Abstracts
PURPOSE: To compare the hemodynamic repercussions following a toxic dose of levobupivacaine and bupivacaine intravascularly injected in swines. Methods: Large White pigs were anesthetized with thiopental, tracheal intubation was performed and mechanical ventilation was instituted. Hemodynamic variables were recorded with invasive pressure monitoring and pulmonary artery catheterization (Swan-Ganz catheter). After a 30-minute resting period, the animals were randomly divided into two groups in a double-blinded fashion and received a bolus injection of 4 mg/kg of either agent for intoxication. Hemodynamic results were then evaluated at 1, 5, 10, 15, 20 and 30 minutes. RESULTS: Levobupivacaine had greater hemodynamic repercussions than racemic bupivacaine. These results disagree with those found when the levorotatory isomer of bupivacaine was used in humans, but are in agreement with recently reported findings in animals. CONCLUSION: Levobupivacaine was shown to be more toxic in pigs than racemic bupivacaine when large doses are injected intravenously.
Bupivacaine; Anesthesia, Local; Swine
OBJETIVO: Comparar as repercussões hemodinâmicas da levobupivacaína e bupivacaína após uma dose tóxica intravascular em suínos. MÉTODOS: Porcos Large-White foram anestesiados com tiopental, entubados e mantidos em ventilação controlada mecânica. Parâmetros hemodinâmicos foram registrados através de pressão invasiva e cateterização da artéria pulmonar (cateter de Swan-Ganz). Após 30 minutos de repouso, os animais foram divididos aleatoriamente em dois grupos e receberam injeção endovenosa em duplo-cego de 4 mg/kg de um ou outro agente anestésico simulando uma intoxicação. Foram avaliados resultados hemodinâmicos aos 1, 5, 10, 15, 20 e 30 minutos. RESULTADOS: Levobupivacaína teve maiores repercussões hemodinâmicas que a bupivacaína racêmica. Estes resultados discordam daqueles encontrados com o isômero levógiro em humanos, mas estão de acordo com resultados recentemente informados em animais. CONCLUSÃO: Levobupivacaína mostrou ser agente mais tóxico em suínos do que a bupivacaína racêmica quando grandes doses são injetadas por via intravascular.
Bupivacaína; Anesthesia, Local; Suínos
ORIGINAL ARTICLE
ANESTHESIA
Hemodynamic effects of local anesthetics intoxication. Experimental study in swine with levobupivacaine and bupivacaine1 1 Research performed at Laboratory of Experimental Anesthesia, Center for Medicine and Experimental Surgery, School of Medicine, State University of Campinas (UNICAMP), São Paulo, Brazil.
Efeitos hemodinâmicos da intoxicação com anestésicos locais. Estudo experimental em suínos com levobupivacaína e bupivacaína
Artur UdelsmannI; Sílvia Elaine Rodolfo de Sá LorenaII; Samira Ubaid GirioliIII; William Adalberto SilvaIV; Ana Cristina de MoraesIV; Nelson Adami AndreolloV
IAssistant Professor, Department of Anesthesiology, Laboratory of Experimental Anesthesia, Center for Experimental Medicine and Surgery, School of Medicine, UNICAMP, Campinas, São Paulo, Brazil
IIFellow Master degree, Department of Surgery, UNICAMP, Campinas, São Paulo, Brazil
IIIFellow Master degree, Department of Pharmacology, UNICAMP, Campinas, São Paulo, Brazil
IVBiologist, Center for Medicine and Experimental Surgery, UNICAMP, Campinas, São Paulo, Brazil
VFull Professor, Head Department of Surgery, UNICAMP, Campinas, São Paulo, Brazil
Correspondence Correspondence: Artur Udelsmann Dept. Anesthesiology FCM / UNICAMP Av. Prof. Atílio Martini, 213 13083-830 Campinas-São Paulo, Brazil Phones: (19) 9105.8304 / 3521.9560 audelsmann@yahoo.com.br
ABSTRACT
PURPOSE: To compare the hemodynamic repercussions following a toxic dose of levobupivacaine and bupivacaine intravascularly injected in swines.
