Abstracts
PURPOSE: The aim of this study was to investigate the effects of the combined use of glutamine (GL) and growth hormone (GH) in the intestine of rats submitted to 80% small bowel resection. METHODS: [24] Twenty four Wistar rats were randomized to receive either a standard rat chow - control group (CG, n=12) or the same diet added to 4% glutamine - GL-GH group (n=12) after 80% enterectomy. The latter group received subcutaneously 0,6UI/day of GH. Groups of six rats in each group were killed on the 5th and 14th days. The following variables were studied: body weight, mucosal weight, histomorphometry and DNA content in the resected specimen and in the adapted intestines after necropsy. RESULTS: All animals lost weight stabilizing after the 5th PO day in both groups. There was not any statistical difference in the mucosal weight associated to groups and dates. However, ileal mucosal weight decreased from basal to final results when compared to jejunal mucosa (p= 0.02). The DNA content increased from the initial to the final results (p <0.001) in both groups, though, this increase was greater in GL-GH animals (CG = 0.53 [95% CI, 0.44-0.62] g/cm-1 vs. GL-GH= 0.85 [95%CI, 0.76-0.94] g/cm-1; p<0.01), especially at the 14th day. Ileal DNA content was significantly greater than jejunal (p=0.01). There was a significant increase in the intestinal wall width and crypt depth in the control group (p<0.01). CONCLUSION: Gut adaptation after massive resection is improved with the combined use of glutamine and GH.
Glutamine; Growth hormone; Intestinal mucosa; Short bowel syndrome; DNA; Rats
OBJETIVO: Investigar os efeitos do uso combinado da glutamina (GL) e do hormônio do crescimento (GH) no intestino de ratos submetidos a ressecção de 80% do intestino delgado. MÉTODOS: Vinte e quatro ratos Wistar foram randomizados para receber uma a dieta padrão- grupo controle (GC, n=12) ou a mesma dieta adicionada de glutamina 4% (isocalórica, isoproteica) - grupo glutamina- hormônio do crescimento (GL-GH, n=12) após a enterectomia à 80%. Este último grupo recebeu por via sub-cutânea, 0,6 UI/dia de GH. Grupos de seis ratos cada foram sacrificados no 5º e 14º dias. As seguintes variáveis foram estudadas: peso corporal, peso de mucosa, histomorfometria e conteúdo de DNA no segmento ressecado inicialmente e no intestino adaptado coletado após o sacrifício. RESULTADOS: Todos os animais perderam peso até o 5º dia, estabilizando-se após esta data em ambos os grupos. Não houve diferença estatística no peso da mucosa associada a grupos ou datas. O peso da mucosa do íleo diminuiu dos dados iniciais para os finais, quando comparados a mucosa jejunal (p<0.02). O conteúdo de DNA aumentou dos dados iniciais para os finais (p=0.001) em ambos os grupos, porém, o aumento foi maior nos animais do grupo GL-GH (CG = 0.53 [95% CI, 0.44-0.62] g/cm-1 vs. GL-GH= 0.85 [95%CI, 0.76-0.94] g/cm-1; p<0.01), especialmente no 14º dia. O conteúdo de DNA no íleo foi significativamente maior que no jejuno (p=0.01). Houve um aumento significativo na espessura da parede e na profundidade da cripta, no grupo controle (p<0.01). CONCLUSÃO: A adaptação intestinal após ressecção extensa é melhorada com o uso combinado de glutamina e GH.
Glutamina; Hormônio do crescimento; Mucosa intestinal; Síndrome do intestino curto; DNA; Ratos
8 - ORIGINAL ARTICLE
Effects of the combined use of glutamine and growth hormone in the intestinal adaptation after massive resection of the small bowel in rats11. Department of Surgery , Medical Sciences School, Federal University of Mato Grosso.
