Abstracts
PURPOSE: Amphotericin B (AmB), an antifungal agent that presents a broad spectrum of activity, remains the gold standard in the antifungal therapy. However, sometimes the high level of toxicity forbids its clinical use. The aim of this work was to evaluate and compare the efficacy and toxicity in vitro of Fungizon™ (AmB-D) and two new different AmB formulations. METHODS: three products were studied: Fungizon™, and two Fungizon™ /Lipofundin™ admixtures, which were diluted through two methods: in the first one, Fungizon™ was previously diluted with water for injection and then, in Lipofundin™ (AmB-DAL); the second method consisted of a primary dilution of AmB-D as a powder in the referred emulsion (AmB-DL). For the in vitro assay, two cell models were used: Red Blood Cells (RBC) from human donors and Candida tropicallis (Ct). The in vitro evaluation (K+ leakage, hemoglobin leakage and cell survival rate-CSR) was performed at four AmB concentrations (from 50 to 0.05mg.L-1). RESULTS: The results showed that the action of AmB was not only concentration dependent, but also cellular type and vehicle kind dependent. At AmB concentrations of 50 mg.L-1, although the hemoglobin leakage for AmB-D was almost complete (99.51), for AmB-DAL and AmB-DL this value tended to zero. The p = 0.000 showed that AmB-D was significantly more hemolytic. CONCLUSION: The Fungizon™-Lipofundin™ admixtures seem to be the more valuable AmB carrier systems due to their best therapeutic index presented.
Fungizon™; Lipofundin™; amphotericin B; Fungal infection; Drug delivery; Red Blood Cells
OBJETIVO: A anfotericina B é um agente antifúngico de largo espectro bastante empregado na terapia antifúngica. Entretanto, esta molécula apresenta um alto nível de toxicidade que, na maioria das vezes, impede o seu uso contínuo na terapêutica médica. O objetivo deste artigo foi comparar a eficácia e a toxicidade in vitro do Fungizon™ (AmB-D) e de dois sistemas carreadores de AmB. MÉTODOS: Três produtos foram avaliados: o Fungizon™ , e dois sistemas oriundos da mistura entre o Fungizon™ e o Lipofundin™ , uma emulsão de uso parenteral. Tais sistemas foram obtidos por duas técnicas: Na primeira diluiu-se previamanete o Fungizon™ com água para injetáveis e em seguida inseriu-se o Lipofundin™ (AmB-DAL); o segundo método consistiu na diluíção extemporânea do Fungizon™ com a referida emulsão (AmB-DL). Dois modelos celulares foram empregados no estudo: os eritrócitos (RBC) oriundos de doadores humanos e a Candida tropicalis (Ct). A avaliação in vitro (liberação de K+ e hemoglobina, e o índice de sobrevivência celular-CSR) foi realizado com quatro concentrações de AmB (entre 50 e 0.05mg.L-1). RESULTADOS: Os resultados demonstram que a ação da AmB não só foi dependente da concentração como também variou de acordo com o modelo celular e o veículo que diluiu o Fungizon™ . Nas concentrações de 50 mg.L-1, apesar da liberação de hemoglobina ser quase que total para AmB-D (99.51), para a AmB-DAL e AmB-DL este valor tendeu a zero. Um p = 0.000 demonstrou que AmB-D foi significativamente mais hemolítico. CONCLUSÃO: A mistura Fungizon™ -Lipofundin™ aparenta ser um bom sistema para carrear a AmB tendo em vista seu elevado índice terapêutico demonstrado.
