Abstracts
PURPOSE: To study the repair of bone defect filled with autograft or bovine bone devitalized matrix in rats under anti-inflammatory action. METHODS: Two hundred and forty Wistar rats were distributed to two groups of 120 animals each. A 2mm-diameter defect was created in the femoral diaphysis. Animals of Group I had the bone defect filled with autograft and those of Group II, with bovine bone devitalized matrix. Animals of each group were redistributed to four subgroups according to the intramuscular administration of anti-inflammatory drug or saline solution: A - diclofenac sodium; B - dexamethasone; C - meloxicam; D - saline solution. Evaluation periods were 7, 14 and 30 days. Histological evaluation consisted of quantifying the inflammatory process, the bone neoformation, the collagen formation and the presence of macrophages. RESULTS: Animals of Group I did not show significant difference considering inflammatory reaction. Significant and progressive increase of bone neoformation was observed in both groups. The animals that received meloxicam and autograft showed less collagen formation at 14 and 30 days. The number of macrophages was higher in Group II than in Group I. The animals treated with dexamethasone and saline solution did not show statistically significant difference. CONCLUSIONS: Diclofenac sodium and meloxicam delayed bone graft repair and dexamethasone did not interfere in it.
Bone Transplantation; Anti-Inflammatory Agents; Rats, Wistar
OBJETIVO: Estudar o reparo do defeito ósseo preenchido com enxerto ósseo autógeno ou matriz óssea bovina desvitalizada sob ação de antiinflamatórios em ratos. MÉTODOS: 240 ratos Wistar, distribuídos em dois grupos de 120 animais. Confeccionou-se defeito de 2 mm de diâmetro na diáfise femoral. Os animais do Grupo I tiveram o defeito ósseo preenchido com enxerto ósseo autógeno e os do Grupo II com matriz óssea bovina desvitalizada. Cada grupo foi redistribuído em quatro subgrupos segundo a administração intramuscular de antiinflamatório ou solução salina: A - diclofenaco de sódio; B - dexametasona; C - meloxicam; D - solução salina. Os períodos de avaliação foram de 7, 14 e 30 dias. A avaliação histológica constou da quantificação do processo inflamatório, osso neoformado, formação de colágeno e macrófagos. RESULTADOS: Os animais do Grupo I não mostraram diferença significante em relação à reação inflamatória. Observou-se aumento significante e progressivo da neoformação óssea nos Grupos I e II. Os animais que receberam meloxicam e enxerto autógeno mostraram menor aporte de colágeno aos 14 e 30 dias de observação. Os macrófagos apresentaram-se em maior quantidade no Grupo II que no Grupo I. Os animais tratados com dexametasona e solução salina não demonstraram diferença estatisticamente significante entre os Grupos I e II. CONCLUSÕES: O diclofenaco de sódio e o meloxicam retardam a reparação do enxerto ósseo. A dexametasona não interfere na reparação do enxerto ósseo.
Transplante Ósseo; Antiinflamatório; Ratos Wistar
ORIGINAL ARTICLE
TRANSPLANTATION
Effect of anti-inflammatory agents on the integration of autogenous bone graft and bovine bone devitalized matrix in rats1 1 Research performed at Post-Graduate Program in Surgery and Experimentation, Federal University of São Paulo (UNIFESP), Brazil.
Efeito de antiinflamatórios na integração de enxerto ósseo autógeno e de matriz óssea bovina desvitalizada em ratos
Roberto Antoniolli da SilvaI; Djalma José FagundesII; Andréia Conceição Milan Brochado Antoniolli SilvaIII; Karin Ellen SistiIV; Themis Maria Milan Brochado de CarvalhoIV; Daniel Nunes e SilvaV
IPhD, Assistant Professor, Department of Surgical Clinics, Federal University of Mato Grosso do Sul (UFMS), Brazil
IIPhD, Coordinator of the Post-Graduate Program in Surgery and Experimentation, School of Medicine, Federal University of São Paulo (UNIFESP), Brazil
IIIPhD, Associate Professor, Department of Surgical Clinics, UFMS, Mato Grosso do Sul, Brazil
IVFellow PhD degree, Post-Graduate of Health and Development Program, Central West Region, UFMS, Mato Grosso do Sul, Brazil
VFellow Master degree, Post-Graduate of Health and Development Program, Central West Region, UFMS, Mato Grosso do Sul, Brazil
Correspondence Correspondence: Roberto Antoniolli da Silva Rua Pitangui, 399 79.103-151 Campo Grande MS Brazil Phone: (55 67)363-6333 Fax: (55 67)321-0453 ianterra@terra.com.br
ABSTRACT
PURPOSE: To study the repair of bone defect filled with autograft or bovine bone devitalized matrix in rats under anti-inflammatory action.
