Abstracts
PURPOSE: To verify the development of blood vessels between the greater omentum and the liver in the presence of distinct liver blood intake blockages. METHODS: Two hundred and eighty conventional male Wistar rats were used, divided into 5 groups: control (n=35), laparotomy (n=35); hepatic artery ligature (n=70), ligature of the right-hand branch of the portal vein (n=70); and ligature of both blood vessels (n=70). The last three groups were divided into two subgroups each (n=35), according to the presence or absence of the transposition of the greater omentum onto the right hepatic lobe. The postoperative periods were 1, 3, 7, 15, 30, 60 and 90 days. At the end of each period, the greater omentum and right hepatic lobe were collected for histopathological examination. The presence of blood vessels between the referred tissues was verified by the administration of Indian ink as a marker of vascular lumen. RESULTS: Macroscopic and microscopic observation and the dye marker demonstrated the distribution of blood vessels between the greater omentum and liver tissues. CONCLUSION: The greater omentum was capable of developing blood vessels when fixed to the parenchyma of the liver after the suppression of hepatic blood flow.
Omentum; Liver; Rats
OBJETIVO: Verificar o desenvolvimento de vasos sanguíneos entre o omento maior e o fígado em presença de diferentes bloqueios do aporte sanguíneo hepático. MÉTODOS: Foram utilizados 280 ratos machos, Wistar, convencional, divididos em 5 grupos: controle (n = 35), com laparotomia (n = 35), com ligadura da artéria hepática própria (n = 70), com ligadura do ramo direito da veia porta (n = 70) e com ligadura de ambos vasos sanguíneos (n = 70). Os três últimos grupos foram divididos em dois subgrupos (n = 35), de acordo com a transposição ou não do omento maior no lobo direito do fígado. Os períodos de pós-operatório foram de 1, 3, 7, 15, 30, 60 e 90 dias. Em cada período foram coletados o omento maior e o lobo direito do fígado para exame histopatológico. Presença de vasos sanguíneos entre os referidos tecidos foi verificada pela administração da tinta nanquim como marcador de lúmen vascular. RESULTADOS: As observações macroscópicas, microscópicas e do marcador tintorial demonstraram a distribuição dos vasos sanguíneos entre o omento maior transposto e o tecido hepático. CONCLUSÃO: O omento maior foi capaz de desenvolver vasos sanguíneos quando fixado junto ao parênquima do fígado, após supressão do fluxo sanguíneo hepático.
Omento; Fígado; Ratos
ORIGINAL ARTICLE
Development of blood vessels of the greater omentum in the hepatic lobe after vascular ligation. An experimental model in the rats 1 1 . Research performed at Department of Surgery, Faculty of Medicine, Federal University of Rio de Janeiro (UFRJ). Brazil.
Desenvolvimento de vasos sanguíneos do omento maior no lobo hepático após ligadura vascular. Modelo experimental em ratos
Paulo Cesar SilvaI; Nelson JamelII; Ricardo Antonio RefinettiII; Eduardo Ferreira MansoIII; Alberto SchanaiderIII
IPhD, Post-Graduation Program, UFRJ. Brazil
IIChairman Full Professor, Department of Surgery, UFRJ. Brazil
IIIAssociate Professor, Department of Surgery, UFRJ. Brazil
Correspondence Correspondence: Paulo Cesar Silva Av. Acúrcio Torres, 2695 24358-080 Niterói RJ Brazil Phone: (55 21) 9958-8714 prsilva@nitnet.com.br
ABSTRACT
PURPOSE: To verify the development of blood vessels between the greater omentum and the liver in the presence of distinct liver blood intake blockages.
METHODS: Two hundred and eighty conventional male Wistar rats were used, divided into 5 groups: control (n=35), laparotomy (n=35); hepatic artery ligature (n=70), ligature of the right-hand branch of the portal vein (n=70); and ligature of both blood vessels (n=70). The last three groups were divided into two subgroups each (n=35), according to the presence or absence of the transposition of the greater omentum onto the right hepatic lobe. The postoperative periods were 1, 3, 7, 15, 30, 60 and 90 days. At the end of each period, the greater omentum and right hepatic lobe were collected for histopathological examination. The presence of blood vessels between the referred tissues was verified by the administration of Indian ink as a marker of vascular lumen.