Fungizon™; Lipofundin™; Anfotericina B; Infecção fúngica; Sistemas de liberação de fármacos; Eritrócitos
Similarity between the in vitro activity and toxicity of two different fungizone / lipofundin admixtures
Semelhança entre atividade in vitro e toxicidade de duas diferentes misturas de fungizoneä / lipofundinä
Ivonete Batista AraújoII; C. Ramon N. BritoI; Isabel A. UrbanoI; Victor A. DominiciI; Miguel A. Silva FilhoI; Walteçá L. L. SilveiraI; Bolívar P. G. L. DamascenoI; Aldo Cunha MedeirosIII; E. Sócrates T. EgitoIV
IPharmacy undergraduate student, Laboratory of Dispersed Systems (LASID) UFRN Brazil
IIProfessor, M. Sc., Department of Pharmacy - LASID, UFRN-Brazil
IIIProfessor, Doctor, Chief of Nucleus of the Experimental Surgery UFRN-Brazil
IVProfessor, Doctor, Department of Pharmacy - Coordinator of the LASID, UFRN-Brazil
Correspondence Correspondence to Prof. E. Sócrates Tabosa do Egito Rua Praia de Areia Branca, 8948 59094-450 Natal RN Phone: (55) (84) 9431 8816 Fax: (55) (84) 215 4355 E-mail: socrates@digi.com.br
ABSTRACT
PURPOSE: Amphotericin B (AmB), an antifungal agent that presents a broad spectrum of activity, remains the gold standard in the antifungal therapy. However, sometimes the high level of toxicity forbids its clinical use. The aim of this work was to evaluate and compare the efficacy and toxicity in vitro of Fungizon (AmB-D) and two new different AmB formulations.
METHODS: three products were studied: Fungizon, and two Fungizon /Lipofundin admixtures, which were diluted through two methods: in the first one, Fungizon was previously diluted with water for injection and then, in Lipofundin (AmB-DAL); the second method consisted of a primary dilution of AmB-D as a powder in the referred emulsion (AmB-DL). For the in vitro assay, two cell models were used: Red Blood Cells (RBC) from human donors and Candida tropicallis (Ct). The in vitro evaluation (K+ leakage, hemoglobin leakage and cell survival rate-CSR) was performed at four AmB concentrations (from 50 to 0.05mg.L-1).
RESULTS: The results showed that the action of AmB was not only concentration dependent, but also cellular type and vehicle kind dependent. At AmB concentrations of 50 mg.L-1, although the hemoglobin leakage for AmB-D was almost complete (99.51), for AmB-DAL and AmB-DL this value tended to zero. The p = 0.000 showed that AmB-D was significantly more hemolytic.
CONCLUSION: The Fungizon-Lipofundin admixtures seem to be the more valuable AmB carrier systems due to their best therapeutic index presented.
Keywords: Fungizon. Lipofundin. amphotericin B. Fungal infection. Drug delivery. Red Blood Cells
RESUMO
OBJETIVO: A anfotericina B é um agente antifúngico de largo espectro bastante empregado na terapia antifúngica. Entretanto, esta molécula apresenta um alto nível de toxicidade que, na maioria das vezes, impede o seu uso contínuo na terapêutica médica. O objetivo deste artigo foi comparar a eficácia e a toxicidade in vitro do Fungizon (AmB-D) e de dois sistemas carreadores de AmB.
MÉTODOS: Três produtos foram avaliados: o Fungizon , e dois sistemas oriundos da mistura entre o Fungizon e o Lipofundin , uma emulsão de uso parenteral. Tais sistemas foram obtidos por duas técnicas: Na primeira diluiu-se previamanete o Fungizon com água para injetáveis e em seguida inseriu-se o Lipofundin (AmB-DAL); o segundo método consistiu na diluíção extemporânea do Fungizon com a referida emulsão (AmB-DL). Dois modelos celulares foram empregados no estudo: os eritrócitos (RBC) oriundos de doadores humanos e a Candida tropicalis (Ct). A avaliação in vitro (liberação de K+ e hemoglobina, e o índice de sobrevivência celular-CSR) foi realizado com quatro concentrações de AmB (entre 50 e 0.05mg.L-1).
RESULTADOS: Os resultados demonstram que a ação da AmB não só foi dependente da concentração como também variou de acordo com o modelo celular e o veículo que diluiu o Fungizon . Nas concentrações de 50 mg.L-1, apesar da liberação de hemoglobina ser quase que total para AmB-D (99.51), para a AmB-DAL e AmB-DL este valor tendeu a zero. Um p = 0.000 demonstrou que AmB-D foi significativamente mais hemolítico.
CONCLUSÃO: A mistura Fungizon -Lipofundin aparenta ser um bom sistema para carrear a AmB tendo em vista seu elevado índice terapêutico demonstrado.