Efeitos do uso combinado da glutamina oral e hormônio do crescimento na adaptação intestinal após ressecção extensa do intestino delgado em ratos
Joaquim M. SpadoniI; José Eduardo de Aguilar-NascimentoII; Maria H.G. Gomes da SilvaIII; Bruno Spadoni-NetoIV; Priscila Arruda Thulio F. Batista da CostaV; Denise Maria T. AléssioV
IAssistent Professor; Department of Surgery; University of Cuiabá (UNIC)
IIProfessor of Surgery; Federal University of Mato Grosso (UFMT)
IIIAssociate Professor; Nutrition School , Federal University of Mato Grosso (UFMT)
IVSurgery Resident; Department of Surgery; University of Cuiabá (UNIC)
VMedical Students; University of Cuiabá (UNIC)
CorrespondenceCorrespondence to José Eduardo de Aguilar-Nascimento Rua Estevão de Mendonça, 81/801 78043-330 Cuiabá - MT Phone: (65)623-7183 Fax: (65)623-4020 aguilar@cpd.ufmt.br
ABSTRACT
PURPOSE: The aim of this study was to investigate the effects of the combined use of glutamine (GL) and growth hormone (GH) in the intestine of rats submitted to 80% small bowel resection.
METHODS: [24] Twenty four Wistar rats were randomized to receive either a standard rat chow - control group (CG, n=12) or the same diet added to 4% glutamine - GL-GH group (n=12) after 80% enterectomy. The latter group received subcutaneously 0,6UI/day of GH. Groups of six rats in each group were killed on the 5th and 14th days. The following variables were studied: body weight, mucosal weight, histomorphometry and DNA content in the resected specimen and in the adapted intestines after necropsy.
RESULTS: All animals lost weight stabilizing after the 5th PO day in both groups. There was not any statistical difference in the mucosal weight associated to groups and dates. However, ileal mucosal weight decreased from basal to final results when compared to jejunal mucosa (p= 0.02). The DNA content increased from the initial to the final results (p <0.001) in both groups, though, this increase was greater in GL-GH animals (CG = 0.53 [95% CI, 0.44-0.62] g/cm-1 vs. GL-GH= 0.85 [95%CI, 0.76-0.94] g/cm-1; p<0.01), especially at the 14th day. Ileal DNA content was significantly greater than jejunal (p=0.01). There was a significant increase in the intestinal wall width and crypt depth in the control group (p<0.01).
CONCLUSION: Gut adaptation after massive resection is improved with the combined use of glutamine and GH.
Key words: Glutamine. Growth hormone. Intestinal mucosa. Short bowel syndrome. DNA. Rats.
RESUMO
OBJETIVO: Investigar os efeitos do uso combinado da glutamina (GL) e do hormônio do crescimento (GH) no intestino de ratos submetidos a ressecção de 80% do intestino delgado.
MÉTODOS: Vinte e quatro ratos Wistar foram randomizados para receber uma a dieta padrão- grupo controle (GC, n=12) ou a mesma dieta adicionada de glutamina 4% (isocalórica, isoproteica) - grupo glutamina- hormônio do crescimento (GL-GH, n=12) após a enterectomia à 80%. Este último grupo recebeu por via sub-cutânea, 0,6 UI/dia de GH. Grupos de seis ratos cada foram sacrificados no 5º e 14º dias. As seguintes variáveis foram estudadas: peso corporal, peso de mucosa, histomorfometria e conteúdo de DNA no segmento ressecado inicialmente e no intestino adaptado coletado após o sacrifício.