RESULTS: Macroscopic and microscopic observation and the dye marker demonstrated the distribution of blood vessels between the greater omentum and liver tissues.
CONCLUSION: The greater omentum was capable of developing blood vessels when fixed to the parenchyma of the liver after the suppression of hepatic blood flow.
Key words: Omentum. Liver. Rats.
RESUMO
OBJETIVO: Verificar o desenvolvimento de vasos sanguíneos entre o omento maior e o fígado em presença de diferentes bloqueios do aporte sanguíneo hepático.
MÉTODOS: Foram utilizados 280 ratos machos, Wistar, convencional, divididos em 5 grupos: controle (n = 35), com laparotomia (n = 35), com ligadura da artéria hepática própria (n = 70), com ligadura do ramo direito da veia porta (n = 70) e com ligadura de ambos vasos sanguíneos (n = 70). Os três últimos grupos foram divididos em dois subgrupos (n = 35), de acordo com a transposição ou não do omento maior no lobo direito do fígado. Os períodos de pós-operatório foram de 1, 3, 7, 15, 30, 60 e 90 dias. Em cada período foram coletados o omento maior e o lobo direito do fígado para exame histopatológico. Presença de vasos sanguíneos entre os referidos tecidos foi verificada pela administração da tinta nanquim como marcador de lúmen vascular.
RESULTADOS: As observações macroscópicas, microscópicas e do marcador tintorial demonstraram a distribuição dos vasos sanguíneos entre o omento maior transposto e o tecido hepático.
CONCLUSÃO: O omento maior foi capaz de desenvolver vasos sanguíneos quando fixado junto ao parênquima do fígado, após supressão do fluxo sanguíneo hepático.
Descritores: Omento. Fígado. Ratos.
Introduction
The greater omentum is a highly vascularized peritoneal fold, which extends from the greater curvature of the stomach to the proximal abdominal viscera. This tissue, when applied to ischemic tissues has shown great potential for neovascularization by means of angiogenesis. Adipocytes and endothelial cells of the omental blood vessels are involved in this process 1. The surgical applicability of the greater omentum in the form of a pediculated or free graft to provide vascular support has been developed in diverse organs and tissues2. The liver is an organ which offers good possibilities for the realization of neovascularization studies, as it presents a double blood flow intake, through the portal vein and hepatic artery. The angiogenic capacity of the greater omentum was demonstrated in human liver transplants that presented spontaneous vascularization adherence in this tissue3. Experimental studies suggested the possibility of angiogenesis, when ligature of the hepatic artery was realized during liver transplant followed by implantation of the greater omentum4. The objective of the present study was to verify the development of blood vessels between the transposed greater omentum and hepatic tissue in distinct situations of liver blood intake blockages.
Methods
Two hundred and eighty conventional male adult rats (Rattus norvegicus albinus) of the Wistar strain, with a mean body weight of 250g, maintained at the Animal Center of the Experimental Surgery Center of the School of Medicine, Rio de Janeiro Federal University (UFRJ), Brazil. The environmental and feeding conditions were adequate for the species, with free access to feed and water. The present study was approved by the Ethics Commission for Laboratory Animal Use in Research, Teaching and Extension, CEPAL, UFRJ, project number 01/04. The realization of the experiments obeyed the Ethical Principals in Animal Experimentation of the Brazilian College of Animal Experimentation (COBEA)5. The rats were randomly placed into five groups: Group 1 Control (n=35); Group 2 Laparotomy (n=35); Group 3 Hepatic artery (HA) ligature (n=70), subdivided into two groups (n= 35), one with and the other without transposition of the greater omentum onto the right hepatic lobe; Group 4 Portal vein right branch (PVRB) ligature (n=70), subdivided into two groups (n=35), one with and the other without transposition of the greater omentum onto the right hepatic lobe; Group 5 HA and PVRB ligatures (n=70), subdivided into two groups (n=35), one with and the other without transposition of the greater omentum onto the right hepatic lobe.