Descritores: Fungizon . Lipofundin. Anfotericina B. Infecção fúngica. Sistemas de liberação de fármacos. Eritrócitos
Introduction
The effectiveness of amphotericin B (AmB) in the treatment of systemic fungal infections, whose incidence has been considerably increasing in patients with immunodeficiency, has been attracting several researchers' interest in the world. Attempts have focused on finding a formulation as effective as FungizonÔ, but inducing a low toxicity level and presenting a smaller cost than others commercially available formulations. An alternative could be a new delivery system based on the lipid emulsions used for parenteral nutrition in clinical practice (Intralipidor Lipofundin). This system has been showing to reduce the AmB toxicity with little damage in its efficacy1. In fact, the reduction of the toxicity of FungizonÔ - IntralipidÔ admixtures was observed in several clinical trials1,6. In 2002, an in vitro evaluation of FungizonÔ and FungizonÔ/ Lipofundinä admixture revealed that the latter one was less toxic against red blood cells, presenting no hemolytic activity7.
Recently, our group has shown that the way in which FungizonÔ is incorporated into the emulsion (Intralipidor Lipofundin) did not change the profile of activity or toxicity found for the AmB admixtures7,8. Therefore, the response against mammalian or fungal cells was remained.
The aim of this work was to correlate the efficacy and toxicity of FungizonÔ (AmB-D) and two AmB emulsion admixtures against two cell models, a cholesterol containing membrane and an ergosterol one.
Methods
In this research work, two cell models were used for the in vitro assay: Red Blood Cells (RBC), from human healthy donor, and Candida tropicalis (Ct). Representing respectively a mamalian (cholesterol containing) and a fungal (ergosterol containing) model. Potassium (K+) and hemoglobin leakage from RBC were monitored, respectively, as a measure of acute and chronic toxicity. K+ leakage or cell survival rate (CSR) from Ct, was used to evaluate the pharmacological activity of the products. Three pharmaceutical products were evaluated: AmB-D, a micellar solution of AmB containing sodium deoxycholate and marketed as Fungizonä (Bristol - Myers Squibb, São Paulo/SP - Brazil), and two preparations obtained through the admixture of Fungizonä and a parenteral emulsion (Lipofundinä LCT/MCT - 20%, B.BRAUN - Brazil). Based on the literature, such admixtures were prepared by two methods: in the first one, AmB-D was previously reconstituted with distilled water for injection and then, added to the parenteral emulsion (AmB-DAL) (9-11). The second method consisted of a primary dilution of AmB-D as a powder in the referred emulsion (AmB-DL) (12). For each preparation, the concentrations of 50, 5, 0.5 and 0.05 mg.L-1 of AmB (5.10-5, 5.10-6, 5.10-7, 5.10-8M, respectively) were used for the in vitro profile of activity or toxicity. To evaluate the effectiveness and toxicity of AmB, 4mL of RBC (5 x 107 cell/ml) or 2mL of Ct (5 x 107 cfu/ml) suspension were incubated by one hour at 37ºC with AmB-D, AmB-DL or AmB-DAL, respectively. Each experiment was accomplished in triplicate and repeated three times (7, 13). The results were expressed in percentage of hemoglobin and K+ leakage by the RBC and the percentage of K+ release and CSR for Ct.
Statistical analysis
All potassium, hemoglobin release and CFU viability data were expressed as the mean ± SD. Statistical analysis was performed using ANOVA test and the significance was defined as P<0.05.
Ethics
This study was performed by written informed consent from the female healthy donor.
Results
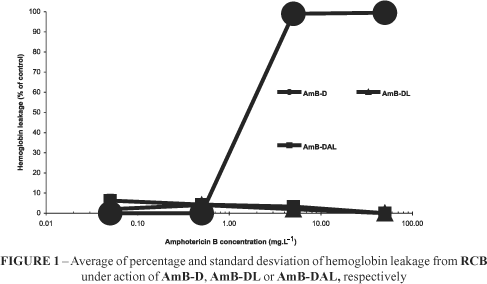
The action of AmB was not only concentration dependent (Figures 1 to 3), but also cellular type (Figure 2 and 3) and vehicle kind dependent (Figures 1 to 3). AmB at the concentrations of 50 mg.L-1 induced an almost complete (99.51) hemoglobin leakage for AmB-D. However, for AmB-DAL and AmB-DL this value tended to zero (Figure 1). In fact, the p = 0.000 showed that AmB-D was significantly more hemolytic.
Concerning K+ leakage, AmB-D was also more toxicic against RBC than AmB-DAL and AmB-DL (Figure 2). Although at AmB concentration of 5 mg.L-1 100% of leakage was observed for AmB-D, for AmB-DAL and AmB-DL products it tended to 50%. In fact, the K+ release induced by AmB-D was significantly different from AmB-DAL and AmB-DL (p<0.0001).
