Abstracts
PURPOSE: The objective of this study was to develop a rat lung tumor model for anticancer drug testing. METHODS: Sixty-two female Wistar rats weighing 208 ± 20 g were anesthetized intraperitoneally with 2.5% tribromoethanol (1 ml/100 g live weight), tracheotomized and intubated with an ultrafine catheter for inoculation with Walker's tumor cells. In the first step of the experiment, a technique was established for intrabronchial implantation of 10(5) to 5×10(5) tumor cells, and the tumor take rate was determined. The second stage consisted of determining tumor volume, correlating findings from high-resolution computed tomography (HRCT) with findings from necropsia and determining time of survival. RESULTS: The tumor take rate was 94.7% for implants with 4×10(5) tumor cells, HRCT and necropsia findings matched closely (r=0.953; p<0.0001), the median time of survival was 11 days, and surgical mortality was 4.8%. CONCLUSION: The present rat lung tumor model was shown to be feasible: the take rate was high, surgical mortality was negligible and the procedure was simple to perform and easily reproduced. HRCT was found to be a highly accurate tool for tumor diagnosis, localization and measurement and may be recommended for monitoring tumor growth in this model.
Lung neoplasms; Walker-256 carcinoma; rats
OBJETIVO: O objetivo foi desenvolver um modelo de tumor de pulmão em rato que permita o teste de fármacos no tratamento deste câncer. MÉTODOS: Sessenta e dois ratos Wistar fêmeas, peso médio de 208±20 g, foram anestesiados com tribromo-etanol 2,5% IP (1ml/100g de rato), traqueostomizados e intubados com cateter ultrafino para injetar células do tumor de Walker. Na 1ª etapa, estabeleceu-se a técnica do implante de células tumorais por via intrabrônquica e o índice de pega tumoral, usando-se de 10(5) a 5×10(5) células. Na 2ª, avaliou-se o volume tumoral e a correlação dos achados obtidos na tomografia computadorizada de alta resolução (TCAR) de tórax com os da necropsia e verificou-se a sobrevida. RESULTADOS: O índice de pega foi de 94,7, com o implante de 4×10(5) células do tumor; as medidas do tumor feitas na TCAR e comparadas com as da necropsia foram semelhantes (r=0, 953, p<0,0001); a sobrevida mediana foi de 11 dias; e a mortalidade cirúrgica de 4,8 %. CONCLUSÃO: O modelo mostrou-se viável, com alto índice de pega, mortalidade cirúrgica desprezível, de execução simples e fácil reprodutibilidade. A TCAR revelou alta acurácia no diagnóstico, localização e mensuração das lesões tumorais, credenciando-se para a monitorização de crescimento tumoral nesse modelo.
Neoplasias Pulmonares; Carcinoma 256 de Walker; Ratos
ORIGINAL ARTICLE
MODELS, BIOLOGICAL
Experimental rat lung tumor model with intrabronchial tumor cell implantation1 1 Study performed at Department of Physiology and Pharmacology and Department of Surgery of Faculty of Medicine, Federal University of Ceará (UFC), Brazil.
Modelo experimental de tumor de pulmão em rato por via intrabrônquica
Antero Gomes NetoI; Antônio Felipe Leite SimãoII; Samuel de Paula MirandaII; Lívia Talita Cajaseiras MourãoIII; Nilfácio Prado BezerraII; Paulo Roberto Carvalho de AlmeidaIV; Ronaldo de Albuquerque RibeiroV
IMD, PhD, Federal University of Ceará, Brazil. Thoracic Surgeon of Messejana Hospital, Ceará, Brazil
IIAcademic Students of Medicine, Federal University of Ceará, Brazil
IIIAcademic Student of Pharmacy, Federal University of Ceará, Brazil
IVMD, PhD, Professor of Pathology, Department of Pathology, Federal University of Ceará, Brazil
VMD, PhD, Professor of Pharmacology and Oncology, Department of Physiology and Pharmacology and Department of Surgery, Federal University of Ceará, Clinical Oncologist of Cancer Institute of Ceará (ICC), Brazil
Correspondence Correspondence: Antero Gomes Neto Hospital de Messejana Frei Cirilo Avenue, 3480 60864-190 Fortaleza - Ceará, Brazil e-mail: anterogn@terra.com.br
ABSTRACT
PURPOSE: The objective of this study was to develop a rat lung tumor model for anticancer drug testing.
