Abstracts
PURPOSE: To investigate clinical and histologically the bone repair in treated animals with calcitonin and sodic diclofenac. METHODS: Ninety-six femoral defects were created in forty-eight animals distributed in four groups (n=24): either left untreated, treated with the sodic diclofenac or calcitonin or both. Follow-up was 7, 14 and 21 days. Histological sections stained by haematoxylin-eosin was observed under light microscopy (100X) and quantitatively scored for their trabecular formation. The groups and subgroups were compared being used the Kruskall-Wallis test. RESULTS: Smaller trabecular formation was observed in the animals of the group II and larger trabecular formation in the animals of the group III. Was found significant differences in the comparison between all the groups (Kruskall-Wallis, p <0.05). CONCLUSION: The obtained data suggest that the bone repair is a time-dependent process, which can be delayed by the sodic diclofenac and accelerated by the calcitonina, when used separately. The associated use of calcitonina and sodic diclofenac didn't show to be the best therapeutic option in the treatment of bone defects surgically created.
Osteogenesis; Anti-inflammatory agents; Calcitonin; Rats
OBJETIVO: Investigar clínica e histologicamente o reparo ósseo em animais tratados com calcitonina e diclofenaco sódico. MÉTODOS: Foram criados 96 defeitos femorais, em 48 animais distribuídos em quatro grupos (n = 24): não tratados, tratados com diclofenaco sódico ou calcitonina ou ambos. O período de seguimento foi 7, 14 e 21 dias. As secções coradas por hematoxilina e eosina foram observadas sob microscopia óptica (100x) e analisadas quantitativamente em relação à neoformação trabecular. Os grupos e subgrupos foram comparados utilizando-se o teste de Kruskall-Wallis. RESULTADOS: Foi observada menor formação de trabéculas ósseas nos animais do grupo II e maior formação de trabéculas ósseo nos animais do grupo III. Foram encontradas diferenças significantes na comparação entre todos os grupos (Kruskall-Wallis, p <0.05). CONCLUSÃO: Os dados obtidos sugerem que o reparo ósseo é um processo tempo-dependente, que pode ser retardado pelo diclofenaco e acelerado pela calcitonina, quando utilizados isoladamente. O uso associado de calcitonina e diclofenaco sódico não mostrou ser a melhor opção terapêutica no tratamento de defeitos ósseos criados operatoriamente.
Osteogênese; Anti-inflamatórios; Calcitonina; Ratos
ORIGINAL ARTICLE
Bone repair in rats treated with sodic diclofenac and calcitonin1 1 Postgraduate Program on Health Sciences in the West Central Region Federal University of Brasília (UnB) and Federal University of Mato Grosso do Sul, (UFMS), Campo Grande, Brazil
Reparo ósseo em ratos tratados com diclofenaco sódico e calcitonina
Maria Cristina Pita SassiotoI; Celso Massaschi InouyeII; Ricardo Dutra AydosIII; Arthur Silveira de FigueiredoIV
IMaster, Fellow PhD degree in Post Graduate of Health and Development in the West Central Region, UFMS, Brazil
IIPhD, Division of Orthopedics, UFMS, Brazil
IIIPhD, Division of Surgery, UFMS, Brazil
IVPhD, Chairman and Head, Division of Orthopedics, UFMS, Brazil
Correspondence Correspondence:
ABSTRACT
PURPOSE:To investigate clinical and histologically the bone repair in treated animals with calcitonin and sodic diclofenac.
METHODS:Ninety-six femoral defects were created in forty-eight animals distributed in four groups (n=24): either left untreated, treated with the sodic diclofenac or calcitonin or both. Follow-up was 7, 14 and 21 days. Histological sections stained by haematoxylin-eosin was observed under light microscopy (100X) and quantitatively scored for their trabecular formation. The groups and subgroups were compared being used the Kruskall-Wallis test.
RESULTS: Smaller trabecular formation was observed in the animals of the group II and larger trabecular formation in the animals of the group III. Was found significant differences in the comparison between all the groups (Kruskall-Wallis, p <0.05). CONCLUSION: The obtained data suggest that the bone repair is a time-dependent process, which can be delayed by the sodic diclofenac and accelerated by the calcitonina, when used separately. The associated use of calcitonina and sodic diclofenac didn't show to be the best therapeutic option in the treatment of bone defects surgically created.
Key words: Osteogenesis. Anti-inflammatory agents. Calcitonin. Rats.
RESUMO
OBJETIVO: Investigar clínica e histologicamente o reparo ósseo em animais tratados com calcitonina e diclofenaco sódico.