RESULTADOS: Todos os animais perderam peso até o 5º dia, estabilizando-se após esta data em ambos os grupos. Não houve diferença estatística no peso da mucosa associada a grupos ou datas. O peso da mucosa do íleo diminuiu dos dados iniciais para os finais, quando comparados a mucosa jejunal (p<0.02). O conteúdo de DNA aumentou dos dados iniciais para os finais (p=0.001) em ambos os grupos, porém, o aumento foi maior nos animais do grupo GL-GH (CG = 0.53 [95% CI, 0.44-0.62] g/cm-1 vs. GL-GH= 0.85 [95%CI, 0.76-0.94] g/cm-1; p<0.01), especialmente no 14º dia. O conteúdo de DNA no íleo foi significativamente maior que no jejuno (p=0.01). Houve um aumento significativo na espessura da parede e na profundidade da cripta, no grupo controle (p<0.01).
CONCLUSÃO: A adaptação intestinal após ressecção extensa é melhorada com o uso combinado de glutamina e GH.
Descritores: Glutamina. Hormônio do crescimento. Mucosa intestinal. Síndrome do intestino curto. DNA. Ratos.
Introduction
Intestinal adaptation is the name given to a series of events that occurs in the remnant gut after extensive resection of the small bowel. These alterations initially reported by Koeberle1 have been extensively studied because the survival of these patients in dependent on that, mainly those one who lose more than 2/3 of the small bowel2-6. A resection greater than 70% of the length of the small bowel leads to serious disabsortive syndrome characterized by intratable diarrhea, severe malnutrition, loss of weight, anemia, gastric hypersecretion, crisis of tetany, and renal and biliar stones. Sometimes, there may be even behavioural and psychotic manifestations7. This clinical picture is called short bowel syndrome (SBS). With the recent knowledge of the intestinal physiopathology and the progresses of the nutritional support, it has been possible to improve and to prolong the lives of these patients enhancing the understanding of the adaptative processes that happen in the intestinal mucosa, mainly, with the specific mucosal nutrients8-10. Various luminal factors enhance the adaptative process of the intestines. It seems that not absorbed nutrients are not so effective as the absorbed ones8. Among the lipids, the long and the short chains triglycerides are considered trophics to the enterocytes, while the median chains are not. Proteins are also important and some amino-acids and oligopeptides are considered powerful stimulants9. As for carbohydrates it is known that bacterial fermentation of both fibers and sugars produces short-chain fatty acids that are trophic for the intestinal mucosa, mainly at the colon10. There are evidences that glutamine (GL) is the principal fuel for enterocytes11-13. Curiously, some experimental works14,15 using diets rich in GL have not achieved good results in experimental SBS. However, the combined use of GL and GH may induce important trophic effects on the intestinal mucosa. Byrne et al.16 reported an improved absorption of nutrients, gain of weight, and better life conditions with a high-carbohydrate, low-fat diet, combined with high doses of GH and GL in most of their patients that were depending on total parenteral nutrition. Waitzberg et al.17 observed an enhanced intestinal adaptation with glutamine and GH in rats submitted to 95% resection of the small bowel. The combined use of GL and GH, or even only GH increases IGF-1 in animal model of SBS18,19. Several other studies have shown that a synergistic action between GL and GH may occur in the process of intestinal adaptation16-19.
Several questions however have not been answered until now on this issue. Thus, further studies are necessary to decide for instances, when would be the proper time to begin, or for how long, or even which would be the correct dosages of both substances20-23. Furthermore, most of the studies have evaluated the intestinal adaptation process only on one time point. It would be interesting to observe the process evolution in two phases, such as in the first and second weeks.
The aim of this experiment was to investigate the effects of the combined use of oral glutamine and growth hormone in the intestinal adaptation on the 5th and the 14th days after massive enterectomy in rats.
Methods
Animals, groups and diets
Twenty-four Wistar rats (252±7.4g) entered the experiment and were housed for three days in individual metabolic cages in the laboratory, to be adapted to the diet in a controlled environment. In this phase, all rats received AIN-93 diet (American Institute of Nutrition) and water ad libitum. The animals were then submitted to 80% mid-bowel resection and after that randomized to two groups to receive postoperatively either the same diet (control group CG; n=12) or an isocaloric (approximately 345 kcal/100g) and isonitrogenous (2.24 gN/day) diet containing 4% L-glutamine (Rhoster Lab., São Paulo, Brazil) (GL-GH group; n=12). This latter group received subcutaneously daily injections doses of 0.6 UI of recombinant growth hormone (Hormotrop, Laboratório Bergamo; Brazil) since the operation day. All animals that died were substituted by similar others.