METHODS: Sixty-two female Wistar rats weighing 208 ± 20 g were anesthetized intraperitoneally with 2.5% tribromoethanol (1 ml/100 g live weight), tracheotomized and intubated with an ultrafine catheter for inoculation with Walker's tumor cells. In the first step of the experiment, a technique was established for intrabronchial implantation of 105 to 5×105 tumor cells, and the tumor take rate was determined. The second stage consisted of determining tumor volume, correlating findings from high-resolution computed tomography (HRCT) with findings from necropsia and determining time of survival.
RESULTS: The tumor take rate was 94.7% for implants with 4×105 tumor cells, HRCT and necropsia findings matched closely (r=0.953; p<0.0001), the median time of survival was 11 days, and surgical mortality was 4.8%.
CONCLUSION: The present rat lung tumor model was shown to be feasible: the take rate was high, surgical mortality was negligible and the procedure was simple to perform and easily reproduced. HRCT was found to be a highly accurate tool for tumor diagnosis, localization and measurement and may be recommended for monitoring tumor growth in this model.
Key words: Lung neoplasms; Walker-256 carcinoma; rats.
RESUMO
OBJETIVO: O objetivo foi desenvolver um modelo de tumor de pulmão em rato que permita o teste de fármacos no tratamento deste câncer.
MÉTODOS: Sessenta e dois ratos Wistar fêmeas, peso médio de 208±20 g, foram anestesiados com tribromo-etanol 2,5% IP (1ml/100g de rato), traqueostomizados e intubados com cateter ultrafino para injetar células do tumor de Walker. Na 1ª etapa, estabeleceu-se a técnica do implante de células tumorais por via intrabrônquica e o índice de pega tumoral, usando-se de 105 a 5×105 células. Na 2ª, avaliou-se o volume tumoral e a correlação dos achados obtidos na tomografia computadorizada de alta resolução (TCAR) de tórax com os da necropsia e verificou-se a sobrevida.
RESULTADOS: O índice de pega foi de 94,7, com o implante de 4×105 células do tumor; as medidas do tumor feitas na TCAR e comparadas com as da necropsia foram semelhantes (r=0, 953, p<0,0001); a sobrevida mediana foi de 11 dias; e a mortalidade cirúrgica de 4,8 %.
CONCLUSÃO: O modelo mostrou-se viável, com alto índice de pega, mortalidade cirúrgica desprezível, de execução simples e fácil reprodutibilidade. A TCAR revelou alta acurácia no diagnóstico, localização e mensuração das lesões tumorais, credenciando-se para a monitorização de crescimento tumoral nesse modelo.
Descritores: Neoplasias Pulmonares; Carcinoma 256 de Walker; Ratos.
