Abstracts
PURPOSE: To assess the importance of sentinel lymph node biopsy in patients with cutaneous melanoma. METHODS: Ninety consecutive non-randomized patients with stages I and II melanoma who underwent sentinel lymph node biopsy were followed up prospectively for six years. RESULTS: Patients were followed up for a mean period of 30 months. Their mean age was 53.3 years, ranging from 12 to 83 years. Thirty patients were male (37.5%) and 50, female (62.5%). Sentinel lymph node was positive in 32.5% and negative in 67.5%. It was found that the thicker the tumor, the greater the incidence of positive sentinel lymph nodes. In the group of patients with positive sentinel lymph nodes, recurrence occurred in 43.5%, but in those with negative sentinel lymph nodes, in only 7%, what points out to the association of tumor recurrence and positive sentinel lymph nodes. There were no major postoperative complications. CONCLUSION: Sentinel lymph node biopsy was demonstrated to be a safe method for selecting patients who need therapeutic lymphadenectomy.
Melanoma; Lymph Nodes; Lymphatic Vessels; Diagnosis
OBJETIVO: Avaliar a importância da biópsia do linfonodo sentinela em pacientes com melanoma cutâneo MÉTODOS: Noventa pacientes com estadiamento I e II foram acompanhados prospectivamente no período de seis anos, de forma consecutiva e não randomizada e submetidos a biópsia do linfonodo sentinela. RESULTADOS: Os pacientes foram acompanhados durante tempo médio de 30 meses. A média de idade dos pacientes foi de 53,3 anos, variando de 12 a 83. Quanto ao sexo, foram avaliados 30 pacientes do sexo masculino (37,5%) e 50 do sexo feminino (62,5%).32,5% dos pacientes apresentaram linfonodo sentinela positivo e 67,5% linfonodo sentinela negativos. Comparando-se a espessura tumoral com a positividade do LS, verificou-se que quanto maior a espessura, maior a incidência de positividade do linfonodo sentinela. No grupo de pacientes com LS positivo a recorrência surgiu em 43,5% dos casos, mostrando a relação entre a recorrência e a positividade do LS. Não houve complicações no pós-operatório. CONCLUSÃO: A biópsia do linfonodo sentinela mostrou-se um método seguro para selecionar os pacientes que necessitam de linfadenectomia terapêutica.
Melanoma; Linfonodos; Vasos Linfáticos; Diagnóstico
ORIGINAL ARTICLE
SURGICAL ONCOLOGY
Sentinel lymph node biopsy in cutaneous melanoma1 1 Research performed at Unit of Skin Tumors, Plastic Surgery Division, Surgery Department, Federal University of São Paulo (UNIFESP), Brazil.
Biópsia do linfonodo sentinela no melanoma cutâneo
Andrea Fernandes de OliveiraI; Ivan Dunshee de Abranches Oliveira SantosII; Thaís Cardoso de Mello TucunduvaIII; Luciana Garbelini SanchesIII; Renato Santos Oliveira FilhoIV; Mílvia Maria Simões e Silva EnokiharaV; Lydia Masako FerreiraVI
IFellow Master degree, Post-Graduation Program in Plastic Surgery, UNIFESP, Brazil
IIPhD, Associate Professor, Head of Plastic Surgery Division, Surgery Department, UNIFESP, Brazil
IIIGraduate Students, UNIFESP, Brazil
IVAffiliate Professor, Plastic Surgery Division, Surgery Department, UNIFESP, Brazil
VPhysician of Pathology Department, UNIFESP, Brazil
VIPhD, Full Professor, Plastic Surgery, Surgery Department, UNIFESP, Brazil
Correspondence Correspondence: Andréa Fernandes de Oliveira Ivan Dunshee de Abranches Oliveira Santos Napoleão de Barros, 715/4º andar 04024-002 São Paulo SP Brazil Phone/Fax: (55 11)5576-4118 / 5571-6579 drandreafernandes@terra.com.br idaos@terra.com.br
ABSTRACT
PURPOSE: To assess the importance of sentinel lymph node biopsy in patients with cutaneous melanoma.
METHODS: Ninety consecutive non-randomized patients with stages I and II melanoma who underwent sentinel lymph node biopsy were followed up prospectively for six years.