MÉTODOS: Foram criados 96 defeitos femorais, em 48 animais distribuídos em quatro grupos (n = 24): não tratados, tratados com diclofenaco sódico ou calcitonina ou ambos. O período de seguimento foi 7, 14 e 21 dias. As secções coradas por hematoxilina e eosina foram observadas sob microscopia óptica (100x) e analisadas quantitativamente em relação à neoformação trabecular. Os grupos e subgrupos foram comparados utilizando-se o teste de Kruskall-Wallis.
RESULTADOS: Foi observada menor formação de trabéculas ósseas nos animais do grupo II e maior formação de trabéculas ósseo nos animais do grupo III. Foram encontradas diferenças significantes na comparação entre todos os grupos (Kruskall-Wallis, p <0.05).
CONCLUSÃO: Os dados obtidos sugerem que o reparo ósseo é um processo tempo-dependente, que pode ser retardado pelo diclofenaco e acelerado pela calcitonina, quando utilizados isoladamente. O uso associado de calcitonina e diclofenaco sódico não mostrou ser a melhor opção terapêutica no tratamento de defeitos ósseos criados operatoriamente.
Descritores: Osteogênese. Anti-inflamatórios. Calcitonina. Ratos.
Introduction
The regeneration of bony tissues requires the timely recruitment of skeletal progenitor cells to an injury site, the differentiation of these cells into bone or cartilage, and the re-establishment of a vascular network to maintain cell viability. Disturbances in any of these cellular events can have a detrimental effect on the process of bone repair1,2. The regenerating potential of human bone is limited. The repair of large bone defects often associated with trauma or bone tumor resections is not observed, and nonunion or delayed union of bone is a serious problem3. The bone repair happens by means of answer tissue composed of three phases, that don't happen separately. Those phases can be described as inflammation, repair and remodelation. The inflammatory answer is considered indispensable to the repair process; without the inflammation, the bone would never heal2,3. Several factors can influence in a positive or negative way the organization and result end of the process of bone repair. Some of them posses influence that can only be investigated in experimental studies4,5. The factors that can influence positive or negatively the reparative osteogenesis composes the objective of several experimental studies and they should be analyzed by different angles, because many of these aspects are put upon, and this perspective is crucial to its understanding6. The nonsteroidal anti-inflammatory drugs are used broadly in all the medical specialties and they head the drugs relationship used by the Brazilian population in self-medication act7. Clinical studies suggest a possible role for cyclooxygenases, that modulates the synthesis of some mediators of the inflammation, as the prostaglandin's, in bone repair and create concerns about the use of nonsteroidal anti-inflammatory drugs in patients with skeletal injury8. Studies demonstrate the delayer effect of nonsteroidal anti-inflammatory drugs in the repair and bony remodelation; the daily use of sodic diclofenac harms the bony repair, taking to the instability of the bony callus; the meloxicam increases the neutrofil's flow with decrease of osteoclast; and the tenoxican presents important inhibitory effect on the osteogenesis8. Other drugs same tends anti-inflammatory, they seem to stimulate the osteogenesis, as it is the case of the calcitonin, a polypeptide composed for 32 amino acids secreted by the C cells of the thyroid, that acts directly on the osteoclasts, inhibiting the bony re-absorption and promoting analgesic action for decrease of the local synthesis of prostaglandins5,9. The calcitonina participates actively of the skeletal homeostasis as regulator of the mineral and bony metabolism, interfering in the action of the parathyroid hormone in the maintenance of the bony mass. The calcitonina decrease the levels of the seric calcium, six hours after the administration. The main factor related to the hypocalcaemia of the calcitonin effect is its union to the osteoclastic cells, causing morphologic and chemotaxis alterations and reducing them to cells mononucleares. The connection of the calcitonina to the osteoclasts is followed by elevation of the intracellular level of cAMP and calcium, suggesting that these act as messengers of the calcitonin action, which also reduces the necessary enzymatic processes to the bony re-absorption5,9. When is used drugs with capacity of reducing the osteoclastic action, the tendency it is that the process of bony deposition is increased, for the osteoblastic action, because both cells participate actively of the formation process and dynamic re-absorption of the bone. Such aspect was observed by Sassioto et al.5, in accomplished study using the same experimental model used in this work. For its anti-inflammatory, analgesic and osteoclastic inhibitory properties, the calcitonina is used in the treatment of diseases characterized by excessive bony remodelation, as the menopause osteoporosis, Paget' disease and malign hypercalcaemia. The animal experimentation and the clinical studies showed that the salmon's calcitonin is the more it activates up to now of the isolated calcitonins and absolutely it exempts of exogenous animal proteins. In spite of the evidence of the anti-osteoclastic action of the calcitonina, the anabolic function of that hormone is still controversial, mainly in relation to the fibroblasts and/or osteoblasts5,9. Considering to be the bony repair a cellular function of osteoclast and osteoblast cells, involved directly in the bony mineral metabolism, with direct regulation for the calcitonin, a hormone broadly used in the treatment of diseases related with the bony formation alterations and affected for several drugs used for pain control, we took place a experimental study standardized with the purpose of observing the use of anti-inflammatory drugs associated to the calcitonin would promote incentive of the osteogenesis without delay of the analgesic and anti-inflammatory function, in rats.