Introduction
Lung cancer has been the main cause of death from cancer worldwide over the past decade. Each year over 170,000 new cases are diagnosed in the U.S. alone leading to 160,000 deaths and representing approximately 28% of deaths from cancer1. In Brazil lung cancer was likewise the most frequent cause of death among men in the year 2000, although prostate cancer is expected to surpass the incidence of lung cancer in 20062. Screening with low-dose helicoidal computed tomography, biological markers and other methods for early diagnosis of lung cancer currently represent an important field of study, especially since chances of cure are considerably greater when patients are submitted to surgery while the disease is still localized3. Apart from the newly introduced chemotherapy drugs, other treatment forms for patients with lung cancer are now becoming available, including immunomodulating, antiangiogenic and targeted drugs.4,5 However, experimental models are necessary to study the biological behavior of lung tumors and the effects of novel anticancer drugs. Preclinical tests evaluating new drugs in vivo are usually performed with immunodeficient mice receiving an ectopic, subcutaneous graft of human cancer cells4. Lung cancer developing in murine models is genetically similar to human tumors, although tumor growth and response to treatment interventions depend on whether the tumor is implanted ectopically under the skin or orthotopically in the lung5. Therefore many authors have given attention to the development and validation of orthotopic tumor models using human tumor cells6,7. In such models, instead of inoculating tumor cells under the skin of rats and mice, tumors are implanted in the organ corresponding to the organ of origin. Moreover, orthotopic lung tumor models using human cancer cells require the use of cell lineages from primary lung tumors as well as immunosuppressed animals, since this type of tumor will not take or grow in immunocompetent animals8. In lung tumor models cells may be inoculated intrabronchially in the pulmonary parenchyma7,8 or may be implanted directly by puncture during open thoracotomy9,10.The orthotopic lung tumor model using intrabronchial inoculation of tumor cells was originally developed by McLemore et al.11 and involved inserting a catheter by tracheal puncture. Later Howard et al.8 and Johnston et al.7 improved the technique by using cervical tracheotomy for the insertion of an ultrafine intrabronchial catheter, thus making it possible to implant cells on the periphery of the pulmonary parenchyma. None of the studies above used computed tomography (CT) to detect the presence of lung tumors in the animals, although a few other and more recent experimental studies have reported using CT scans with small animals10,12,13. The objectives of this study were a) to develop a technically simple rat lung tumor model with intrabronchial implantation of cells of Walker's carcinosarcoma by cervical tracheotomy, and b) to diagnose tumors in vivo using high-resolution computed tomography (HRCT) with subsequent correlation of findings from necropsy and histopathological examination.
Methods
Experimental animals
Experiments were carried out with 62 female Wistar rats weighing 208 ± 20 g reared at the laboratory of the Federal University of Ceará (UFC). During the study the animals were kept in cages at the physiology and pharmacology laboratory in groups of up to six individuals. The temperature was maintained at 24° C and the animals were exposed to a 24-hour circadian rhythm with free access to water and food. The study was previously approved by the UFC Ethics Committee for Animal Research (CEPA protocol #33/06), and all experiments were performed in accordance with sound ethical principles.
Origin and preparation of suspension of neoplastic cells
The experiment used cells from the Walker-256 carcinosarcoma14. The neoplasm is cultivated at the laboratory by weekly intramuscular injections with a suspension of 106 tumor cells in the inner thigh of Wistar rats. The cells were prepared for the present study as described in the literature10. Tumor cell viability was assessed with trypan blue staining and the number of cells per 1 ml of suspension was determined using a Neubauer chamber. The suspension was subsequently kept at 4°C during the entire experiment.
Technique of intrabronchial tumor cell implantation
The animals were anesthetized intraperitoneally with 2.5% tribromoethanol at a concentration of 1 ml/100 g live weight, then placed in dorsal decubitus and submitted to hair clipping and antisepsis with Povidine® (polyvinylpyrrolidone) in the cervical area. Cervical tracheotomy was performed as described by Howard et al. (1991) 8, beginning with a skin incision just above the manubrium sterni and dissecting the muscle layers until uncovering the trachea (Figure 1). A small incision was made with a size 11 blade into the trachea by the 2nd and 3rd ring to allow for the introduction of a size 16G polyethylene catheter. By tilting it to the right, the catheter was led through the trachea into the left bronchus. A size 22G ultrafine polyethylene catheter (Figure 2) was then guided through the first catheter and advanced until detecting resistance from the lung periphery. At this location 70100 µl of tumor cell suspension was inoculated. The catheters were subsequently removed and the trachea and skin were closed with a single stitch of Prolene® 7-0 thread and Mononylon® 4-0 thread, respectively (Figure 3). The procedure lasted 56 minutes, after which the animal was placed in left lateral decubitus until emergence from anesthesia, in order to confine the inoculum to the site of implantation, as recommended by Wang et al. (1997) 9.