Preoperative and operative procedures
In preoperative procedures on the rats, anesthetic induction and maintenance were realized with diethyl ether by an open inhalatory system and progressive administration. The rats were then fixed in decubitus and trichotomy was realized in the abdominal region, followed by antisepsis. The Group 1 suffered no surgical intervention and those from Group 2 underwent only a median incision in the abdominal wall. In the remaining groups, the rats were submitted to median laparotomy, with exteriorization and placement to the left of the intestinal folds, wrapped in gauze moistened with 0.9% physiological serum. This maneuver permitted the exposure of the midbody together with the HA, portal vein and biliary duct, as well as the right hepatic lobe. Following this maneuver, in Group 3 the adjacent tissue was dissected and the portal vein deviated at the hepatic hilum, 1 cm from the insertion of the hepatic artery, followed by simple ligation of this artery with 6.0 mononylon thread. In Group 4 , due to the anatomical disposition, it was necessary to simultaneously separate the right branch of the biliary duct and the hepatic artery from the RBPV. Following this, the RBPV was separated from the caudal vena cava and simple ligation of the RBPV was realized using the same type of thread. Finally, in the Group 5, both procedures were realized during the same operative period. Transfixation of the hepatic parenchyma of the right hepatic lobe was performed with the aid of a 3 cm fragment of infant urethral probe no. 10, which produced a circular incision in the middle third of the right lobe, forming an intra-hepatic canalicular trajectory from the visceral to the diaphragmatic surface. In the corresponding subgroups, the transposition of a portion of approximately 8 mm of the pediculated greater omentum was performed in this region (Figure 1).
Postoperative procedures
To accompany the development of blood vessels in the omentum-hepatic interposition region, macroscopic observations were realized consisting of the formation of adherences, coloration, tissue size and vascularization; microscopic analyses regarding the presence of polymorphonuclear infiltrates (PMNI), fibroblasts and vascularization; and, finally, blood flow verification by the administration of Indian ink as a vascular lumen marker. In this stage, five rats from each group/subgroup were anesthetized, after the following periods: 1, 3, 7, 15, 30, 60 and 90 postoperative days (POD). After the macroscopic observations were completed, three of these rats were randomly chosen for sacrifice by ethylic ether vapor saturation for microscopic analysis. From these rats, the stomach, spleen, greater omentum and liver were removed in block and fixed in a solution of 10% formalin buffered with saline. Later these materials were included in paraffin blocks, cut into slices of 5ì, stained with hematoxylin-eosin (HE) and examined under an optical microscope. In the subgroups with omentum-hepatic interposition, the two remaining rats underwent exposure of the spleen, the transposed greater omentum and the hepatic lobe, which was sectioned and isolated from the remainder of the liver. These structures were placed on a microscope slide in order to maintain them separated from the remaining viscera. Posteriorly, administration of a 2.5 mL solution of black Indian ink and 0.05 mL of sodium heparin (1000 UI/Kg of body weight) was realized via intrasplenic injection. Up on completion of this procedure, the rats were sacrificed and the region of omentum-hepatic interposition was removed in block and processed as previously described for microscopic analyses (Figure 2).