METHODS: Two hundred and forty Wistar rats were distributed to two groups of 120 animals each. A 2mm-diameter defect was created in the femoral diaphysis. Animals of Group I had the bone defect filled with autograft and those of Group II, with bovine bone devitalized matrix. Animals of each group were redistributed to four subgroups according to the intramuscular administration of anti-inflammatory drug or saline solution: A diclofenac sodium; B - dexamethasone; C - meloxicam; D - saline solution. Evaluation periods were 7, 14 and 30 days. Histological evaluation consisted of quantifying the inflammatory process, the bone neoformation, the collagen formation and the presence of macrophages.
RESULTS: Animals of Group I did not show significant difference considering inflammatory reaction. Significant and progressive increase of bone neoformation was observed in both groups. The animals that received meloxicam and autograft showed less collagen formation at 14 and 30 days. The number of macrophages was higher in Group II than in Group I. The animals treated with dexamethasone and saline solution did not show statistically significant difference.
CONCLUSIONS: Diclofenac sodium and meloxicam delayed bone graft repair and dexamethasone did not interfere in it.
Key words: Bone Transplantation. Anti-Inflammatory Agents. Rats, Wistar.
RESUMO
OBJETIVO: Estudar o reparo do defeito ósseo preenchido com enxerto ósseo autógeno ou matriz óssea bovina desvitalizada sob ação de antiinflamatórios em ratos.
MÉTODOS: 240 ratos Wistar, distribuídos em dois grupos de 120 animais. Confeccionou-se defeito de 2 mm de diâmetro na diáfise femoral. Os animais do Grupo I tiveram o defeito ósseo preenchido com enxerto ósseo autógeno e os do Grupo II com matriz óssea bovina desvitalizada. Cada grupo foi redistribuído em quatro subgrupos segundo a administração intramuscular de antiinflamatório ou solução salina: A diclofenaco de sódio; B dexametasona; C meloxicam; D solução salina. Os períodos de avaliação foram de 7, 14 e 30 dias. A avaliação histológica constou da quantificação do processo inflamatório, osso neoformado, formação de colágeno e macrófagos.
RESULTADOS: Os animais do Grupo I não mostraram diferença significante em relação à reação inflamatória. Observou-se aumento significante e progressivo da neoformação óssea nos Grupos I e II. Os animais que receberam meloxicam e enxerto autógeno mostraram menor aporte de colágeno aos 14 e 30 dias de observação. Os macrófagos apresentaram-se em maior quantidade no Grupo II que no Grupo I. Os animais tratados com dexametasona e solução salina não demonstraram diferença estatisticamente significante entre os Grupos I e II.
CONCLUSÕES: O diclofenaco de sódio e o meloxicam retardam a reparação do enxerto ósseo. A dexametasona não interfere na reparação do enxerto ósseo.
Descritores: Transplante Ósseo. Antiinflamatório. Ratos Wistar.
Introduction
In orthopedics, repair of bone defects is frequently difficult because a fast growth of connective tissue inside them impedes the formation of bone tissue, making bone graft the classical method of treatment in anatomo-functional reconstruction2,3. However, the use of autogenous bone graft presents as limitation an inadequate amount when the donor source is scarce.
Studies on bone graft and reparative osteogenesis have been focused on the search for substitutes for autogenous bone or pharmacological factors and agents that interfere in such processes. Bovine devitalized matrix is one of the options; when used to fill bone defects, it acts as a mold for fibroblast cells of the host bone to invade and differentiate in cartilaginous and bone tissues5.
A comparative study on implants of devitalized bovine bone, autogenous bone, coral hydroxyapatite and castor-oil polyurethane in standardized bone defects of femoral condyles in rabbits demonstrated that although bovine devitalized matrix implant has osteoconductive property, it causes more intense inflammatory reaction and presents minor osteogenic potential as well as slower bone tissue recovery, when compared to autogenous bone graft, which demonstrated greater osteogenic potential and less intense inflammatory reaction3.
The action of non-hormonal anti-inflammatory (NHAI) agents is based on the inhibition of prostaglandin synthesis which, besides producing analgesic and anti-inflammatory effect, decreases the number of macrophages, fibroblasts and collagen fibers6, as previously demonstrated in studies about the healing of several tissues such as abdominal wall, alimentary canal, muscles, cartilages and bones7.