High-resolution computed tomography (HRCT)
The animals were anesthetized intraperitoneally with 10% chloral hydrate at a dosage of 0.1 ml/30 g live weight in order to maintain hypnosis during HRCT. During the scan the animals were kept in ventral decubitus with the aid of a cloth, avoiding the use of adhesive tape. Following scanning with HRCT the animals were placed in cotton-wadded boxes to keep them warm until emergence from anesthesia and transference to cages. Scanning was performed with a Siemens tomograph (SOMATON AR.TX: 130 KV, 50 mA, average FOV 5 cm, high-resolution filter for 2-mm sections, scanning time 3 seconds per section [150 mAS]). On the average, six 2-mm sections separated by 2-mm intervals were made of the lower half of the chest where the tumor was located. Images were captured in wide window mode for the lung study and in narrow window mode for the study of the mediastinum. Tumors were measured in two dimensions (axial and perpendicular) in wide window mode (Figure 4).
Experimental design
The experimental model was developed in two steps with the animals assigned to experimental groups at random. Animals that died during follow-up of non-tumor-related causes or presented no tumors upon necropsia and histopathological examination were excluded from the analysis.
Step 1 (n=32): Establishment of intrabronchial tumor cell implantation technique and subsequent take rate.
The animals were randomly assigned to four groups and, using the technique described above, inoculated intrabronchially with different concentrations of Walker's tumor cells in order to establish the number of cells required for tumor take: Group 1 (n=8), 105 cells; Group 2 (n=8), 2 x 105 cells; Group 3 (n=10), 4 x 105 cells; and Group 4 (n=6), 5 x 105 cells. On the sixth day the animals were euthanized with chloral hydrate and submitted to necropsis through median sternotomy and laparotomy for the joint excision of trachea, lungs and heart in order to verify the presence of tumors in the chest and abdomen (liver and adrenal gland tumors). Lung sections were fixed in buffered isotonic formaldehyde (100 mL of 37% formaldehyde solution, 900 mL distilled water, 4 g monobasic sodium phosphate and 6.5 g dibasic sodium phosphate). Twenty-four hours later samples were immersed in 70% alcohol, stained with hematoxylin-eosin and examined histopathologically by a blinded pathologist.
Step 2 (n=30): Assessment of tumor volume, correlation of HRCT findings with findings from necropsia, and determination of time of survival.
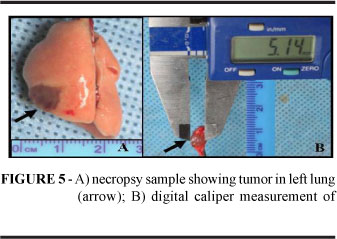
The animals were randomly assigned to one of two protocols, A and B, both of which with intrabronchial inoculation of 4 x 105 tumor cells. Although the highest take rate in Step 1 was observed after inoculation with 5 x 105 tumor cells, a smaller number of cells was used in Step 2 in order to make tumor cells available for a greater number of experiments. The animals assigned to Protocol A (n=16) were HRCT scanned on the 5th day of implantation and subsequently euthanized with chloral hydrate and submitted to necropsis. HRCT sectioning was set to 2 mm and tumors were measured in two dimensions, as described above. At necropsy tumors were measured manually, registering the two largest diameters with a digital caliper (Figure 5). Tumor volumes were calculated in cm3 using Steel's formula: (Dxd2)/2, where D is the largest diameter and d the smallest. The animals assigned to Protocol B (n=14; survival group) were weighed daily until spontaneous death and then submitted to necropsy as described above.
Statistical analysis
Tumor volumes obtained from HRCT and necropsy and expressed as average values ± standard error were compared using simple linear regression. Survival rates were determined with the Kaplan-Meier test. The statistical analysis was carried out with the SPSS software and the level of statistical significance was set at 5%.