When the cell model was the fungal one, a similar profile of activity was detected for all tested products, but after incubation with AmB-D at 0.05 mg.L-1 a larger K+ release (Figure 2) was found compared to the AmB-DAL and AmB-DL (p<0.0001).
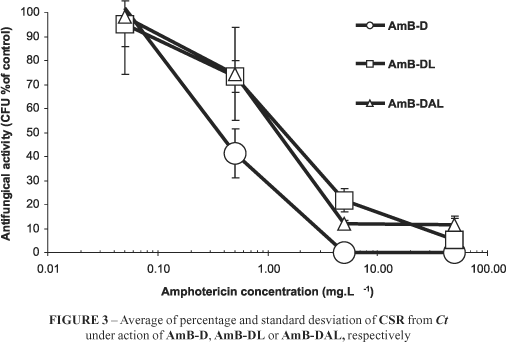
Such activity profile was also confirmed by the CSR data. AmB-D, AmB-DAL and AmB-DL showed a high effectiveness from 0.5 mg.L-1 that tended to 0% as soon as a ten fold concentration was achieved (Figure 3). Additionally, the CSR showed that whereas AmB-D was able to kill all seeded fungal cells from a concentration of 5 mg.L-1, AmB-DAL and AmB-DL were discreetly less effective showing a small number of CFU (Figure 3) not statistically significant, at 50 mg.L-1.
Discussion
The development of new drug delivery systems for AmB remains a great challenger for several pharmaceutical research groups. The aim is to develop less toxic formulations by changing the AmB physico-chemical behavior, and consequently, its biological properties in vitro and in vivo. In fact, the main mechanism of action (and toxicity) of this molecule consists on the formation of ion chanels by its interaction with the membrane sterols14. Depending on the size of the ion chanels formed, they allow the leakage of the internal constituents like potassium and hemoglobin on the mamallian cells. This is followed by a disturbance in the enzyme activity of the cells inducing their death.
Lipidic systems like liposomes or emulsions are able to strongly interact with the AmB molecule and change its physico-chemical properties. This is mainly due for the formation of reservoir systems that release monomeric species of AmB, which are less toxic to mammalian cells, but high actif against fungal cells15. Therefore, the use of an in vitro methodology that contemplates both cell models seems to be a valuable tool for the evaluation of new AmB delivery carriers.
On the other hand, the mixture of parenteral emulsion with Fungizon has been largely accepted as a way to reduce the AmB toxicity in the clinical therapy. In fact, several clinical trials reveal the efficacy of such strategy2,4,12. However, additional studies should be addressed to this "new pharmaceutical entity" to clearly understand its mechanism of action. Recentrly we have developed some physico-chemical studies on this AmB-lipid admixture system and showed, by spectrophotometric studies, that the presence of oligomeric AmB species is reduced in such preparation7.
This work permitted us to evaluate the real correlation between two different AmB-Lipofundin admixtures, and by consequence, to determine if the way how the mixture should be carried out interferes in their final in vitro behavior of AmB. The data presented above (Figures 1 to 3) confirm the results that were published previously by our group7,8. They relate the influence of FungizoneTM / LipofundinTM admixtures in decreasing RCB hemolysis. Besides, they indicate that although these admixtures reduced the permeability (K+ leakage) on RBC membranes, they increase such permeability against ergosterol containing membranes, and also promoting a slight decrease in their percentage of CSR.
All the results together suggest that parenteral emulsions can be able to reduce the Fungizon toxicity probably by changing the behavior of the AmB molecule into the dispersed emulsion system. The profile of activity, which was similar to other lipid-based forms of AmB1, indicates that AmB-emulsion admixtures could be an eligible carrier for such molecule. These systems may have some practical advantages. To name a few, they do not include expensive semi-synthetic lipids and are currently in use on clinical trials.
Conclusion
This paper showed that the methodology adopted to evaluate the pharmacological action and toxicity of different AmB carrier systems was fully satisfactory. The results showed that the action of AmB was not only concentration dependent, but also cellular type and vehicle kind dependent. The Fungizon-Lipofundin admixtures seem to be a good system for future use due to their best therapeutic index presented. However, it is important to stand out that the AmB admixtures should be considered as a new pharmaceutical dosage form; therefore, their use should be made with caution and after additional pre-clinical and clinical studies to validate them.