Results
Macroscopic observations
The rats included in the control and laparotomy groups showed no macroscopic alterations in the right hepatic lobe or the greater omentum. Those which underwent HA ligation without omental transposition presented spontaneous adherence of the omentum to the right hepatic lobe between POD 7 and 90. However, rats with the omentum transposed showed a diffuse presence of calibrous blood vessels around the right hepatic lobe in the same period. The group of rats which underwent PVRB ligation without omental transposition presented no macroscopic alterations in the omentum up to POD 7, though from POD 15 to 90 they presented spontaneous adherence to the right hepatic lobe. In this latter period, the presence of lighter colored areas in the central portion was noted, surrounded by areas of normal coloration. The referred lobe presented normal size on POD 7 and atrophied with spontaneous adherence to the medial lobe from POD 15 to 90. In the subgroup with omental transposition, the greater omentum presented calibrous blood vessels around the right hepatic lobe from POD 7 to 90 and showed normal coloration and reduced sized from POD 15 to 90. When the rats underwent simultaneous HA and PVRB ligation without omental transposition, observation of the greater omentum showed adherence to the right hepatic lobe in all postoperative periods and intense vascularization from POD 15 to 90. The right lobe also presented a lighter coloration, atrophy and adherence to the middle lobe from POD 1 to 90. In rats with omental transposition, the greater omentum presented intense vascularization and calibrous blood vessels around the right hepatic lobe from POD 7 to 90. On POD 7, this lobe showed lighter coloration and hemorrhagic areas, more intense at the organ periphery, and atrophy with normal coloration from POD 15 to 90 (Figure 3).
Microscopic observations
At all postoperative times, the rats of the control and laparotomy groups presented preserved hepatic and omental architecture; in those which underwent HA ligation without omental transposition, preserved omental histological characteristics were observed. However, in the right hepatic lobe, preserved histology was observed up to POD 7, necrotic areas were located on the periphery of the lobe on POD 15, areas with degeneration on POD 30 and normal hepatocytes and areas of fibrosis on POD 90. In the subgroup with omental transposition, the omentum-hepatic region presented PMNI, fibroblasts and intense vascularization on POD 7, the presence of fibroblasts and blood vessels on POD 15 and calibrous blood vessels on POD 60 and 90. In the hepatic parenchyma the presence of necrotic foci and fibrosis were observed on POD 7, areas of fibrosis on POD 15 and preserved architecture from POD 30 to 90. In the group of rats which underwent PVRB ligation without omental transposition, spontaneous adherence of the greater omentum to the right hepatic lobe was observed, with the presence of fibroblasts and blood vessels from POD 15 to 90. The right hepatic lobe presented necrosis in the central areas of the hepatic lobes from POD 1 to 15; in the latter period, an association with fibrosis occurred and from POD 30 to 90, fibrosis was observed. In the subgroup with omental transposition, the presence of fibroblasts and vascularization was observed from POD 3 to 15 and intense vascularization around the hepatic parenchyma from POD 30 to 90. In the right lobe, the hepatic parenchyma presented areas of necrosis up to POD 7, which were substituted by areas of fibrosis and PMNI from POD 30 onwards, with a reduction in the areas of fibrosis on POD 30. From POD 60 to 90, preserved architecture was observed in this lobe. In rats which underwent simultaneous HA and PVRB ligation without omental transposition, the absence of any histological alteration in the greater omentum was observed up to POD 3, however from POD 1 to 90, spontaneous adherence to the middle lobe was noted. On POD 7, the presence of PMNI was noted, fibroblasts and intense vascularization on POD 15 and blood capillaries around the hepatic parenchyma from POD 30 to 90. The right hepatic lobe presented alternating hemorrhagic and ischemic areas on POD 1, areas of necrosis in the central zone to the intermediary zones of the lobes on POD 3, PMNI and a reduction in the necrotic areas on POD 7 the onset of areas of fibrosis on POD 15, PMNI surrounding areas of necrosis on POD 30 and fibrosis from POD 60 to 90. In the subgroup with omental transposition, the presence of omental blood vessels around the hepatic parenchyma of the right lobe was observed in the omentum-hepatic region on POD 1, followed by fibroblasts and intense vascularization on POD 3, PMNI, fibroblasts and neoformed blood vessels on POD 7, the intense presence of fibroblasts and the proliferation of blood vessels in areas of hepatic parenchyma fibrosis on POD 15 and intense vascularization, with calibrous blood vessels from POD 30 to 90. In the right liver lobe, the hepatic parenchyma presented hemorrhagic areas close to the omentum-hepatic region on POD1, areas of necrosis on POD3, the persistence of necrotic areas close to the referred region, PMNI and the neoformation of blood vessels on POD 7, areas of necrosis with intense fibroplasia and PMNI on POD 15, areas of necrosis surrounded by PMNI on POD 30 and preserved architecture from POD 60 to 90 (Figure 4).