Results
Step 1 (n=32): Establishment of intrabronchial tumor cell implantation technique and subsequent take rate.
The procedure posed no technical difficulty and lasted 5-6 minutes per animal on the average, making it possible to inoculate 12 animals in one hour. Two surgical deaths were registered during Step 1, corresponding to a mortality rate of 6.2% (2/32). On the sixth day, when the animals were euthanized and submitted to necropsy, macroscopic nodules were observed in the lower left lung (Figure 6) at a development rate proportional to the number of cells inoculated (Table1). The histopathological tests confirmed macroscopic findings in all cases. Most tumors formed a dense mass around the bronchus or bronchiole and were characterized by the presence of polygonal cells, visible nucleoli, loose chromatin and widespread atypical mytosis (Figures 7 and 8).
Step 2, Protocol A (n=16): Assessment of tumor volume and correlation of HRCT findings with findings from necropsy.
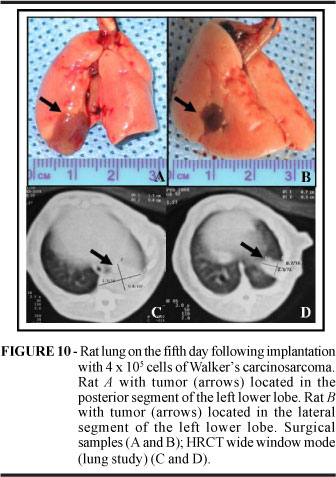
On the fifth day following implantation animals inoculated with 4 x 105 tumor cells and submitted to HRCT and necropsy presented a tumor take rate of 100%. One surgical death was registered. The correlation between findings of tumor volume (cm3) obtained with HRCT and necropsy was positive (r=0.953; p<0.0001) (Figures 9 and 10). The necropsy confirmed HRCT findings with regard to tumor volume and location (lateral or posterior segment of the left lower lobe). The microscopic examination yielded findings similar to those obtained in Step 1.
Step 2, Protocol B (n=14): Time of survival for rats inoculated with 4 x 105 cells of Walker's carcinosarcoma.
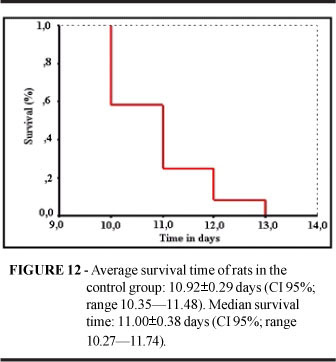
An animal which died of an unknown cause on the third day following tumor cell implantation was excluded from the analysis. Of the remaining 13 animals submitted to necropsy after spontaneous death in consequence of the neoplasm, only one presented no tumor. Tumors confirmed by necropsy and histopathological examination displayed the same level of differentiation as tumors examined during Step 1. In almost all cases, the tumor occupied most of the lung with large areas of necrosis and hemorrhagic foci (Figure 11). Eight animals (66%) presented loco-regional tumor dissemination either towards the mediastinum (n=6; 49.5 %) or the pleura (n=2; 16.5%). No distant metastases were detected in the lung, liver or adrenal glands, as opposed to what is commonly observed with lung cancer in humans. All animals died within two weeks; the median survival time was 11.00±0.38 days (Figure 12).
The overall surgical mortality was 4.8 % (3/62) with a variation of 0% (Step 2, Protocol B) to 6.2% (in both Step 1 and Step 2, Protocol A) as shown in Table 2, indicating that the intrabronchial lung tumor implantation model tested in this study is simple to reproduce.
The histopathological examination confirmed the presence in the left lower lobe of a nodule or large tumor mass occupying most of the lung in 36 of the 38 animals submitted to intrabronchial implantation with 4 x 105 cells of Walker's carcinosarcoma, thus yielding an overall tumor take rate of 96% (Table 3).