Surgical technique
Under inhalatory anesthesia with ethilic ether, all the rats were submitted to 80% resection of the mid-small bowel, leaving 10% of both the proximal jejunum and the distal ileum. The bowel extremities were end-to-end anastomosed with 7-8 stitches of 6.0 polygalactin interrupted sutures. The abdominal cavity was closed in two layers with 4.0 nylon. All the rats received subcutaneous injections containing 10 ml of saline solution for the next 3 days.
Histomorphometric evaluation
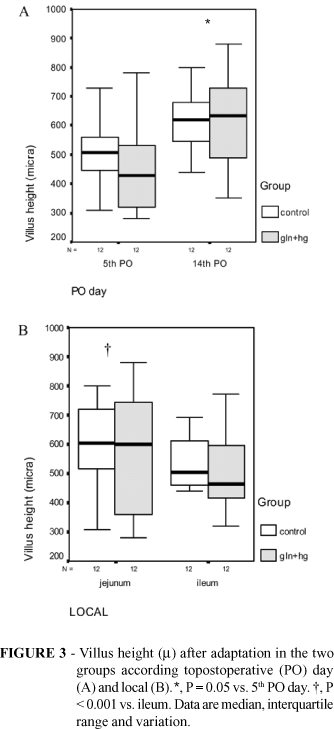
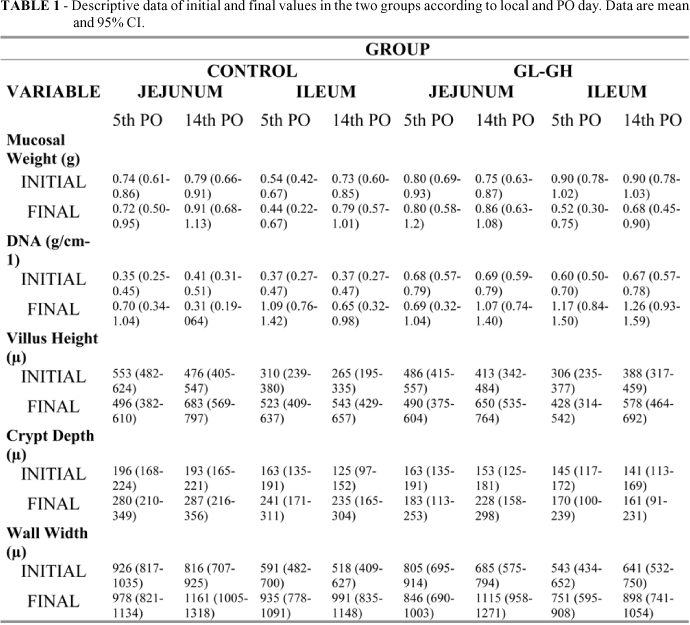
Full-thickness biopsies of two cm each from both extremities of the specimen, corresponding to the jejunum and the ileum, were collected, fixed on poster paper with pins and immediately placed in 10% formalin. In each specimen, five slides stained with hematoxylin-eosin were examined by two observers blinded to the study design. The mean value of the five best-oriented glands was considered to represent the data from each specimen. Wall width (WW), villus height (VH), and crypt depth (CD) were measured using a micrometer rule as published elsewhere24.
DNA content assay
The rest of the specimen corresponding to the jejunum and ileum (approximately 15 cm each) was opened longitudinally through the anti-mesenteric border and the mucosal layer was stripped out using a glass slide. This material was weighed and wrapped in aluminum foil and kept in a refrigerator (-20ºC) until the moment of the DNA assay using the Giles and Myers technique25.