Methods: Large White pigs were anesthetized with thiopental, tracheal intubation was performed and mechanical ventilation was instituted. Hemodynamic variables were recorded with invasive pressure monitoring and pulmonary artery catheterization (Swan-Ganz catheter). After a 30-minute resting period, the animals were randomly divided into two groups in a double-blinded fashion and received a bolus injection of 4 mg/kg of either agent for intoxication. Hemodynamic results were then evaluated at 1, 5, 10, 15, 20 and 30 minutes.
RESULTS: Levobupivacaine had greater hemodynamic repercussions than racemic bupivacaine. These results disagree with those found when the levorotatory isomer of bupivacaine was used in humans, but are in agreement with recently reported findings in animals.
CONCLUSION: Levobupivacaine was shown to be more toxic in pigs than racemic bupivacaine when large doses are injected intravenously.
Key words: Bupivacaine. Anesthesia, Local. Swine.
RESUMO
OBJETIVO: Comparar as repercussões hemodinâmicas da levobupivacaína e bupivacaína após uma dose tóxica intravascular em suínos.
MÉTODOS: Porcos Large-White foram anestesiados com tiopental, entubados e mantidos em ventilação controlada mecânica. Parâmetros hemodinâmicos foram registrados através de pressão invasiva e cateterização da artéria pulmonar (cateter de Swan-Ganz). Após 30 minutos de repouso, os animais foram divididos aleatoriamente em dois grupos e receberam injeção endovenosa em duplo-cego de 4 mg/kg de um ou outro agente anestésico simulando uma intoxicação. Foram avaliados resultados hemodinâmicos aos 1, 5, 10, 15, 20 e 30 minutos.
RESULTADOS: Levobupivacaína teve maiores repercussões hemodinâmicas que a bupivacaína racêmica. Estes resultados discordam daqueles encontrados com o isômero levógiro em humanos, mas estão de acordo com resultados recentemente informados em animais.
CONCLUSÃO: Levobupivacaína mostrou ser agente mais tóxico em suínos do que a bupivacaína racêmica quando grandes doses são injetadas por via intravascular.
Descritores: Bupivacaína. Anesthesia, Local. Suínos.
Introduction
High doses of local anesthetic agents are sometimes required for surgery following the administration of local and regional anesthesia and there is always the potential risk for toxic reactions. Both the cardiovascular and central nervous systems are the primary target organs of local anesthetic toxicity in case of inadvertent intravascular injection. Bupivacaine is currently one of the most widely used local anesthetics, due to its quality of anesthesia and prolonged duration of action.1,2 Nevertheless, an Anesthesiology editorial on the severe cardiovascular effects of bupivacaine intoxication was published in 1979,3 and since then research has focused on discovering new long-acting local anesthetics with lower toxicity. Although bupivacaine is synthesized in the form of its two dextrorotatory R(+) and levorotatory L(-) isomers4, until recently it had only been marketed as a racemic mixture, containing 50% of each of the two enantiomers. However, evidence that the levorotatory isomer caused less toxicity dates back to 1972.5,6 Levobupivacaine, the levorotatory isomer, has recently begun to be more widely studied. In some animal models, the lethal dose of levobupivacaine was shown to be up to 1.6 times higher than that of the racemic mixture7! In humans, levobupivacaine would be less potent at producing negative inotropic effects and prolonging the PR and QT intervals of the ECG, typical of racemic mixture intoxication8. However, the drawback with this new agent would be its less intense motor block10, despite having equal analgesic potency to racemic bupivacaine.9The aim of this study was to simulate acute intoxication with either agent in pigs, as might accidentally occur during local and regional anesthesia with high doses of local anesthetics and to evaluate its hemodynamic repercussions.
Methods
After approval from the Ethics Committee for Animal Experiments, forty healthy Large White pigs (both sexes, body weight 20-27 kg) underwent the following protocol:
1. The pigs were fasted on the night before the procedure and had free access to water.
2. In the morning of the procedure, they were weighed, an auricular vein was cannulated and anesthesia was induced with a 25 mg.kg-1 IV dose of sodium thiopental 2.5% solution 11.