Discussion
The lung tumor model described in the present study was shown to be simple to use and easy to reproduce. The procedure demanded 56 minutes for each animal, making it possible to inoculate 2024 animals in 2 hours. Tracheotomy and intrabronchial cell implantation caused minimal trauma and mortality was negligible (4.8%) compared to the mortality rates reported in the literature (510%) for similar models8,9,11. Our model was developed with cells from Walker's carcinosarcoma, which may be described as an adenocarcinoma of the rat mammary gland, although tumor cells from the lungs of rodents and humans may also be used. However, experiments with human tumor cells require the use of athymic mice or immunosuppressed rats. With experimental animals of this type it is possible to develop orthotopic models in which tumor cells are implanted in the organ corresponding to the organ of origin7,8. The fact that orthotopic models may be developed with any lineage of human tumor cells regardless of phenotype makes it easier to measure response to anticancer therapy. The authors are presently developing an orthotopic rat lung tumor model based on the model described in this study. During Step 1, tumors turned into nodules in 90% of the animals, as documented by HRCT and necropsy performed on the 6th day following intrabronchial implantation with 4 x 105 cells. The overall tumor take rate with this number of cells was 94.7 %. This is similar to the tumor take rate (94.5%) observed for an earlier model developed in our laboratory10 and inoculated via thoracotomy with 2 x 105 cells of the same lineage. In a study by Wang et al. 9, tumor cells implanted by direct puncture of the parenchyma via thoracotomy yielded a higher take rate (100%) than cells inoculated intrabronchially (95%), illustrating the influence of the microenvironment upon tumor development. Other workers implanting cells in the lung transtracheally and intrabronchially found tumor take rates of 80100% and very low mortality8, lending support to the overall tumor take rate observed in the present study (94.7%). Models based on implantation via thoracotomy may present high tumor take rates, but require a high level of surgical skill, are more difficult to reproduce and are associated with higher rates of surgical mortality. The thoracotomy-based model developed by Gomes-Neto et al.10 presented a relatively high surgical mortality rate (14.3%), in contrast with the present model of intrabronchial implantation (4.8%). The aggressive nature of Walker's carcinosarcoma is evident in the high tumor take rates observed and the rapid growth of the neoplasm characterized by the presence of large nodules in the lung (average volume: 0.118 ± 0.108 cm3) as early as the 5th day following cell implantation. In a study inoculating rats with different lineages of tumor cells intrabronchially, Howard et al.8 reported no tumor growth until the third week following implantation. Other workers implanting human cancer cell lineages intrabronchially in nude rat lung tumor models reported observing the first small lung nodules (<13 mm diameter) during the 5th week following inoculation with 20 x 106 cells.15 Using Walker's carcinosarcoma, our model presented lung nodules on the 5th day following inoculation, and in less than two weeks all animals in the control group had died. Howard et al.16 developed an orthotopic nude rat lung tumor model with endobronchial implantation of human lineages of lung carcinoma (NCI-H460) and found distant metastases in several organs, the earliest of which in the mediastinal lymph nodes. The most severely affected areas were the contralateral lung, kidneys, brain, bones and (less frequently) the adrenal glands. However, no metastases were observed until the 14th day following implantation. Dissemination to the mediastinal lymph nodes was detected on the 21st day and distant metastases only by the 28th day. In a study using the same lineages in nude rats and the same model, Johnston et al.7 found metastases in the mediastinal lymph nodes in 100% of the animals in the control group, as well as systemic metastases in the bones (95%), kidneys (83%), brain (48%) and contralateral lung (82%). The authors found the lung cancer model useful, in spite of the aggressiveness of the tumor causing the animals to die by the 5th week