RESULTS: Patients were followed up for a mean period of 30 months. Their mean age was 53.3 years, ranging from 12 to 83 years. Thirty patients were male (37.5%) and 50, female (62.5%). Sentinel lymph node was positive in 32.5% and negative in 67.5%. It was found that the thicker the tumor, the greater the incidence of positive sentinel lymph nodes. In the group of patients with positive sentinel lymph nodes, recurrence occurred in 43.5%, but in those with negative sentinel lymph nodes, in only 7%, what points out to the association of tumor recurrence and positive sentinel lymph nodes. There were no major postoperative complications.
CONCLUSION: Sentinel lymph node biopsy was demonstrated to be a safe method for selecting patients who need therapeutic lymphadenectomy.
Key words: Melanoma. Lymph Nodes. Lymphatic Vessels. Diagnosis.
RESUMO
OBJETIVO: Avaliar a importância da biópsia do linfonodo sentinela em pacientes com melanoma cutâneo
MÉTODOS: Noventa pacientes com estadiamento I e II foram acompanhados prospectivamente no período de seis anos, de forma consecutiva e não randomizada e submetidos a biópsia do linfonodo sentinela.
RESULTADOS: Os pacientes foram acompanhados durante tempo médio de 30 meses. A média de idade dos pacientes foi de 53,3 anos, variando de 12 a 83. Quanto ao sexo, foram avaliados 30 pacientes do sexo masculino (37,5%) e 50 do sexo feminino (62,5%).32,5% dos pacientes apresentaram linfonodo sentinela positivo e 67,5% linfonodo sentinela negativos. Comparando-se a espessura tumoral com a positividade do LS, verificou-se que quanto maior a espessura, maior a incidência de positividade do linfonodo sentinela. No grupo de pacientes com LS positivo a recorrência surgiu em 43,5% dos casos, mostrando a relação entre a recorrência e a positividade do LS. Não houve complicações no pós-operatório.
CONCLUSÃO: A biópsia do linfonodo sentinela mostrou-se um método seguro para selecionar os pacientes que necessitam de linfadenectomia terapêutica.
Descritores: Melanoma. Linfonodos. Vasos Linfáticos. Diagnóstico.
Introduction
Lymph nodes are the first site of cutaneous melanoma dissemination1. Dissemination is sequentially ordained and melanoma progresses according to a pattern in most cases2. A sentinel lymph node (SL) is the first lymph node to where the primary tumor drains3. The biopsy technique was first described by Morton, who confirmed the hypothesis, by means of clinical experience, that the sentinel lymph node histopathology mirrors what is occurring in the remaining lymph nodes of the group assessed4. Sentinel lymph node biopsy for patients with localized cutaneous melanoma, that is, with no clinical evidence of locoregional or distant metastases, is also confined for patients whose primary lesion suggests the risk of lymph node metastases, such as Breslow thicker than 1.0 mm or despite thinner than 1.0mm, displays other histopathological risk features, such as: Clark IV or V and ulceration5. Other risk factors, such as regression, high mitotic index (more than 6 mitoses/mm³), vertical growth phase and lymphatic invasion are controversial6. This procedure has been included in cutaneous melanoma staging since 2002, after the AJCC/UICC consensus7. In the same year, in Brazil, after the meeting of the Brazilian Melanoma Group Consensus, sentinel lymph node biopsy was indicated in cases with Breslow thickness equal to or greater than 0.76 mm8. Sentinel lymph node biopsy results define which patients are the proper candidates for complete lymphadenectomy, sparing from it the individuals with unaffected sentinel lymph nodes9. It encompasses three fundamental steps:
-
preoperative lymphocintigraph, performed by a nuclear medicine physician.
-
sentinel lymph node biopsy itself, utilizing vital stain lymphatic mapping and intraoperative gamma detection. This is done by a surgeon trained in the procedure.
-
histopathological examination of the sentinel lymph node, with appropriate techniques, by a pathologist.
The lymphocintigraph localizes the regional drainning lymph nodes and allows localizing the sentinel lymph node projection under the skin. It is essential for accomplishing good results with the procedure. Patent blue lymphatic mapping simulates the lymphatic pathway the tumor cell could move between the primary skin lesion and the corresponding regional lymph nodes, staining the sentinel lymph node. Intraoperative gamma detection enables easier and less aggressive dissecting of the sentinel lymph node10. Breslow thickness and the presence of ulcerations in the primary lesion are the main independent indicators of the presence of sentinel lymph node metastases1. The finding of micrometastases in the sentinel lymph node is considered the most important prognostic factor for melanoma recurrence5. Sentinel lymph node biopsy is a reproducible method for regional microstaging of melanoma in patients with clinically undetectable lymph node metastases9.