Weight evolution
Animals were weighed on five dates during the experiment as follows: day 3: adaptation period; day 0: enterectomy day; day 5: end of experiment for half of the rats; day 10; and, day 14: end of experiment for the other 50% rats. Animals were killed on the 5th and the 14th days. During the necropsy the same procedure was repeated to collect material of the remnant small bowel for mucosal weight, histomorphometry and DNA content assay.
Statistical analysis
The data analysis was performed using Anova for repeated measures. Data were expressed as mean and 95% CI. Boxplots for continuous variables show the median, interquartil interval and variation. A significant level of 5% (p<0.05) was established.
Results
Weight evolution
It was observed that until the 5th day all the animals lost weight similarly (p<0.01). After that, the weight stabilized identically for both groups (p>0.05) (Figure 1).
Mucosal weight
The findings of the mucosal weight can be seen in Table 1 and Figure 2.
Within-subjects comparisons showed that there were not any statistical differences in the mucosal weight associated to groups and data. However, ileal mucosal weight decreased from basal to final results when compared to jejunal mucosa (p= 0.02). All other comparisons were not significant. Between-groups comparisons showed that there was no difference between the two groups (CG = 0.71 [95% CI, 0.64-0.77]g vs. GL-GH= 0.78 [95%CI, 0.71-0.84]g; p=0.11). The mucosa was heavier in the jejunum than in the ileal (Jejunum = 0.80 [95% CI, 0.73-0.86]g vs. ileum = 0.69 [95%CI, 0.62-0.75]g; p=0.01), and at the 14th when compared to the 5th PO day (5th PO day = 0.68 [95% CI, 0.62-0.75]g vs. 14th PO day = 0.80 [95%CI, 0.74-0.86]g; p=0.01). There were no interactions between PO days, regions or groups.
Histomorphometry
Villus height
There was an increase in villus height over time in all groups (p<0.001), except at the jejunum on the 5th PO day, where it significantly decreased from initial to final results (p=0.01) (Figure 3 and Table 1). There was no difference between groups (CG = 481 [95% CI, 444-518] µ vs. GL-GH= 468 [95%CI, 431-505]µ; p=0.59). Data from the 14th PO day were borderline significantly higher than those from the 5th PO day (p=0.05). Jejunal villi was significantly higher when compared to ileal in both groups (Jejunum = 531 [95% CI, 494-568] µ vs. ileum = 418 [95%CI, 381-454] µ; p<0.001). There was no other interaction of statistical significance.
Crypt depth
There was an increase of the crypt depth during the experiment in both groups (p<0.001) though significantly greater in controls (p=0.03). The crypts at the jejunum were deeper than at the ileum (Jejunum = 210 [95% CI, 192-229] µ vs. ileum = 173 [95%CI, 154-191] µ; p<0.01). Comparisons between factors showed that control animals had greater CD than GL-GH animals (CG = 215 [95% CI, 196-234] µ vs. GL-GH= 168 [95%CI, 149-186] µ; p<0.01). There was no difference on the findings from the 5th and 14th PO day (p=0.86). No significantly interactions between groups, PO day and region of the gut occurred (Figure 4 and Table 1).
Wall width
The WW augmented over time in both groups (p<0.01) and this increase was greater on the 14th PO than on the 5th PO day (p<0.01). However, the findings at the jejunum on the 5th PO day were not significantly different from the initial condition. Between-groups comparison showed that controls had the gut wall wider than GL-GH group (CG = 865 [95% CI, 815-914] µ vs. GL-GH= 786 [95%CI, 736-835] µ;p=0.02); and the WW at the jejunum was greater than at the ileum (Jejunum = 917 [95% CI, 867-966] µ vs. ileum = 734 [95%CI, 684-783] µ; p<0.001). There was no other interaction of significance (Figure 5 and Table 1).