Methods
This study was approved by the Animal Use Ethics Commission from Federal University of Mato Grosso do Sul (37/2002) and all procedures were performed according to the Animal Experimentation Ethics Committee. Forty-eight male, adult Wistar rats with mean weight of 355g were used, distributed into four groups of 12 animals each, forming a control group (Group I), a diclofenac group (Group II), a calcitonin group (Group III) and a diclofenac-calcitonin group (Group IV). All animals were anesthetized with a solution 1:1 with xylazine (20 mg/mL) and ketamine (50mg/mL), intramuscularly of 0,1mL per 100g of weight. The anesthesic plan was evaluated through the following parameters: corneal reflex and caudal pinch reflex. After shaving, a 20mm longitudinal incision was performed at the lateral aspect of the thigh, followed by blunt muscle divulsion and femoral shaft exposure, periosteal incision and elevation, and creation of a bone defect in both femora at the lateral cortex and bone marrow with a 2mm electric metal saw. The place of the operation was washed with saline solution; the muscle was approximated with 3.0 simple catgut stitches, followed by cuticular continuous stitches with 4.0 monofilament polyamide. The animals of the group I didn't receive any postoperative medication; the animals of the group II received 0,05mL of sodic diclofenac (100mg/mL); the animals of the group III received 0,05mL (2UI) of calcitonina; and the animals of the group IV received sodic diclofenac and calcitonin in similar doses to the one of the groups II and III. Whole the drugs injected intramuscularly right after the procedure. Animals were allowed immediate weight bearing of operated limbs after anesthetic recovery, with no kind of external immobilization whatsoever. They were kept in cages, with unrestricted water and food consumption until accomplishment of seven, 14, and 21 days of follow-up, when four animals of each group were weighted and sacrificed by continuous ethyl ether inhalation.Femora were dissected and after macroscopic evaluation for morphological changes of femoral structure fracture, heterotopic ossification and bone cortex defect persistence they were fixed in 10% buffered formaline, and processed under standard histological technique. Four-micra histological cuts were stained with hematoxylin-eosin and observed under light microscopy for histomorphological trabecular structure. Three photomicrographies were taken from each histological cuts and were submitted to quantitative analysis of above-mentioned parameter with image analysis software (ImageLab2.0), considering as final result the obtained average of the three readings. The quantitative procedures followed the model published by Sassioto et al.5 Results were submitted to statistical analysis by Kruskal-Wallis variance analysis to compare all study groups in each period (seven, 14 and 21 days). The null hypothesis rejection level was set to 5%.
Results
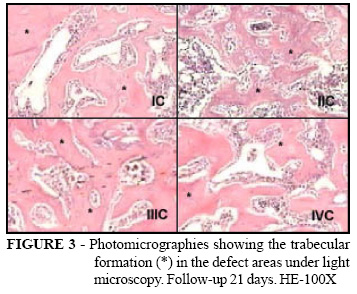
All animals gained weight and no death occurred during the experiment. Macroscopically, neither the presence of femoral fracture nor heterotopic ossification was observed. The bone cortex defect remained visible in all follow-up periods. Under light microscopy, areas of defect in all groups, at seven days of follow-up, were filled up by connective stroma with vascular neoformation, islets of newly formed bone tissue and the presence of inflammatory reaction (Figure 1). At 14 days, areas of defect showed connective stroma, the presence of moderate inflammatory reaction and newly formed bone laid in thin trabeculae in all groups (Figure 2). At 21 days, all groups showed the presence of dense bone trabeculae with lamellar bone aspect and incomplete medullary space reconstitution (Figure 3). The data of quantitative analysis were in Table 1.