Epidemiologic studies reveal differences among melanoma case reports regarding type and lesion thickness. In Brazil, several public university hospitals report a higher incidence of thick and of acral lentiginous melanomas than what is reported from countries with predominantly Caucasian population. With the aim of assessing the method in a general university hospital, sentinel lymph node biopsies were performed as from 1999, in melanoma patients with Breslow thickness greater than 1.0 mm (and as from 2002, greater than 0.76 mm), who were prospectively followed up and the results were compared to those of the international literature.
Methods
The study group was composed of 90 patients who underwent sentinel lymph node biopsy from 1999 to 2005, as previously approved by the Ethics Committee of the Institution. The patients signed informed consent forms. Patient selection was consecutive and non-randomized. They were prospectively followed up. Biopsy of the primary lesion was initially performed for diagnostic confirmation and assessment of Breslow thickness. All patients with tumor thickness equal to or greater than 1.0 mm and with no clinical signs of lymph node metastases were included in the study.
Procedures
Preoperative lymphocintigraph was performed on the day prior to surgery, with 250 micro-Curies of the radioisotope (technetium) injected in the dermis, in the four cardinal points respective to the lesion or in the scar when a previous biopsy had been done, at a total dose of 1000 micro-Curies. Images were obtained using the SPX 4, Elscint scintillation camera. Patent blue (1 to 2 ml) was injected in the same cardinal points in the dermis on the benning of the surgery. A gamma detector was used intraoperatively, what helps to identify the lymph node and reduces the error margin. The sentinel lymph node was examined at pathology with hematoxillin-eosin stain and immunohistochemistry for HMB 45 and S-100 protein, markers of melanoma, as an attempt to identify micrometastases.
Follow-up
All data were recorded in the protocol forms as well as in the patients' hospital charts, for the entire follow-up time. All participants attended the skin tumors outpatient clinic of the Plastic Surgery Division every three months in the initial three years and every six months up to five-postoperative years.
Analysis
The following data were assessed: age, sex, information on the primary tumor (localization, histopathological type, ulceration, Breslow and Clark), and histopathological diagnosis of SL, time of follow-up, recurrence and current patient status. Breslow thickness was categorized in four groups: A (0 to 1mm), B (1.01 to 2.0mm), C (2.01 to 4.0 mm) and D (>4.0mm).
Results
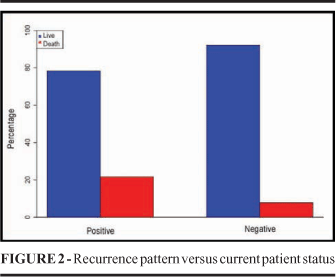
Of the 90 patients studied, ten were excluded: in two, the sentinel lymph node was not found; in two, lymphadenectomy was done due to macroscopic involvement of the SL; four were lost to follow-up and two patients had in situ melanoma, which did not meet the study criteria. The mean follow-up time was 30 months. Patients lost to follow-up and with recurrences were uniformly distributed in the study period, as seen in the Kaplan-Meier estimator (Figure 1). The patients' mean age was 53.3 years, ranging from 12 to 83 years. Thirty patients were male (37.5%) and 50, female (62.5%). Among the 80 patients studied, in 26 (32.5%) the sentinel lymph node was positive and in 54 (67.5%), negative (Table 1). In the group of patients with positive SL, tumor thickness was between 0 1.0 mm in three, between 1.01 2.0 mm in four, between 2.01 4.0 mm in seven and greater than 4.0 mm in eleven. In the group of patients with negative SL, on the other hand, Breslow 0 1 mm was found in nine; 1.01 2 mm, in 25; 2.01 3.0 mm, in 13 and greater than 4.0 mm, in seven. Of the patients with positive SL, 45.9% had Breslow greater than 4.0 mm, while of those with negative SL only 13.0% fitted this category. Tumor thickness was significantly different between the groups with positive and negative SL, what confirms such association (p=0.008) (Table 1). The mean tumor thickness was 3.11 mm, and 48.1% of them were thicker than 2.0 mm. Ulceration occurred in 29 patients (37.2%). All patients with Breslow thickness equal to or grater than 0.76 mm were included in the study, complying with the recommendations of the Brazilian Consensus on Sentinel Lymph Node, published by the Brazilian Melanoma Group, in 20038. Histological type distribution was as follows: in the group with positive SL, five were superficial spreading, ten, nodular, ten, acral lentiginous and one, malignant lentigo. And in the group with negative SL, 26 were superficial spreading, 17, nodular and four, acral lentiginous. Of the group of patients with negative SL, disease recurrence occurred in only 13.5% while in the one with positive SL, in 43.5%, what points to the association of tumor recurrence and positive SL (p=0.007) (Table 1). Of the patients with positive SL, in five (21.7%) the disease progressed to death; this was found in four (7.7%) of the ones with negative SL. There was no statistically significant association of tumor recurrence and deaths (p=0.122) (Figure 2). No local or systemic adverse reactions were observed after the injection of the radioisotope or of the vital stain. There was no dehiscence, infection or seroma at the sentinel lymph node biopsy site. By analyzing the patients of our service, it becomes evident that they present poor prognostic indicators, especially the thick Breslow measurement. However, of the 80 patients, 55 had negative sentinel lymph nodes.