3. The body surface area of the animal (BSA) was calculated in square meters using the classic formula from the literature12: BSA= (9 x weight in grams 2/3) x 10-4, introducing the value into the Engstrom AS/3 multiparametric monitor for calculation of body index values.
4. A tracheal tube was inserted and connected to a pneumatically driven ventilator using a partial re-breathing system and CO2 absorbent. Tidal volume was 15ml.kg-1 and respiratory rate was adjusted to maintain ETCO2 at 32 34 mmHg. Fresh oxygen flow was 1 l/min and hemoglobin oxygen saturation was measured, placing the sensor on the animal tongue, aimed at achieving a value above 97%. ECG was also monitored.
5. Anesthesia was maintained with an intravenous infusion of sodium thiopental 2.5% (5mg.kg-1.h-1).
6. Subsequently, an incision was made along the inner aspect of the thigh under local anesthesia (5 ml 1% lidocaine hydrochloride without vasoconstrictor) for catheterization of the femoral artery and continuous measurement of arterial blood pressure. Through the same incision, the femoral vein was dissected and a 7F Swan-Ganz catheter was inserted. The catheter was advanced into a branch of a pulmonary artery and proper catheter placement was confirmed by the pressure waveform obtained. Cardiac output (CO) was then measured by the thermodilution method. In addition, mean arterial pressure (MAP), mean pulmonary artery pressure (mPAP), central venous pressure (CVP) and pulmonary capillary wedge pressure (PCWP) were measured. Using classic formulas in the literature, the monitor also performed calculations of the remaining hemodynamic parameters, e.g.: cardiac index (CI), stroke index (SI), systemic vascular resistance index (SVRI), pulmonary vascular resistance pulmonary index (PVRI), left ventricular stroke work index (LVSWI), right ventricular stroke work index (RVSWI). At this early phase, blood samples were drawn from the animals for dosage of hematocrit and hemoglobin levels.
7. After a resting period of about 30 minutes to ensure stabilization, baseline hemodynamic measurements (T0) were recorded.
8. Then the animals were randomly divided into 2 groups in a double-blinded fashion: bupivacaine group (B) and levobupivacaine group (L). Each group was intravenously injected with a toxic dose of 4 mg.kg-1 of either local anesthetic13 during 30 seconds and the experimenter was blinded to the study code.
11. Additional hemodynamic measurements were recorded at 1, 5, 10, 15, 20 and 30 minutes after intoxication (at T1 to T6, respectively).
12. At the completion of the experiment, each animal was sacrificed while under anesthesia by a 10 ml intravenous injection of 19.1% potassium chloride solution.
Subsequently, the double-blinded protocol was disclosed and data was statistically treated. Categorical variables received statistical treatment using the chi-square test. Variables without normal distribution were log-transformed for the tests in order to reduce skewness and variability. To compare the distribution of single numerical variables (measured at a single time point), the Student's t-test was used. To study the behavior of the hemodynamic parameters measured at several time points, analysis of variance (ANOVA) for repeated measures was performed, followed by Duncan's post-hoc test for multiple comparisons to compare the groups at each time point (between groups comparisons), and by the profile test by contrasts to analyze the changes in the parameters of each group (within groups comparisons).The significance level was set at 5%, i.e., p < 0.05.
Results
The following table demonstrates the distribution by sex, mean and standard deviations of weight, hematocrit levels, hemoglobin levels and body surface areas found in both groups.
The groups were homogeneous regarding distribution by sex (p=0.736), weight (p=1), hematocrit level (p=0.334), hemoglobin level (p=0.099) and body surface area (p=0.95). There was no difference in any of the hemodynamic parameters measured at rest between the groups.
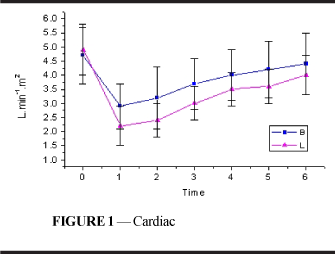
Following intoxication, there was a reduction in cardiac index (Figure 1 and Table 1) in both groups and these values did not return to those similar to resting values until the end of the experiment (p<0.001). The decrease in L was statistically more important than in B and was maintained until T4 (p<0.001).