Blood flow verification
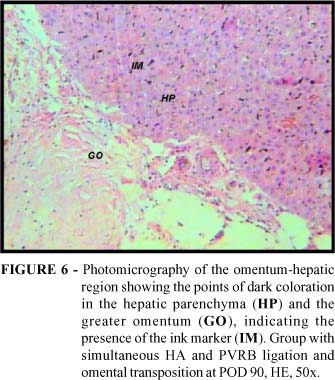
In approximately 15 sec., the Indian ink-heparin solution was capable of revealing a dark coloration in the omental vascular network and in the right hepatic lobe, which had been sectioned and isolated from the remaining hepatic lobes. Thus, in the subgroups with omental transposition a dark coloration both beneath the hepatic capsule and on the incision surface that separated the right hepatic lobe from the rest of the liver was observed (Figure 5). Among the materials processed for microscopic observation, points of dark coloration suggestive of the presence of the ink marker in the hepatic parenchyma and greater omentum were highlighted (Figure 6).
Discussion
In rats with arterial and/or portal blood suppression included in the subgroups without omental transposition, spontaneous adherences between the greater omentum and right hepatic lobe were observed; results that are in agreement with the findings of Yedlicka et al.3. These adherences are the result of circulatory alterations promoted by insufficient blood delivery to the hepatic tissue7. Since they are vascularized adherences, it is possible that these are a compensatory form of establishing hepatic blood circulation. Moreover, lobe atrophy was noted among the rats of these subgroups, indicative of acute ischemia, corroborating the findings of Fornander et al. 8, as well as the presence of areas of necrosis followed by fibrosis in the hepatic parenchyma, caused by acute hepatocellular lesions. These observations are in agreement with the findings of Silva Jr. et al.7. In rats included in the subgroups with omental transposition after hepatic vascular ligation, the presence of omental blood vessels around the right lobe of the liver were observed. This fact reinforces the description made by Liebermann-Meffert2 regarding the capacity of the greater omentum to provide vascular support to adjacent tissues with circulatory deficiency. The suppression of blood flow in an acute manner proportioned the appearance of an ischemic state and hepatic tissue hypoxia. According to Yancopoulos et al.9, these events are responsible for the onset of angiogenesis, because they promote the liberation of vascular growth promoters, like Fibroblast Growth Factor (FGF) and Vascular Endothelial Growth Factor (VEGF). Moreover, the transfixation of the hepatic parenchyma followed by omental tissue interposition promoted the appearance of an inflammatory process in the region. The presence of fibroblasts and neoformed blood vessels conferred characteristics of repair to this region10. Fibroblasts are stimulatory elements for FGF, which in turn promotes granulation and angiogenesis tissue growth11. It should be noted that these finding are in agreement with the immunological and antiinflammatory properties of omental tissue2. According to Shi et al. 12, a fuller understanding of how the angiogenic factors act in the proliferation of hepatic sinusoid endothelial cells is still unknown, though they are probably VEGF-dependent. According to these authors, the appearance of areas of necrosis and fibrosis promotes a reduction in the liberation VEGF, with a consequent diminished number of capillaries around the hepatic sinusoids. In the present study, both areas of necrosis and fibrosis were observed in the hepatic parenchyma, which were found not to endure for longer postoperative periods. This could be explained by the omentum-hepatic vascular interaction. The appearance and persistence of blood vessels in the referred area indicate the possibility of neovascularization, according to Liebermann-Meffert 2 and Lynch et al. 11, which occurs by angiogenic cytokine action and by the growth factors present in the lipidic fraction of the greater omentum. In the present work, omental blood vessels were capable of realizing the maintenance of the hepatic parenchyma after arterial and portal blood flow suppression. These results corroborate the findings of Ishikawa et al.4. The blood vessels presented increased diameter and branching; amplifying their distribution in the hepatic tissue. The distribution of the ink marker in the omental blood vessels around the hepatic tissue, suggests the occurrence of communication with the hepatic sinusoids. The mechanism by which blood flow is established between the greater omentum and liver was not fully clarified. The vascular structural similarity of the greater omentum and the liver, consisting of capillaries2, 13, could have contributed to neovascularization. The results obtained with this experimental model indicate the possibility of new studies involving angiogenesis and surgical revascularization.