DNA content
The DNA content increased from the initial to the final results (p <0.001) in both groups. This increase in DNA content occurred similarly at the 5th and 14th PO day (p=0.29) though greater in the ileum than in the jejunum (p< 0.01). However, animals of GL-GH group showed higher increase from the 5th to the 14th PO day than controls (p=0.01). All other within-subjects comparisons were not significant (Figure 6 and Table 1).
The between-subjects comparisons showed that GL-GH rats had higher DNA content in both examined regions when compared to controls (CG = 0.53 [95% CI, 0.44-0.62] g/cm-1 vs. GL-GH= 0.85 [95%CI, 0.76-0.94] g/cm-1; p < 0.01). The DNA content at the ileal mucosa was significantly greater than at the jejunum (Jejunum = 0.61 [95% CI, 0.52-0.70] g/cm-1 vs. ileum = 0.77 [95%CI, 0.68-0.86] g/cm-1; p=0.01). There was no difference in time points though an interaction has occurred showing that the DNA content at the 14th PO day was higher than the 5th PO day only in GL-GH group (p=0.01). These findings can be seen in Figure 6 and Table 1.
Discussion
Our results showed that the combined use of oral glutamine and the growth hormone has benefited the intestinal adaptation process after massive small bowel resection. A significant gain in the gut mucosa cellularity ocurred, mainly expressed by the increased content of the DNA in the remnant intestines of the rats treated with GL-GH. This finding possibly reflects the synergistic action of an energetic substrate for the epithelium and a trophic hormone for the intestinal wall. In fact, other experiments have already demonstrated an enhanced intestinal adaptation with this association17-19. A significant increase in cellularity between the 5th and the 14th days was verified only in GL-GH group, suggesting that this process was still taking place after two weeks when the studied association was used. The higher augment of DNA in distal portions of the gut confirms the important adaptation of the ileum after massive resection of the small bowel5-6.
Interesting histomorphometrics changes were observed along the experiment. While ileal adaptation was intense and active in both groups on the 5th PO day, it was almost not noticed at the jejunum (Figures 3-5). However, contrasting with the mucosal DNA content findings, the WW and CD were greater in controls than in treated animals, especially at the ileum. This was difficult to understand or interpret, and it was not described in the literature whatsoever. However, these measurements (an average of 5 slides/per specimen) are subjective and localized, and thus, errors may possible exist. In fact, criticisms for the use of histological parameters are exactly due to subjectivism of the method. On the other hand, DNA content assay is potentially less correlated to biases, is not subjective, and therefore most confident. Between histology and DNA assay we assume that the latter should be most reliable.
Normal intestinal function also depends on the unique capacity of the terminal ileum to absorb vitamin B12 and bile salts. The distal 100 cm of the ileum is the only region of the small bowel where vitamin B12-intrinsic factor complex is absorbed 26. Thus, the adaptation of the distal small bowel is crucial. Our findings showed that the DNA content of the ileal mucosa increased higher than at the jejunum and this effect was most intense in GL-GH group. Contrasting with these results, it was interesting to notice that the histological findings both in control group and in the jejunum presented greater values than in GL-GH group and in the ileum. This suggests that the increased values of wall width and crypt depth may not necessarily means an increased number of cells and could be related to oedema.