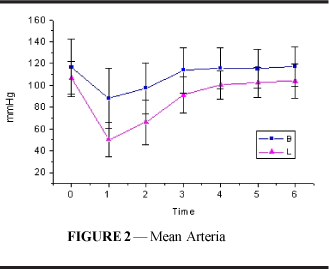
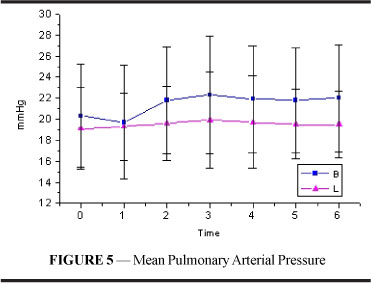
Mean arterial pressure (Figure 2 and Table 2) decreased significantly in both groups; values returned to those similar to resting values at T3 in group B and at T4 (p<0.001) in group L. Starting from T1, values in L were significantly lower than those in B until T4 (p<0.001).
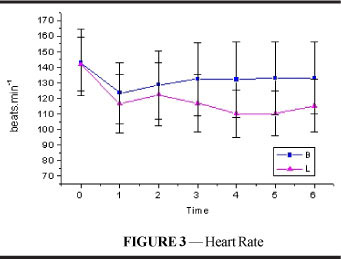
There was a significant decrease in heart rate (Figure 3 and Table 3) following intoxication in both groups, although the reduction was more considerable in E and this difference was maintained until the end of the experiment (p<0.001). In both groups, HR did not return to values comparable to resting values until T6 (p=0.003).
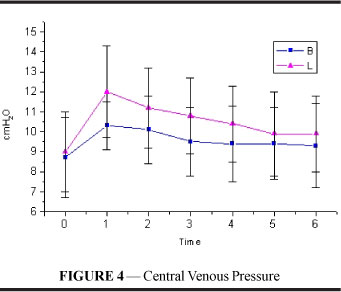
Central venous pressure (Figure 4 and Table 4) increased significantly in both groups and the increase was maintained until the end of the experiment (p<0.001) with no difference between the groups.
Mean pulmonary artery pressure (figure and table 5) showed no significant alterations or differences between both groups.
Pulmonary capillary wedge pressure (figure and table 6) showed increased values in both groups following intoxication and these values were maintained until the end of the experiment (p<0.001). No difference between the groups was found.
Stroke index (figure and table 7) demonstrated a significant decline. In both groups, these values remained lower than resting values until T4 (p<0.001). The fall in L, however, was significantly more important at T1 and T2 (p=0.019).
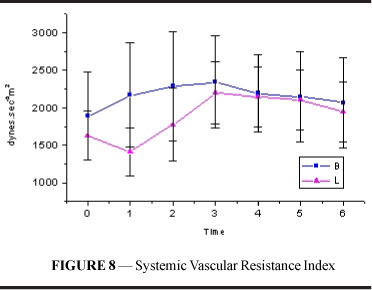
Systemic vascular resistance index (figure and table 8) in group B increased, maintaining values higher than resting values until T6. In L, SVRI initially showed a decrease followed by a significant increase beginning at T2 and maintained values higher than resting values until T6 (p<0.001). B values were higher than L values at T1 and T2 (p=0.004).
Pulmonary vascular resistance index (figure and table 9) showed values higher than resting values from T2 to T5 in both groups (p=0.001). No difference was found between B and L.
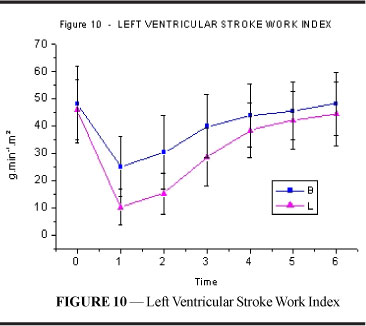
Left ventricular stroke work index (figure and table 10) showed a decrease after intoxication in both groups. However, the fall was more significant in L, and this difference was maintained until T3 (p=0.001). Values returned to those similar to resting values at T4 in group B and T5 (p<0.001) in group L.