Conclusion
The greater omentum is capable of developing blood vessels when fixed to the parenchyma of the right hepatic lobe after alterations in blood intake in this lobe in the rat.
Received: July 12, 2006
Review: August 18, 2006
Accepted: September 24, 2006
Conflict of interest: none
Financial source: none
- 1. Goldsmith HS, Griffith AL, Kupferman A, Catsimpoolas N. Lipid angiogenic factor from omentum. J Am Med Assoc.1984;252(15):2034-46.
- 2. Liebermann-Meffert D. The greater omentum: Anatomy, Embryology and Surgical applications. Surg Clin.North Am. 2000;80(1):275-91.
- 3. Yedlicka JW, Halloran J, Payne WD, Hunter DW, Castaneda-Zuniga WR, Amplatz K, Letourneau JG. Angiogenesis after hepatic arterial occlusion in liver transplant patients. JVIR.1991;2(2):235-40.
- 4. Ishikawa M, Fjii M, Iuchi M, Miyauchi T, Tashiro S. Effect of intrahepatic omental implantation on angiogenesis in rat liver with hepatic artery ligation. Clin Exp Med. 2001;1 (1):27-33.
-
5Legislation and Ethic.[homepage on the Internet]. S.Paulo: Brazilian College of Animal Experimentation (COBEA); 2000 [cited 10 Dec 2005]. Available from http://www.COBEA.org.br
- 6. Ren J, Wold LE. Measurement of cardiac mechanical function in isolated ventricular myocytes from rats and mice by computerized video-based imaging. Biol Proced. 2001;3(1): 43-53.
- 7. Silva JrOC, Soares AF, Franco CFF, Souza MEJ, Picinato MANC, Roselino JES, Ceneviva R. Alterações agudas hepatocelulares após ligadura da artéria hepática em ratos com colestase extra-hepática. Acta Cir Bras.1990;5(2):55-8.
- 8. Fornander J, Seeman T, Hasselgren PO. Changes of protein synthesis in liver tissue following ligation of hepatic artery or portal vein in rats. Eur J Surg . 1985;17(2):101-8.
- 9. Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000; 407(14):242-8.
- 10. Petroianu A, Silva RTF, Parreira LM, Barbosa AJA. Alterações morfológicas do fígado após secção hepática parcial e omentoplastia. Rev Col Bras Cir. 1998;25(1):15-7.
- 11. Lynch SE, Colvin RB, Antoniades HN. Growth factors in wound healing: Single and synergistic effects on partial thickness porcine wounds. J Clin Invest. 1989; 84(8):640-6.
- 12. Shi BM, Wang XY, Mu QL, Wu TH, Liu HJ, Yang Z. Angiogenesis effect on rat liver after administration of expression vector encoding vascular endothelial growth factor D. World J Gastroenterol. 2003;9(2):312-5.
- 13. Ekataksin W. The isolated artery: An Intrahepatic arterial pathway that can bypass the lobular parenchyma in mammalian livers. Hepatology. 2000;31(1):269-79.
Publication Dates
-
Publication in this collection
04 Dec 2006 -
Date of issue
Dec 2006
History
-
Accepted
24 Sept 2006 -
Reviewed
18 Aug 2006 -
Received
12 July 2006