Acknowledgements
This work was funded by the grant number 47836/01-7 NV from CNPq.
Study from the Healthy Science Postgraduate Program (PPGCSa) - Federal University of Rio Grande do Norte (UFRN).
- 1. Jensen GM, Skenes CR, Bunch TH, Weissman CA, Amirghahari N, Satorius A. Determination of the relative toxicity of amphotericin B formulations: A red blood cell potassium release assay. Drug Deliv 1999;6:81-88.
- 2. Chavanet PY, Garry I, Charlier N, Caillot D, Kisterman JP, Dathis M. Trial of Glucose Versus Fat Emulsion in Preparation of Amphotericin for Use in Hiv Infected Patients with Candidiasis. Br Med J 1992;305:921-5.
- 3. Moreau P, Milpied N, Fayette N, Ramee JF, Harousseau JL. Reduced Renal Toxicity and Improved Clinical Tolerance of Amphotericin-B Mixed with Intralipid Compared with Conventional Amphotericin-B in Neutropenic Patients. J Antimicr Chemother 1992;30:535-41.
- 4. Caillot D, Reny G, Solary E, Casasnovas O, Chavanet P, Bonnotte B. A Controlled Trial of the Tolerance of Amphotericin-B Infused in Dextrose or in Intralipid in Patients with Hematological Malignancies. J Antimicr Chemother 1994;33:603-13.
- 5. Joly V, Aubry P, Ndayiragide A, Carriere I, Kawa E, MlikaCabanne N. Randomized comparison of amphotericin B deoxycholate dissolved in dextrose or intralipid for the treatment of AIDS-associated cryptococcal meningitis. Clin Infec Dis 1996;23:556-62.
- 6. Hiemenz JW, Walsh TJ. Lipid formulations of amphotericin B: Recent progress and future directions. Clin Infec Dis 1996;22:S133-S144.
- 7. Egito EST, Araujo IB, Damasceno BPGL, Price JC. Amphotericin B/emulsion admixture interactions: An approach concerning the reduction of amphotericin B toxicity. J Pharm Sci 2002;91:2354-66.
- 8. Araujo IB, Damasceno BPGL, Medeiros TMD, Soares LAL, Egito EST. Decrease in Fungizone toxicity induced by the use of Lipofundim as a diluent: an in vitro study. Curr Drug Deliv 2005;2:in press.
- 9. Puigventos-Latorre F, Delgado-Sanches O, Alvarez-Rabanal MV. Paciente com aspergilosis pulmonar: Indicação de la administração de uma mezcla IV de anfotericina B en intralipid 20%. Farm Clín 1995;12:31-2.
- 10. Joly V, Farinotti R, Saintjulien L, Cheron M, Carbon C, Yeni P. In-Vitro Renal Toxicity and in-Vivo Therapeutic Efficacy in Experimental Murine Cryptococcosis of Amphotericin-B (Fungizone) Associated with Intralipid. Antimicr Agents Chemother 1994;38:177-83.
- 11. Joly V, Geoffray C, Reynes J, Goujard C, Mechali D, Maslo C, et al. Amphotericin B in a lipid emulsion for the treatment of cryptococcal meningitis in AIDS patients. J Antimicr Chemother 1996;38:117-26.
- 12. Caillot D, Casasnovas O, Solary E, Chavanet P, Bonnotte B, Reny G, et al. Efficacy and Tolerance of an Amphotericin-B Lipid (Intralipid) Emulsion in the Treatment of Candidaemia in Neutropenic Patients. J Antimicr Chemother1993;31:161-9.
- 13. Egito EST, Appel M, Fessi H, Barratt G, Puisieux F, Devissaguet JP. In-vitro and in-vivo evaluation of a new ampbotericin B emulsion-based delivery system. J Antimicr Chemothe 1996;38:485-97.
- 14. Bolard J. How Do the Polyene Macrolide Antibiotics Affect the Cellular Membrane-Properties. Bioch Biophy Acta 1986;864:257-304.
- 15. Legrand P, Romero EA, Cohen BE, Bolard J. Effects of Aggregation and Solvent on the Toxicity of Amphotericin-B to Human Erythrocytes. Antimicr Agents Chemother 1992;36:2518-22.
Correspondence to
Publication Dates
-
Publication in this collection
28 May 2007 -
Date of issue
2005