Experimental and clinical studies have demonstrated the possible benefits with the use of this association in the treatment of intestinal insufficiency, after extensive resection of the small bowel13-20. Iglesias and Zucoloto27 had indicated that the adaptative intestinal reactions initiate in the first few days after enterectomy. In this experiment, it was observed that the adaptative process on the 5th day, expressed by the DNA content, was almost complete in the control group though still on progress in the GL-GH group until the 14th day, possibly due to a continuous trophic action of the treatment. An important finding of this experiment was that it is possible to have an enhanced adaptation with the use of oral glutamine. It was demonstrated before that the oral glutamine was not efficient when used alone. Therefore, the addition of the GH contributed and was more important than the association with short chain fatty acids for this process28. Considering that GH increases the absorption of aminoacids from 20% to 70%, mainly glutamine and leucine, the association of these two trophic factors seems to be very attractive11. Our experiment showed that the adaptative process especially at the ileum begins almost immediately after the enterectomy and is already expressive on the 5th post-operative day. At the jejunum however, the process seems to be much slower. This finding suggests that glutamine should be used in this phase, probably associated with a trophic hormone as GH. In this experiment, a large dose of GH (0,6 UI/day or 0,2 g/day in rats with ± 250 g) was used, based on the literature18,19. However, smaller doses for a longer period of time are also associated with enhanced adaptation20. In summary, the findings of this experiment showed that gut adaptation after massive resection could be improved with the combined use of glutamine and GH. Furthermore, the treatment with GL-GH has assured a continuous adaptation in a faster rhythm during all the experiment. Another important issue aroused is that the early use of oral glutamine and GH could be of great utility in this phase of high energetic consumption and great trophism. Although caution is always necessary to extrapolate findings of an animal experiment to the clinical setting we conclude that the early combined use of oral glutamine and growth hormone improves intestinal adaptation after massive small bowel resection.
Conclusion
Gut adaptation after massive resection is improved with the combined use of glutamine and GH.
Received: April 12, 2005
Revised: May 9, 2005
Accepted: June 14, 2005
Conflict of interest: none
Financial source: none
- 1. Coleman EP, Bennet DA. Massive intestinal resection. Am J Surg. 1993; 59:429-32.
- 2. Wilmore DW, Dudrick SJ, Daly JM, Vars HM. The role of nutrition in the adaptation of the small intestine after massive resection. Surg Gynecol Obstet. 1971; 132:673-80.
- 3. Schapiro M. Interposição de um segmento de cólon distal entre cotos remanescentes de intestino delgado após ressecção de 90% do jejuno-íleo [Tese-Doutorado].Universidade Federal de São Paulo-Escola Paulista de Medicina; 1974.
- 4. Koruda MJ, Rolandelli RH, Settle RG, Saul SH, Rombeau JL. The effect of a pectin-suplemented elemental diet on intestinal adaptation to massive small bowel resection. JPEN J Parenter Enteral Nutr. 1986; 10:343-50.
- 5. Iglesias ACRG. Mecanismos de adaptação intestinal e suas relações com a ressecção extensa do intestino delgado. Rev Col Bras Cir. 1993; 20:34-41.
- 6. Scolapio JS, Fleming CR. Short bowel syndrome. Gastroenterol Clin North Am. 1998; 37:467-79.
- 7. Jackson WPV. Ressección massiva del intestino delgado. In:Jones FA. Recientes avances en gastroenterologia. Barcelona: Ed. Toray; 1960.
- 8. Stragand JJ, Hageman RF. Effect of luminal contents on colonic cell replacement. Am J Physiol. 1977; 233:208-11.
- 9. Sturm A, Layer P, Goebell H, Dignass AV. Short Bowel Syndrome: An update on the therapeutic approach. Scand J Gastroenterol. 1997; 32: 289-96.
- 10. Cook SI, Sellin JH. Review article: short-chain fatty acids in health and disease. Aliment Pharmacol Ther. 1998; 12:499-507.
- 11. Souba WW, Herskowitz K, Austgen TR, Chen MK, Salloum RM. Glutamine nutrition: theoretical considerations and therapeutic impact. JPEN J Parenter Enteral Nutr. 1990; 14:237-41.
- 12. Campos FG, Waitzberg DL, Logulo AF, Mucerino DR, Habr-Gama A. Importância da glutamina em nutrição na prática clínica. Arq Gastroenterol. 1996; 33:86-92.