The daily use of diclofenac sodium may impair bone integration. Experiments with mice demonstrated that the use of diclofenac sodium interfered in the fractures remodeling, leading to instability of the bone callus9. Meloxicam, another NHAI agent, increased neutrophils flow and decreased osteoclasts on the seventh day of observation11. Tenoxicam presented inhibitory effect on osteogenesis. In osteogenesis, steroidal anti-inflammatory agents have been considered inhibitors of phosphatase-kinase1, an essential enzyme for the triggering of osteoblasts proliferation with time-dependent characteristics12.
The use of anti-inflammatory drugs in orthopedics as well as in traumatology during immediate post-operative period, the absence of papers in literature about the effect of such medicines on bone repair using graft, and the result of research indicating that bovine bone devitalized matrix in spite of presenting osteoconductive potential leads to slower tissue repair and more intense inflammation stimulated the present investigation, which used a standard experimental model and aimed at histologically studying the repair of a bone defect that was created in rat femoral diaphysis and filled with autogenous bone graft or bovine bone devitalized matrix under the action of anti-inflammatory agents.
Methods
The present experiment used 240 adult male Wistar-EPM rats (weighed 250300g, aged 7890 days old), which were randomly distributed to two groups: Group I, in which the standardized bone defect was filled with autogenous bone graft, and Group II, in which the standardized bone defect was filled with bovine bone devitalized matrix.
Both groups were redistributed to four subgroups of 30 animals each, according to the anti-inflammatory agent used: A - diclofenac sodium (Voltaren®), 2mg.Kg-1 a day; B - dexamethasone (Decadron®), 0.5 mg.Kg-1 a day; C - meloxicam (Movatec®), 0.5mg.Kg-1 a day; and D - isotonic saline solution as control. The anti-inflammatory drugs and the saline solution were intramuscularly (IM) administered at the immediate post-operative period and for six consecutive days.
Intraperitoneal injection of pentobarbital sodium (Tiopental®), at the dose of 50mg.Kg-1, was used as anesthetic procedure. The animal was positioned in left lateral decubitus in order to let its right inferior member exposed for the operation. For antisepsis, 2% iodized alcohol and sterile operative cloth were used; then, longitudinal incision was made in the lateral surface of the right thigh (30mm length), from the saliency of the right lateral femoral condyle, aimed at reaching the skin and subcutaneous cells.
After rupture of the muscle and exposure of the right femoral diaphysis, an incision was made and the periosteum was delicately removed by using a bistoury and a metallic trephine (2mm diameter) coupled to a low-rotation electric perforator; the lateral cortical layer was crossed, reaching the marrow layer, thus creating the standardized defect.
As it is a cortico-spongy tissue, the bone content removed was fragmented during the operation. It was collected to immediately fill the defects in animals from Group I. Bone defects of animals from Group II were filled with bovine bone devitalized matrix.
The muscular plan was closed by using 4-0 catgut interrupted simple stitches and the skin by using 4-0 polyamide monofilament thread and continuous suture; then the anti-inflammatory drug or the saline solution was IM administered at the immediate post-operative period and for six consecutive days, comprising seven days of treatment.
After daily clinical observation for 7, 14 and 30 days, ten animals of each subgroup received anesthesia similar to that received during operation, had the right posterior limb disarticulated and the femur dissected. Euthanasia was obtained by the continuous inhalation of ethyl ether under fume hood.
The pieces were immersed in individual flasks containing 10% buffered formaldehyde and sent to the Department of Pathological Anatomy, São Paulo State University (UNESP), Botucatu, for histological processing. From each block, three slides were prepared: the first was stained with Hematoxylin and Eosin (HE) for quantitative analysis of the inflammatory reaction and bone neoformation; the second was stained with Picrossirius Red for analysis of the presence of collagen; and the third was prepared for macrophages identification through immunohistochemical test.
In the qualitative analysis under optical microscopy, the presence of inflammatory cells like neutrophils, fibroblasts and macrophages in the area of the defect filled with graft was considered as inflammatory reaction; bone tissue surrounded by osteoblasts evidencing osteoblastic activity was considered as bone neoformation.
The quantitative analysis was carried out using a computerized program of image analysis (ImageLab 2.3), based on spectrophotometry principles.