Discussion
Bone defect repair involves proliferation and differentiation of several tissue types in a sequence, followed by remodeling. All of these processes may be influenced by drugs10. Some drugs can have effects on the proliferation of early callus tissue, others on the differentiation of chondrocytes or osteoblasts, capillary formation, sensitivity to mechanical input etc. If the repair of long bones is not optimized, it should be quite possible to enhance it by pharmacological or other means11,12. We chose the rat as an animal model because it is easy to obtain a homogenous sample, and it is simple to handle. Weight gain in all sample animals shows that operative procedures and injected drug did not interfere with normal animal development during the studied period5. The visual defect identification at bone cortex during the study period is explained by the centripetal characteristic of bone defect healing, that is, from the periphery to the center of the cavity, having initially a hematoma formation and organization by granulation tissue, non lamellar, immature bone tissue formation; bone trabeculae maturation, osteoclastic activity, and medullary stroma formation. It is thought that the absence of femoral cortical operative access has time dependent features, which means that the follow-up period of this experimental study could have been insufficient for complete bone cortical remodeling5. Absence of fracture or heterotopic calcification on macroscopic evaluation observed in this work suggests that the used operative technique produced minimum damage to the soft tissue related to the femur. Our results are in agreement with the one of Zagaja and Cromie13; they affirmed that the risks factors associated with traumatic heterotopic ossification include prolonged operating time, hematoma formation, degree of bony debris, devitalized muscle and concomitant infection. Prophylaxis with single low dose radiation or nonsteroidal anti-inflammatory drugs has been shown to be effective in the prevention of heterotopic ossification. In this work, the smallest formation of bony trabeculae in the animals of the group II seems to be related with the use of nonsteroidal anti-inflammatory drug (sodic diclofenac) in the beginning phases of the bony repair, where the inflammatory process is of fundamental importance. Our results are similar to the of Neal et al.14, where the inhibition of bone formation in humans only requires treatment with Cox inhibitors during the first 5-7 days after the operation. This suggests that the initial inflammation is crucial for starting the bone formation response. Also, in fracture repair, some data from animal experiments have suggested that Cox inhibitors are most harmful during the early phase of healing, and that remodeling is little affected15. Anti-inflammatory drugs are an important adjunct in controlling postoperative pain. Concerns exist regarding the use of these for postoperative pain management because of the possible deleterious impact on bone healing15. The Cox1 and Cox2 enzymes both catalyze the same rate-limiting enzymatic step in the production of prostaglandins. By and large, Cox1 is responsible for the small production of prostaglandins that is necessary for cell survival, and Cox2 is induced whenever excess prostaglandin is needed during inflammation or repair. Relatively speaking, Cox2 produces huge amounts of prostaglandins, so when Cox2 is turned on, the production from Cox1 becomes neglible. Thus, in fracture repair, both unspecific and specific Cox2 inhibitors dramatically reduce prostaglandin production and should therefore be expected to have similar effects11. There is a substantial amount of literature on the effects of Cox inhibitors on bone repair in experimental animals. Some show inhibition of repair and others do not. In those studies where Cox inhibitors appear harmless, the dosing of the Cox inhibitor may be inadequate. In male rats, the selective Cox2 inhibitors celecoxib and rofecoxib are metabolized so quickly by the liver that also a high dose given once daily will yield too low a serum concentration to be relevant11. In this work, the dense formation of bony trabeculae in the animals of the group III seems to be related with the use of salmon calcitonin right after the surgical procedure. We used intramuscularly administered salmon calcitonin, for facility of drug application, better dosing control, and for difficulty to carry out venous or nasal application in the animals of the sample. We employed a single, intramuscularly applied dose of 2 IU. It was decided for the use of an immediately postoperatively single dose of calcitonin because, according to Ito et al.16, bone formation stimulation occurs when calcitonin is administered before osteogenesis start, yielding an increase in the osteoblastic cell number. With 21 days of following, the averages obtained in the area of the bony defect of the animals of the group IV were inferior to obtained them in the areas of the animals of the groups I and III, and superiors to obtained them in the areas of the animals of the group II, confirming the incentive effect to the osteogenesis of the calcitonina, but not moving away the deleterious effect of the sodic diclofenac. Several drugs have had dramatic effects on fracture repair in animal models and their effects in humans have usually not been studied, and it is possible that in our daily practice, we unknowingly prescribe drugs that affect the healing of fractures and bone defects4. Cox inhibitors have a negative effect, but it is still being debated to what extent and in which situations this is of clinical importance15. The real concept on calcitonin activity in repair of bone defects is still limited, and comparison with literature data is difficult due to different methodology, experimental model, dosage employed, follow-up and calcitonin administration moment, demanding future studies to better define its action on the bone tissue component cells5.