of implantation by way of local and systemic dissemination. In our model tumors developed quickly and disseminated to the mediastinum, but no distant or systemic metastases were found, perhaps because of the early death of the animals (median survival time: 11 days) in consequence of the aggressive nature of the cell lineage inoculated. Not even the contralateral lung displayed metastases, proving that no dissemination or endobronchial leakage of tumor cells occurred at the moment of implantation, a possibility discussed by some authors7,16. The massive mediastinal dissemination observed in the present study resembles findings from human patients with small-cell carcinoma. In fact, small-cell carcinoma behaves very aggressively in humans, usually with early dissemination to the mediastinum, although distant metastases are also frequently observed17. Presently oncological research tends to employ mice inoculated subcutaneously when testing new anticancer drugs18. However, the validity of the results obtained with these models is questionable due to differences in the pharmacodynamic aspects of subcutaneous tumor grafts and tumors in their organ of origin. Because chemosensitivity and response to therapy with anticancer drugs depend on the microenvironmental interaction of stroma and tumor, the choice of anatomical site of implantation is of considerable importance6. Thus, the development of orthotopic animal models is likely to increase our ability to anticipate human response to therapy with anticancer drugs19. The results obtained in the present study demonstrate the feasibility of our rat lung tumor model based on intrabronchial inoculation of tumor cells in the lower left lung and may contribute to the development of an orthotopic lung tumor model using immunosuppressed rats inoculated with human tumor cells. The HRCT technique employed to diagnose lung tumors in rats inoculated with Walker's tumor cells was shown to be time-saving, non-invasive and capable of early tumor detection. HRCT findings regarding tumor size were validated by histopathological examination and correlated closely with necropsy findings (r=0.953; p<0.0001), suggesting that HRCT scanning may be used in the future to evaluate tumor growth and volume without euthanasia, thus avoiding time and resource-consuming procedures. Other recently published studies using experimental models10,12,13 found the HRCT scanning technique to be an efficient tool for diagnosing lung nodules and to be less costly than magnetic resonance imaging20. The efficacy of HRCT scanning in the detection and measurement of tumors, as demonstrated by our study, makes it a suitable and non-invasive technique for diagnosing lung tumors, monitoring tumor growth and evaluating response to anticancer drugs in vivo in animals submitted to implantation with tumor cells.
Conclusion
1. Model of intrabronchial tumor implantation proved feasible: the take rate was high, surgical mortality was negligible and the procedure was simple to perform and easy to reproduce.
2. High-resolution computed tomography was found to be a highly accurate tool for tumor diagnosis, localization and measurement and may be recommended for monitoring tumor growth in this model.
Received: August 20, 2007
Review: October 25, 2007
Accepted: November 26, 2007
Conflict of interest: none
Financial source: CNPq
- 1. Jemal A, Tiwari RC, Murray T, Ghafoor A, Samuels A, Ward E, Feuer EJ, Thun MJ. Cancer statistics. CA Cancer J. Clin. 2004; 54:8-29.
- 2. Instituto Nacional do Câncer. Estimativa 2006: Incidência de câncer no Brasil. Rio de Janeiro, INCA/MS; 2005.
- 3 Libby DM, Wu N, Lee IJ, Farooqi A, Smith JP, Pasmantier MW, Mccauley D, Yankelevitz DF, Henschke CI. CT screening for lung cancer: the value of short-term CT follow-up. Chest. 2006; 129(4):1039-42.
- 4. Ciardiello F, Caputo R, Bianco R, Damiano V, Pomatico G, De Placido S, Bianco AR, Tortora G. Antitumor effect and potentiation of cytotoxic drugs activity in human cancer cells by ZD-1839 (Iressa), an epidermal growth factor receptor-selective tyrosine kinase inhibitor. Clin. Cancer Res. 2000;6:2053-63.
- 5. Masferrer JL, Leahy KM, Koki AT, Zweifel BS, Settle SL, Woerner BM, Edwards DA, Flickinger AG, Moore RJ, Seibert K. Antiangiogenic and antitumor activities of cyclooxygenase-2 inhibitors. Cancer Res. 2000;60(1):130611.