Two sites were analyzed in each slide, allowing the calculation of the arithmetic mean of two parameters: the percentage of the area occupied by the parameter to be analyzed and the light optical density reflected by that parameter. Quantitative measures for the statistical analysis were obtained using Kruskal-Wallis analysis of variance to compare the variables' values at 7, 14 and 30 days for each anti-inflammatory drug in each group separately. The same analysis was used to compare data among the anti-inflammatory agents at each experiment day in each group. The Mann-Whitney test was used to compare the values between Group I and Group II for each anti-inflammatory drug at each day of the experiment. Nullity hypothesis rejection level was set at 5%.
Results
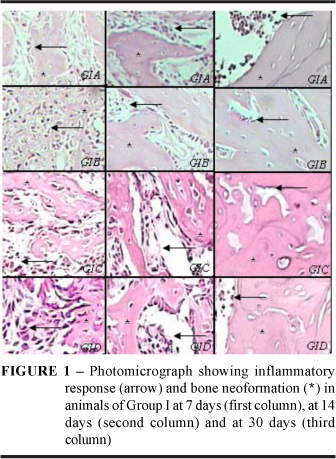
More intense inflammatory reaction was observed at all periods in the animals of Group II, in which bone defect was filled with bovine bone devitalized matrix (Figures 1 and 2). Statistical analysis showed there was no significant difference regarding inflammatory reaction among subgroups whose defects were filled with autogenous graft. However, among subgroups whose defects were filled with bovine devitalized matrix, there was less intense inflammatory reaction in animals that received dexamethasone and saline solution (Figure 3).
A significant and progressive increase of bone neoformation was observed in both Groups I and II at all periods analyzed, indicating bone neoformation had a time-dependent aspect, regardless the use of anti-inflammatory agents. In Group II, the animals that received diclofenac sodium and meloxicam presented less neoformation, but the animals treated with dexamethasone and saline solution showed no significant difference at 14 and 30 days, when comparing Groups I and II (Figure 4).
Collagen formation was time-dependent and was significantly high at all periods studied (Figures 5 and 6). The animals that received meloxicam and autogenous graft showed less collagen formation than the other subgroups at 14 and 30 days. Those that received diclofenac and bovine devitalized matrix presented less collagen concentration at all periods analyzed. The group that received dexamethasone and autogenous graft had less collagen formation than the group that received dexamethasone and bovine bone devitalized matrix at 7 days (Figure 7).
The immunohistochemical study for macrophages identification (Figure 8) showed a higher amount of macrophages in Group II (in animals treated with diclofenac and meloxicam) than in Group I at 7 and 14 days. There was no statistically significant difference between Groups I and II for animals treated with dexamethasone and saline solution. At 30 days, no group presented macrophages (Figure 9).
Discussion
Rats were used in the present experiment because they can be easily obtained and handled, and show anatomical and biological similarities to humans, such as the pharmacokinetics of NHAI agents and the binding of medicines to plasma proteins, which makes rat the appropriate animal model for comparison of NHAI drugs effects as well as for clinical prognostics40. Also, comparative studies among several animal species have established that the pharmacokinetic profile of male rats is the most similar to that of humans.
For homogeneity, only male rats were used, avoiding the factor of estrous cycle inherent to females.
The correction of bone defects with substance loss has in the autogenous bone graft the ideal pattern (gold standard). Its use, however, has some limiting factors such as the amount of material available in patients with low reserve of autogenous bone (children and elderly) or previously subjected to operative procedures using such reserve3, 4, 5.
To fill bone defects as well as to induce bone formation, several synthetic products have been used such as morphogenetic protein49, bone devitalized matrix3,48, castor oil polymer, and coralline hydroxyapatite. These synthetic materials present varied degree of integration according to the implanted site and the animal studied. However, when compared to autogenous graft, those products have not been efficient enough as bone substitutes.
In an attempt to solve such problem, materials of biological origin, known as biomaterials, have been increasingly used to fill bone cavities due to their osteoinduction and/or osteotransport properties in a procedure denominated guided bone regeneration3,5,7,9.
Among grafts derived from biological sources are coral hydroxyapatite, castor oil polyurethane, and inorganic bovine matrix. Figueiredo compared the osteointegration of autogenous bone graft, coral porous hydroxyapatite, castor oil polyurethane and inorganic bovine matrix in femoral bone defects in rabbits, and observed more intense inflammatory reaction and more reabsorption of the graft in defects filled with inorganic bovine matrix.