Conclusion
The set of results from this experimental study suggests that sodic diclofenac and calcitonin action becomes more evident only during the initial phases of osteogenesis, modulating bone formation. But the actions of these drugs, when used in an associated way, didn't show to be the best therapeutic option in the treatment of bone defects surgically created in the animal model and time period studied.
Maria Cristina Pita Sassioto
Av. Joana D'Arc, 954 - Bloco 34 - Apto. 203
79 070-170 - Campo Grande- MS - Brazil
Conflict of interest: none
Financial source: Manoel de Barros Foundation
- 1. Street JT, Wang JH, Wu QD, Wakai A, McGuinness A, Redmond HP. The angiogenic response to skeletal injury is preserved in the elderly. J Orthop Res. 2001;19(6):1057-66.
- 2. Colnot C, Thompson Z, Miclau T, Werb Z, Helms JA. Altered fracture repair in the absence of MMP9. Development. 2003;130(17):4123-33.
- 3. Saito N, Okada T, Horiuchi H, Ota H, Takahashi J, Murakami N, Nawata M, Kojima S, Nozaki K, Takaoka K. Local bone formation by injection of recombinant human bone morphogenetic protein-2 contained in polymer carriers. Bone. 2003;32(4):381-6.
- 4. Mandracchia VJ, Nelson SC, Barp EA. Current concepts of bone healing. Clin Podiatr Med Surg. 2001;18(1):55-77.
- 5. Sassioto MCP, Inouye CM, Aydos RD, Figueiredo AS, Pontes ERJC, Takita LC. Estudo do reparo ósseo com matriz óssea bovina desvitalizada e calcitonina em ratos. Acta Cir Bras [serial online]. 2004 Set-Out. Available in URL:http://www.scielo.br/acb .
- 6. Cao Y, Mori S, Mashiba T, Westmore MS, Ma L, Sato M, Akiyama T, Shi L, Komatsubara S, Miyamoto K, Norimatsu H. Raloxifene, estrogen, and alendronate affect the processes of fracture repair differently in ovariectomized rats. J Bone Miner Res. 2002;17(12):2237-46.
- 7. Sassioto MCP, Massaschi CM, Aydos RD, Silva AR, Takita LC, Figueiredo MJPSS, Bueno TAAO. Estudo morfológico do reparo de defeito ósseo preenchido com enxerto ósseo autógeno ou matriz óssea bovina, em ratos. Ensaios Ci. 2003;7:543-50.
- 8. Zhang X, Schwarz E M, Young D A, Puzas J E, Rosier R N, O'Keefe R J. Cyclooxygenase-2 regulates mesenchymal cell differentiation into the osteoblast lineage and is critically involved in bone repair. J Clin Invest. 2002;109(11):1405-15.
- 9. Azria M. Las calcitoninas. Fisiología y Farmacología. Madrid: Mayo; 1998.
- 10. Pilitsis JG, Lucas DR, Rengachary SS. Bone healing and spinal fusion. Neurosurg Focus. 2002;13(6):e1.
- 11. Aspenberg P. Drugs and fracture repair. Acta Orthop. 2005;76(6):741-8
- 12. Aaron RK, Ciombor DM, Simon BJ. Treatment of nonunions with electric and electromagnetic fields. Clin Orthop. 2004;419:21-9.
- 13. Zagaja GP, Cromie WJ. Heterotopic bone formation in association with pelvic fracture and urethral disruption. J Urol. 1999;161(6):1950-3.
- 14. Neal BC, Rodgers A, Clark T, Gray H, Reid IR, Dunn L, MacMahon SW. A systematic survey of 13 randomized trials of non-steroidal anti-inflammatory drugs for the prevention of heterotopic bone formation after major hip surgery. Acta Orthop Scand. 2000;71(2):122-8.
- 15. Simon AM, Manigrasso MB, O'Connor JP. Cyclo-oxygenase 2 function is essential for bone fracture healing. J Bone Miner Res. 2002;17(6):963-76.
- 16. Ito N, Yamazaki H, Nakazaki M, Miyahara T, Kozuka H, Sudo H. Response of osteoblastic clonal cell line (MC3T3- E1) to [Asu] eel calcitonin at a specific cell density or differentiation stage. Calcif Tissue Int. 1987; 40(4):200-5.
Publication Dates
-
Publication in this collection
07 Feb 2007 -
Date of issue
2006