeno e fibroblastos. O modelo experimental para estudar a resistência da cápsula do expansor de pele mostrou-se factível em cobaias.
Descritores: Cobaia. Contratura capsular. Expansor de pele. HBGF-1.
Introduction
Tissue expansion has been diligently investigated, particularly regarding the ideal shape of the expanders, their surface and the effects of the prosthesis on the nearby tissues. However, few articles have been published about reduction of capsular contracture around the silicone membrane of the expander1-3.
Growth factors are substances capable of modulating cellular mitosis. The Heparin Binding Growth Factor 1 (HBGF-1) regulates the formation of the scar for it modulates the cellular response. This growth factor has a short half-life and needs to bind with heparin or collagen to increase its life span and maintain its integrity in the extra-cellular environment4. There is only one study in the literature concerning the use of HBGF-1 to attenuate the contracture of the capsular reaction to silicone5. A more flexible capsule around the expander would result in: A) A better adjustment of the flap on the covered area; B) Less chance of extrusion; C) Major advancement of the flap without the necessity of resecting the capsule.
Proposition
The purpose of this study is to report the use of guinea pigs as experimental model to study the resistance of the tissue expander capsule.
Method description
Adult guinea pigs weighting from 290 to 340 g, from the University of Alabama at Birmingham, were studied.
Procedure
The animals were divided into two groups. A round 20 cc tissue expander was inserted in all the animals. An irrigation system composed of a multiperforated silicone catheter with a valve at its extremity was fixed around the base of the expander (FIGURE 1).
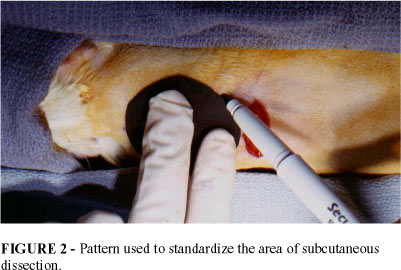
The animals were anesthetized with a solution of Ketamine and Xylazine injected intraperitoneally. A transverse incision was made on the back of the animal, adjacent to the site to be undermined. A flexible pattern was utilized to measure the area to be undermined (FIGURE 2), thus the undermining was standardized. The dissection was carried out under the paniculum carnosum and the subcutaneous tissue, where the tissue expanders were inserted (FIGURE 3).
At this stage, a square and an arc were permanently tattooed on the skin flap. The square tattoo was used to measure the expanded area and the arc tattoo was used to study the linear gain of the expanded skin (FIGURE 4).
The prosthesis was expanded at surgery, in both groups of animals with a saline solution corresponding to a volume of 20% of its total capacity. Starting from the first week of surgery, at every four days, the prosthesis was filled with a volume corresponding to 10% of the expander's capacity, until the whole expander was filled 20 cc (FIGURE 5).
Observing the same time period, together with the expansion, a 3 cc solution of the HBGF-1 diluted in saline was injected into the animals of the experimental group through the capsular irrigation catheter described. The concentration of HBGF-1 was 0.1 ug/ml. In order to simulate the same effect of the irrigation system over the capsule, 3 cc of saline were injected into the animals of the control group through the irrigation system.
After each expansion the pressure was measured with a Striker electronic manometer device (FIGURE 6 and 7).
After the animal's sacrifice, 5 cc of saline were injected into each expander and the pressure was measured after every 1 cc.
Histology
Ten samples were obtained from the base, dome and lateral region of each expander's capsule. Each specimen was submitted to histological examination, after staining with hematoxylin and eosin. The number of blood vessels, fibroblasts, macrophages and neutrophils were counted on each field.
The trichrome stain was employed to count the number of collagen fibers in each field and immunofluorescence was utilized to measure the number of adhesive fibronectin proteins.
Testing the Irrigation Catheter
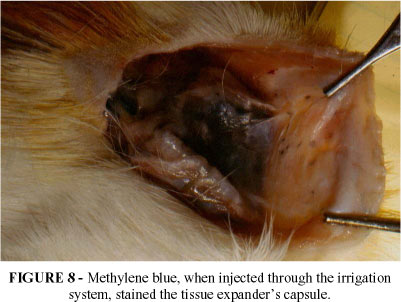
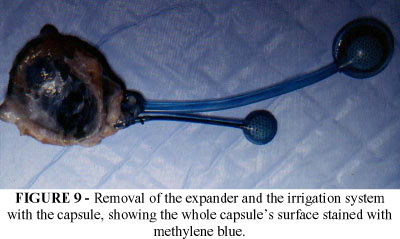
With the purpose of testing the efficacy of the irrigation system, methilene blue was injected into the capsule after the complete expansion of the prosthesis. After the animal's sacrifice, the capsule was dissected and it was found that the stain spread throughout the capsule. (FIGURE 8 e 9).
Testing the Expander's pressure extra-vivo
All expander's pressure was measured before its insertion on the animals. This test was performed to check the basal pressure of the expanders (all were 0 negligible).
Perspectives
The experimental model to study the resistance of the tissue expander's capsule showed to be feasible in guinea pigs. This model can be used to study capsular contracture around breast prosthesis, so that new materials of prosthesis can be tested, as well as in the test of new drugs to avoid capsular contracture.
Nahas FX, Vasconez LO, Ferreira LM. Guinea pigs as experimental model to evaluate the resistance of the tissue expander capsule. Acta Cir Bras [serial online] 2004 Vol 19 Special Edition. Available on URL: http://www.scielo.br/acb.
- 1. Netscher DT, Spira M, Peterson R - Adjunctive Agents to Facilitate Rapid Tissue Expansion. Ann Plast Surg. 1989;23:412.
- 2. Lee P, Squier MA, Bardach J - Enhancement of Tissue Expansion by Anticontractile Agents. Plast Reconstr Surg. 1985;76(4): 604.
- 3. Matt BH, Squier CA, Kelly KM, Bardach J - Enhancement of Expansion of Gunea Pig Skin by Local Delivery of na Anticontractile Agent Using a New Bilumen Expander. Ann Plast Sur. 1990;24:335.
- 4. Burgess WH, Maciag T - The Heparin-Binding (Fibroblast) Growth Factor Family of Proteins. Annu Ver Biochem. 1989;58:575.
- 5. Nahas FX, Vasconez, LO - O Efeito do Fator de Crescimento de Fibroblasto HBGF - 1 na Cápsula do Expansor de Pele. Rev Soc Bras Cir Plast. 1995;10(1):62-8.
- 6. Bartell TH, Mustoe TA - Animal Models of Human Tissue Expansion Plast Reconstr Surg. 1989;83(4):681.
- 7. Thompson JA, Anderson KD, DiPietro JM, Zwiebel JA, Zametta MA, Maciag T - Site-Directed Neovessel Formation in vivo. Science. 1988;241:1349.
Publication Dates
-
Publication in this collection
17 Mar 2005 -
Date of issue
Dec 2004