- 6.Onn A, Isobe T, Itasaka S, Wu W, O'reilly MS, Ki HW, Fidler IJ, Herbst RS. Development of an orthotopic model to study the biology and therapy of primary human lung cancer in nude mice. Clin. Cancer Res. 2003;9:5532-39.
- 7.Johnston MR, Mullen JBM, Pagura ME, Howard RB. Validation of an orthotopic model of human lung cancer with regional and systemic metastases. Ann. Thorac. Surg. 2001;71:1120-25.
- 8.Howard RB, Chu H, Zeligman BE, Marcell T, Bunn PA, Mclemore TL, Mulvin DW, Cowen ME, Johnston MR. Irradiated nude rat model for orthotopic human lung cancers. Cancer Res. 1991;51: 3274-80.
- 9.Wang HY, Ross HM, Ng B, Burt ME. Establishment of an experimental intrapulmonary tumor nodule model. Ann. Thorac. Surg. 1997;64:216-19.
- 10.Gomes-Neto, A.; Pessoa BBGP, Aguiar AS, Furtado BM, Moraes MO, Ribeiro RA. Modelo de tumor de pulmão em rato com o carcinossarcoma de Walker. Acta Cir. Bras. 2002;17(1):12-22.
- 11.Mclemore TL, Liu MC, Blacker PC, Gregg M, Alley MC, Abbott BJ, Shoemaker RH, Bohlman ME, Litterst CC, Hubbard WC. Novel intrapulmonary model for orthotopic propagation of human lung cancers in athymic nude mice. Cancer Res. 1987;47:5132-40.
- 12.De Clerck NM, Meurrens K, Weiler H, Van Dyck D, Vanhoutte G, Terpsra P, Postnov AA. High-Resolution X-ray Microtomography for the Detection of Lung Tumors in Living Mice. Neoplasia. 2004;6:374-9.
- 13.Greschus S, Kiessling F, Lichy MP, Moll J, Mueller MM, Savai R, Rose F, Ruppert C, Gunther A, Luecke M, FusenigNE, Semmler W, Traupe H. Potential applications of flat-panel volumetric CT in morphologic and functional small animal imaging. Neoplasia. 2005;7(8):730-40.
- 14.Earle WR. A study of the Walker rat mammary carcinoma 256, in vivo and in vitro. Am. J. Cancer. 1935;24:566-612.
- 15. March TH, Marron-Terada PG, Belinsky SA. Refinement of an orthotopic lung cancer model in the nude rat. Vet. Pathol. 2001;38:483-90.
- 16.Howard RB, Mullen JBM, Pagura ME, Johnston MR. Characterization of a highly metastatic, orthotopic lung cancer model in the nude rat. Clinical & Experimental Metastasis. 1999;17:15762.
- 17.Capelozzi VL. Anatomia patológica do câncer do pulmão. In: Zamboni M, Carvalho WR. Câncer do pulmão. 1ed. São Paulo: Atheneu, 2005. p.47-68.
- 18.Ciardiello F, Caputo R, Bianco R, Damiano V, Fontanini G, Cuccato S, De Placido S, Bianco AR, Tortora G. Inhibition of growth factor production and angiogenesis in human cancer cells by ZD1839 (Iressa), a selective epidermal growth factor receptor tyrosine kinase inhibitor. Clin. Cancer Res. 2001;7:145965.
- 19. Killion, JJ, Radinsky R, Fidler IJ. Orthotopic models are necessary to predict therapy of transplantable tumors in mice. Cancer Metast. Rev. 1998;17:279-84.
- 20.Kennell SJ, Davis IA, Branning J, Pan HP, Kabalka GW, Paulus MJ. High resolution computer tomography and MRI for lung tumor growth in mice undergoing radioimmunotherapy: correlation with histology. Med. Phys. 2000;27(5):1101-07.
Publication Dates
-
Publication in this collection
27 Mar 2008 -
Date of issue
Feb 2008
History
-
Accepted
26 Nov 2007 -
Reviewed
25 Oct 2007 -
Received
20 Aug 2007