The use of bovine bone devitalized matrix is advantageous due to its easy obtaintion13, great amount of material available for graft, and high osteogenic3 capacity, serving as a mold for bone neoformation. It is a xenogenic, microporous, natural hydroxyapatite with physical properties appropriate for filling cavities; it is adaptable to the irregularities of the implant site, allowing good bone juxtaposition in the repair of defects and facilitating bone growth3, 20. Besides, it has low production cost and can be stored at room temperature3, 20. At bone implantation, it triggers intense inflammatory reaction and can be partially or completely removed from the receptor site through a slow process of surface reabsorption 3, 20.
Reparative osteogenesis of bone defects, with or without the use of bone graft, can be influenced by biological factors12 such as the coexistence of chronic diseases like diabetes mellitus, by the use of chemical substances like calcitonin and biphosphorates, or by physical procedures15 like sterilization17 and techniques for graft preparation and insertion, accelerating or delaying bone repair.
Non-hormonal anti-inflammatory agents are routinely used for 7 to 10 days after bone lesions58, 59 and can interfere in osteogenesis10, delaying and reducing bone neoformation. Experimental studies on the relationships between anti-inflammatory drugs and bone or cartilaginous14,15,16,17,19,20,21 repair have used intramuscular injection of drugs from seven to ten days for later analysis of their effects at three different periods of observation: the first in the acute phase; the second in an intermediate phase; and the third in a late phase of the healing phenomenon.
The inflammatory process triggered by the inorganic matrix hinders and delays graft integration. A drug capable of reducing or abolishing the inflammatory process would be of great value in the study of this type of graft. Non-hormonal anti-inflammatory agents are currently used in medicine, especially in orthopedic surgery, mainly for analgesic and/or anti-inflammatory purpose. The administration period varies from 7 to 10 days13.
Studies on bone/cartilaginous lesions repair in experimental animals using NHAI agents demonstrated that they made the bone callus instable in tibial fractures, delayed bone repair, decreased bone neoformation, delayed organization and remission of blood clot, inhibited bone neoformation in implants of bovine bone devitalized matrix, increased neutrophils flow, and decreased the amount of osteoclasts in the acute phase of the bone repair process19,20.
The aim of the present research was to verify the occurrence of a better graft integration by associating the use of inorganic bovine matrix and anti-inflammatory drugs. The administration period of the NHAI agents was established as 7 days based on previous studies using experimental animals and on the clinical practice. To fulfill the study's objective, two NHAI agents of current use and a hormonal anti-inflammatory drug, which is considered the pattern of steroid hormone, were chosen.
Diclofenac sodium is a non-hormonal anti-inflammatory inhibitor of Type-1 cyclooxygenase (COX-1) and, in spite of presenting an undesired potential effect on gastrointestinal treatment, is the most recommend drug in orthopedics, mainly for low-income population, due to its accessible cost and ready availability at the health units of the Unique Health System [Sistema Único de Saúde]. The dose used was 2mg.Kg-1, which was previously established in other experimental studies 15,16.
Meloxicam, a non-steroid anti-inflammatory drug, is a selective inhibitor of Type-2 cyclooxygenase (COX-2), both in vitro and in vivo, presenting high rate of plasmatic protein binding (99%) and biological half-life around 20 hours. It is metabolized in the liver, and its inactive metabolites are excreted through urine and feces. Its usual dose is from 7.5 to 15mg.day-1, orally, rectally or parenterally13.
Dexamethasone is a corticosteroid of potent anti-inflammatory activity which, at therapeutic doses, does not lead to hydrosodic retention nor worsens the secondary phenomena attributed to continuous administration of several corticoid63,67,68 derivates. It is an anti-inflammatory drug of great therapeutic value for the treatment of rheumatic diseases and other painful syndromes and is currently used in daily orthopedic clinical practice19 at the dose of 0.5mg.Kg-1.day-1.
The standardized defect was created in the rats' femoral diaphysis because they have appropriate dimensions and allow easy and little traumatic seizure. A 2mm-diameter trephine was coupled to an electric perforator of low rotation to avoid thermal necrosis of the bone cells in the transition area between the implant and the receptor bone, which could be caused by an electric perforator of high rotation3, 48, 50. The autogenous bone removed when the standardized defect was created was collected for subsequent filling 3, 47, 48, 50 in control animals of Group I. The technique was easy, efficient for material collection, and adequate for filling the standardized defects. Besides, it could be easily replicated.