Discussion
Sentinel lymph node biopsy was developed by Morton, in 1992, as a minimally invasive surgical alternative for identifying occult micrometastases in patients who could benefit from total lymphadenectomy2. Lymphatic mapping shows the anatomical pathway metastatic cell path from the primary tumor and identifies the lymph node to be meticulously examined. The sentinel lymph node is the first site of regional metastases; if the SL contains metastases, other lymph nodes of the same lymphatic drainage bed may also contain malignant cells. Sentinel lymph node biopsy was added as part of the new melanoma AJCC (American Joint Committee of Cancer) staging system, in 20027. The presence of micro metastases in the regional lymph node is the most important prognostic factor in early stage cutaneous melanoma. Elective lymphadenectomy should improve survival of such patients, as micrometastases are found in 20% of them. However, it was abandoned after sentinel lymph node biopsy was introduced, because of complications due to the procedure, like lymphedema, and to the fact that survival was not improved by it 11,12,13. The intraoperative SL localization is indicated for patients with melanomas equal to or greater than 1.0 mm thick and no clinical evidence of lymph node metastases11. Approximately 20% of patients with melanoma with Breslow thickness between 1.0 and 4.0 mm show metastases in the sentinel lymph node, increasing to 34% in those with thickness greater than 4.0 mm and dropping to 4.7% in individuals with tumor thickness below 1.0 mm associated with ulcerated lesion or Clark IV5. Morton demonstrated that tumor thickness may be used as an indicator of expected lymph node metastases and that the sentinel lymph node biopsy may accurately detect micrometastases of tumors thicker than 2.0 mm. However, in the case of thinner melanomas, the incidence may be underestimated12, 13. In the present study, 13.5% of patients with negative SL had tumor recurrence. There is a direct relation between the presence of lymph node metastases and thickness of the primary tumor12,13,14,15. Similar results were found in the present study, in which nearly half the patients with affected SL had Breslow thickness above 4.0 mm. It was also seen that most lesions in the group with positive SL were of the clinical types nodular or acral lentiginous, that is, lesions in the phase of vertical growth, and thus with greater thickness6. In patients with metastasis-free SL, the predominant lesion type observed was superficial spreading, in which the initial growth phase is radial with no rapid invasion of the deepest skin layers6. In this group, perhaps due to racial admixture, the incidence of acral melanoma found was higher than that reported in the literature. There were no intraoperative or postoperative complications, in agreement with the literature stating that SL biopsy is a low morbidity procedure, important for determining which patients will benefit from complete lymphadnectomy10,12,13. Until recently, SL biopsy was only considered as a staging gold-standard because there were no data proving it would increase patient survival. After the report by Morton et al.13, it became evident that survival of the group of patients with intermediate thickness tumors and positive SL biopsy might be increased by selective lymphadenectomy. Of the 80 patients studied, 49 had lesions of intermediate thickness, 11 of whom had positive SL. These patients were granted with the possibility of extended survival by selective lymphadenectomy.
Received: April 09, 2007
Review: June 14, 2007
Accepted: July 16, 2007
Conflict of interest: none
Financial source: none
- 1. Balch CM. Molecular diagnosis of melanoma. Melanoma Res. 2001;11(suppl 1): S9.
- 2. Reingten D, Cruse W, Wells K, Berman C, Fenske N, Glass F, Schroer K, Heller R, Ross M, Lyman G, Cox C, Rappaport D, Seigler HF, Balch C.. The ordely progression of melanoma nodal metastases. Ann Surg. 1994;220:759-67.
- 3. Chakera AH, Drzewiecki KT, Eigtued A, Juhl BR. Sentinel node biopsy for melanoma: a study of 241 patients. Melanoma Res. 2004;14:521-6.