- 13. Cukier C, Waitzberg DL, Borges VC, Silva MLT, Rodrigues JG, Pinotti HW. Clinical use of growth hormone and glutamine in short-bowel syndrome. Rev Hosp Clin Fac Med São Paulo. 1999; 54: 29-34.
- 14. Wiren ME, Permet J, Scullman SP, Wang F, Larsson J. No differences in mucosal adaptative growth one week after intestinal ressection in rats given enteral glutamine suplementation or deprived of glutamine. Eur J Surg. 1996; 162:489-98.
- 15. Neves JS, Aguilar-Nascimento JE, Salomão AB, Gomes-da-Silva, MH, Nascimento M, Nochi Jr RJ. Influencia da glutamina na mucosa do intestino delgado de ratos submetidos a enterectomia extensa. Rev Col Bras Cir. 2003; 30:406-15.
- 16. Byrne TA, Persinger RL, Young LS, Ziegler TR, Wilmore DW. A new treatment for patients of short-bowel syndrome: Growth hormone, glutamine and a modified diet. Ann Surg. 1995; 222: 243-55.
- 17. Waitzberg DL, Cukier C, Mucerino DR, Logulo AF, Torrinhas RS, de Castro I. Small bowel adaptation with growth hormone and glutamine after massive resection of rat'small bowel. Nutr Hosp. 1999; 14:81-90.
- 18. Zhou X, Li YX, Li N, Li, JS. Glutamine enhances the gut-trophic effect of growth hormone in rats after massive small bowel resection. J Surg Res. 2001; 99: 47-52.
- 19. Gu Y, Wu ZH, Xie JX, Jin DY, Zhuo HC. Effects of growth hormone (rhGh) and glutamine supplemental parenteral nutrition on intestinal adaptation in short bowel of rats. Clin Nutr. 2001; 20:159-66.
- 20. Seguy D, Vahedi K, Kapel N, Souberbielle JC, Messing B. Low-dose growth hormone in adult home parenteral nutrition-dependent short bowel syndrome patients: a positive study. Gastroenterology. 2003; 124:561-4.
- 21. Weiming Z, Ning L, Jieshou L. Effect of recombinant human growth hormone and enteral nutrition on short bowel syndrome. Parenter Enteral Nutr. 2004; 28:377-81.
- 22. Zhou Y, Wu XT, Zhuang W, Wei ML. Clinical evidence of growth hormone, glutamine and a modified diet for short bowel syndrome: meta-analysis of clinical trials. Asia Pac J Clin Nutr. 2005; 14:98-102.
- 23. Matarese LE, Seidner DL, Steiger E. Growth hormone, glutamine and modified diet for intestinal adaptation. J Am Diet Assoc. 2004; 104:1265-72.
- 24. Aguilar-Nascimento JE, Lima SA, Pereira ACC. Effects of oral parenteral nutrition solution on the morphology and mechanical resistance of the small bowel in rats. Acta Cir Bras. 1997; 12:159-62.
- 25. Giles KW, Myers A. An improved diphenylamine method for the estimation of deoxyribonucleic acid. Nature. 1965, 4:206-13.
- 26. Okuda K. Discovery of vitamin B12 in the liver and its absorption factor in the stomach: A historical review. J Gastroenterol Hepatol. 1999;14:301-8.
- 27. Iglesias ACRG , Zucoloto S. Proliferação celular do epitélio intestinal: mecanismo de adaptação e controle após ressecção extensa do intestino delgado. Med Ribeirão Preto. 1994, 27:303-9.
- 28. Aguilar- Nascimento JE ; Neves JS; Salomão AB ; Nascimento M. Oral glutamine and SCFA fail to improve gut mucosal adaptation after extensive enterectomy in rats. Clin Nutr. 2004; 23:805.
Publication Dates
-
Publication in this collection
05 Sept 2005 -
Date of issue
Oct 2005
History
-
Accepted
14 June 2005 -
Reviewed
09 May 2005 -
Received
12 Apr 2005