Right ventricular stroke work index (figure and table 11) also showed a decrease after intoxication in both groups. The fall was more substantial in L and a significant difference between the groups was maintained from T1 to T4 and at T6 (p=0.001). Values returned to those similar to resting values at T3 in B, and only at T5 (p<0.001) in L.
Discussion
The cardiac toxicity of local anesthetics is attributed to a blockade of sodium channels in the heart, leading to a prolonged conduction time with widening of QRS complexes, prolongation of PR interval, AV block and arrythmias14. Furthermore, these drugs have the ability to cause cardiac toxicity by altering mitochondrial metabolism in cardiac cells and thus alter inotropism15. The S(-) isomer may be less cardiotoxic due to its lower affinity for cardiac sodium channels, in comparison to the R(+)isomer, as demonstrated in guinea pigs16. Such data, however, should be viewed with some reservation before it can be extrapolated to humans. The overall pharmacodynamic events seen with levobupivacaine are similar to those seen with bupivacaine but we have found quantitative differences17. Other authors have also compared levobupivacaine, racemic bupivacaine and ropivacaine in animals, demonstrating that levobupivacaine had a similar18 or even greater toxicity.19,20 In agreement with those authors, our results showed that levobupivacaine had greater hemodynamic repercussions on a swine model of acute intoxication, simulating what may occur after accidental intravenous injections of local anesthetics during local and regional anesthesia. Evidence of toxicity was shown in the significantly more important decrease in cardiac index, mean arterial pressure, heart rate and left ventricular stroke work index. In a more homogeneous model, these results confirm those previously obtained with a preparation containing 75% of the levorotatory isomer in dogs21. Results obtained in animals should be viewed cautiously and prudence is of the essence when extrapolating these data to humans. However, further comprehensive studies must be encouraged, since results of this nature are not the first to be observed. Accidental injections of high doses of local anesthetics and toxic reactions during local and regional anesthesia have decreased in the last 30 years, falling from 0.2 to 0.01%. Peripheral nerve blocks still account for the majority of these cases (7.5 per 10.000)22. Such considerations should intensify new efforts to discover drugs and techniques that enable us to achieve a significantly low morbidity and mortality rate, protecting patients from the undesirable and unpredictable effects of local and regional anesthesia techniques with high doses of local anesthetics.
Conclusion
Levobupivacaine was more cardiotoxic than racemic bupivacaine when large doses were injected intravenously in swine, as may accidentally occur during local and regional anesthesia.
Received: August 29, 2007
Review: October 30, 2007
Accepted: November, 26, 2007
Conflict of interest: none
Financial source: FAEPEX, UNICAMP
- 1. Groban L, Deal DD, Vernon JC, James RL, Butterworth John - Cardiac resuscitation after incremental overdosage with lidocaine, bupivacaine, levobupivacaine, and ropivacaine in anesthetized dogs. Anesth Analg. 2001;92:37-43.
- 2. Chang DH-T, Ladd LA, Copeland S, Iglesias MA, Plummer JL, Mather LE -Direct cardiac effects of intracoronary bupivacaine, levobupivaciane and ropivacaine in the sheep. Br J Pharmacol. 2001;132:649-58.
- 3. Albright GA - Cardiac arrest following regional anesthesia with etidocaine or bupivacaine. Anesthesiology. 1979;51:285-7.
- 4. Ohmura S, Kawada M, Ohta T, Yamamoto K, Kobayashi T - Systemic toxicity and resusciation in bupivacaine, levobupivacaine, or ropivacaine-infused rats. Anesth Analg. 2001;93:743-8.
- 5. Åberg G - Toxicological and local anesthetic effects of optically active isomers of two local anesthetic compounds. Acta Pharmacol Toxicol. 1972;31:273-86.
- 6. Luduena FP, Bogado EF, Tullar BF - Optical isomers of mepivacaine and bupivacaine. Arch Int Pharmacodyn. 1972;200:359-69.