- 4. Morton DL, Wen DR, Wong JH, Economou JS, Cagle LA, Storm FK, Foshag LJ, Cochran AJ. Technical details of intraoperative lymphatic mapping for early-stage melanoma. Arch Surg.1992;127:392-9.
- 5. Gershenwald JE, Thompson W, Mansfield PF, Lee JE, Colome MI, Tseng CH. Multi-intistucional melanoma lymphatic mapping expeience: the prognostic value of sentinel lymph node status in 612 stage I or II melanoma patients. J Clin Oncol. 1999;17:976-83.
- 6. Santos IDAO. Melanoma cutâneo. In: Forones NM. Guia de oncologia (guias de medicina ambulatorial e hospitalar. São Paulo: Manole; 2005. p.191-205.
- 7. Balch CM, Buzaid AC, Soong SJ, Atinks MB, Cascinelli M, Coit DG,Fleming ID, Gershenwald JE, Haughton Jr A , Kirkwood JM, McMasters KM, Mihm MF, Morton DL, Reingten DS, Ross MI, Sober A, Thompson JA, Thompson JF. Final version of the American Joint Committee on cancer staging system for cutaneous melanoma. J Clin Oncol. 2001;19(16):3635-48.
- 8. Neves RJ, Belfort FA, Brandão M, Silva DCP, Jorge D, Parro F. Relatório final do consenso nacional sobre linfonodo sentinela do grupo brasileiro de melanoma. Acta Oncol Bras.2003;23:499-503.
- 9. Morton DL, Thompson JF, Essner R, Elashoff R, Stern SL, Nieweg OE, Roses DF, Karakousis CP, Mozzillo N, Reingten D, Wng H, Glass EC, Cochran AJ. Multicenter selective lymphadenectomy trial group: validation of the accuracy of intraoperative lymphatic mapping and sentinel lymphadenectomy for early-stage melanoma. Ann Surg. 1999;230:453-65.
- 10. Oliveira Filho RS, Silva AM, Hochman B, Oliveira RL, Arcuschin L, Yamaga LY, Ferreira LM. Vital dye is enough for inguinal sentinel lymph node in melanoma patients. Acta Cir Bras. 2006;21(1):12-5.
- 11. Sapienza MT, Campos Neto GC, Belfort FA, Marone MMS, Tavares MGM, Lopes MMMF, Soares Junior J, Endo IS, Nakagawa S, Lewin S. Pesquisa do linfonodo sentinela em pacientes com melanoma: experiência com fitato marcado com tecnécio-99m e revisão da literatura. An Bras Dermatol. 2004;79:181-91.
- 12. Cascinelli N, Belli F, Santinami M, Fait V, Testori A, Ruka W, Cavaliere R, Mozzillo N, Rossi CR, MacKie RM, Nieweg O, Pace M, Kirov K. Sentinel lymph node biopsy in cutaneous melanoma: the WHO melanoma program experience. Ann Surg Oncol. 2000;7:469-74.
- 13. Morton DL, Thompson JF, Cochran AJ, Mozzillo N, Elashoff R, Essner R, Nieweg OE, Roses DF, Hoekstra HJ, Karakousis CP, Reintgen DS, Conventry BJ, Glass EC, Wang H. Sentinel-node biopsy or nodal observation in melanoma. N Engl J Med. 2006;355:1307-17.
- 14. Morton DL, Cochran AJ, Thompson JF, Elashoff R, Essner R, Glass EC, Mozzillo N, Niewg OE, roses DF, Hoekstra HJ, Karakousis CP, Reingten DS, Coventry BJ, Wang H. Multicenter selective lymphadenectomy trial group: sentinel node biopsy for early-stage melanoma accuracy and morbidity in MSLT-I. Ann Surg. 2005;242:302-13.
- 15. Morton DL, Hoon DSB, Cochran AJ, Turner RR, Essner R, Takeuchi H, Wanek LA, Glass E, Foshag LJ, Hsueh EC, Bilchik AJ, Elashoff D, Elashoff R. Lymphatic mapping and sentinel lymphadenectomy for early-stage melanoma: therapeutic utility and implications of nodal microanatomy and molecular staging for improving the accuracy of detection of nodal micrometastases. Ann Surg. 2003;238:538-50.
Publication Dates
-
Publication in this collection
27 Sept 2007 -
Date of issue
Oct 2007
History
-
Reviewed
14 June 2007 -
Received
09 Apr 2007 -
Accepted
16 July 2007