- 7. Foster RH, Markham A - Levobupivacaine. A review of its pharmacology and use as a local anesthetic. Drugs. 2000;59:551-79.
- 8. Bardsley H, Gristwood R, Baker H, Watson N, Nimmo W - A comparison of the cardiovascular effects of levobupivacaine and rac-bupivacaine following intravenous administration to healthy volunteers. Br Clin Pharmacol. 1998;46:245-9.
- 9. Lyons G, Columb M, Wilson RC, Johnson RV - Epidural pain relief in labour:potencies of levobupivacaine and racemic bupivacaine. Br J Anaesth. 1998;81:899-901.
- 10. Héctor JL, Columb MO - The relative motor blocking potencies of bupivacaine and levobupivacaine in labour. Anesth Analg. 2003;97:1509-13.
- 11. Smith AC, Ehler WJ, Swindle MMl - Anesthesia and analgesia in swine. In: Kohn DF, Wixson SK, White WJ, Benson JG - Anesthesia and Analgesia in Laboratory Animals, 1st ed., New York, Academic Press,1997;313-36.
- 12. Ettinger SJ: Textbook of Veterinary Internal Medicine, vol. 1, 1st ed., Philadelphia, WB Saunders Co., 1975:146.
- 13. Lefrant JY, Muller L, de La Coussaye JE, Lalourcey L, Ripart J, Peray PA, Mazoit X, Dauzat M, Sassine A, Eledjam JJ - Hemodynamic and cardiac electrophysiologic effects of lidocaine-bupivacaine mixture in anesthetized and ventilated piglets. Anesthesiology. 2003;98:96-103.
- 14. Clarkson CW, Hondeghem LM - Mechanism for bupivacaine depression of cardiac conduction: fast block of sodium channels during the action potential with slow recovery from block during diastole. Anesthesiology. 1985;62:396-405.
- 15. Butherworth JF, Brownlown RC, Leith JP, Prielipp RC, Cole LR - Bupivacaine inhibits cyclic 3,5 adenosine monophosphate production. A possible contribution factor to cardiovascular toxicity. Anesthesiology. 1993;79:88-95.
- 16. Valenzuela C, Snyders DJ, Bennett PB, Tamargo J, Hondeghem LM - Stereoselective block of cardiac sodium channels by bupivacaine in guinea pig ventricular myocytes. Circulation. 1995;92:3014-24.
- 17. Huang YF, Prior ME, Mather LE, Veering B Cardiovascular and central nervous system effects of intravenous levobupivacaine and bupivacaine in sheep. Anesth Analg. 1998;86:797-804.
- 18. Royse CF, Royse AG The myocardial and vascular effects of bupivacaine, levobupivacaine, and ropivacaine using pressure volume loops. Anesth Analg. 2005;101:679-87.
- 19. Masuda R, Takeda S, Yoshii S, Nagano H, Tomaru T Levobupivacaine exerts the most detrimental effect on the cardiovascular system among enantiomers of bupivacaine in anesthetized dogs. Anesthesiology. 2004;101:A652.
- 20. Jung CW, Lee KH, Choe YS, Bae SS Comparison of resuscitative effect of insulin between bupivacaine and levobupivacaine induced cardiovascular collapse in dogs. Anesthesiology. 2004;101:A649.
- 21. Udelsmann A, Munhoz DC, Silva WA, Moraes AC, Marcondes G Comparação entre os efeitos hemodinâmicos da intoxicação aguda com bupivacaína racêmica e a mistura com excesso enatiomérico de 50% (S75-R25). Estudo experimental em cães. Rev Bras Anestesiol. 2006;56:391-401.
- 22. Cox B, Durieux ME, Marcus MA Toxicity of local anesthetics. Best Pract Res Clin Anaesthesiol. 2003;17:111-36.
Publication Dates
-
Publication in this collection
27 Mar 2008 -
Date of issue
Feb 2008
History
-
Accepted
26 Nov 2007 -
Reviewed
30 Oct 2007 -
Received
29 Aug 2007