The inorganic bovine bone matrix easily filled the standardized defects. It was obtained from lyophilized bovine bone, which is marketed as bars composed of small, medium or big sterilized particles. In the present study, the bars used were composed of small particles and were fragmented to allow appropriate filling of the defect.
All groups had weight gain, which indicated that there was no compromising of the animals' health in spite of the operation and the use of implants and drugs.
To evaluate the action of the anti-inflammatory agents on the bone healing process, representative histological parameters were obtained at 7, 14 and 30 days. For data analysis, a histological study was carried out and included slides stained with hematoxylin-eosin for quantification of the inflammatory process and the neoformed bone. Another parameter analyzed was the formation of collagen, which is the main component of the bone organic matrix19. For its quantification, the direct visualization principle and the staining method with Picrossirius Red F3BA derived from picric acid were used. This method is easy to use, selective, allows ready interpretation, and is frequently employed in works involving healing20. In biomedical literature, there is not any paper about its application in the study of organic bone matrix, which is an important component of the bone and, therefore, a relevant parameter to be analyzed when studying bone regeneration.
Macrophages quantification was considered relevant due to the importance these cells have in a certain moment of the healing process, when they generate a stimulus and regulate the proliferation process, modulating stimuli that result in the collagen synthesis23. An immunohistochemical model was adopted for macrophage identification and consisted of antigen-antibody reaction using a standard technique. The antigen CD68, found in the macrophage cytoplasm, was detected by the monoclonal antibody HAM56; to identify the reaction, a discloser that stains the cellular bodies dark-brown was used24. Experiments involving healing had already used such method for macrophage identification; however, all of them involved healing of the abdominal23 wall. A study was carried out in mice to explain the pathogenesis of bone reabsorption associated with periapical lesion, and could quantify macrophages and verify their prevalence until the fifth day of observation as well as the prevalence of osteoblasts after that period. However, a different method was used to identify those cells19.
All those parameters were quantified by using a computer program developed to allow identification, selection and subtraction of structures of the same image through spectrum of 32 x 1,000 shades of colors, defining the morphologic patterns in the area and the perimeter for the calculation of the form factor, which was obtained from the relationship between those two variables35. Such method is already well established and has been used in experimental studies involving abdominal wall healing and bone regeneration3.
More intense inflammatory reaction was observed at all periods in animals whose bone defects were filled with bovine bone devitalized matrix. Such data corroborated those from literature3 which demonstrated intense inflammatory reaction triggered by the presence of bovine bone devitalized matrix.
There was a significant and progressive increase in bone neoformation in both Groups I and II at all periods analyzed, suggesting it was time-dependent, regardless the use of anti-inflammatory drugs.
Collagen formation was also time-dependent and significantly high at all periods studied, suggesting that NHAI agents interfered in such process both in the group that received autogenous graft and in the group that received bovine bone devitalized matrix.
At 7 days, the group that received dexamethasone and autogenous graft showed less collagen formation than the group that received dexamethasone and bovine bone devitalized matrix, which demonstrated that the corticoid interfered in the collagen synthesis only in the initial phase of regeneration.
Studies have shown that corticoid impairs the formation of bone collagen22, 26, indicating the need for additional studies aimed at identifying the type of collagen formed. The organic bone matrix consists of collagen Type I (95%), and the cartilage is mainly composed by collagen Type II, and as the corticoid does not interfere in the formation of cartilaginous collagen, further investigation on the type of collagen formed could contribute for a better understanding of that result.
The immunohistochemical study for macrophage identification showed no statistically significant difference between animals of Groups I and II that were treated with dexamethasone and saline solution. At 30 days of evaluation, no group presented macrophages because these cells are part of the healing initial phase. Similar results were obtained in an experimental study on bone repair carried out by Lin et al24, in which there was macrophage prevalence at 5 days and osteoblast prevalence at 15 and 20 days, a later stage of bone repair.
Higher macrophage concentration was observed in animals of Group II that were treated with diclofenac and meloxicam probably because the bovine bone devitalized matrix caused significant inflammatory reaction, delaying the bone repair process.
Some works in literature suggested that NHAI agents interfere in the bone repair19, 21. Sell et al18 reported that the use of diclofenac sodium, at a daily dose of 50mg, impaired bone integration of non-cemented prostheses in humans, since it interfered in the proliferation and metabolism of fibroblasts and osteoblasts, accelerating the differentiation of the former and the inhibition of the latter.
Akman et al19 studied the effect of diclofenac sodium, IM administered at daily doses of 1mg and 2mg, on the repair process of tibia closed fracture in 55 Wistar rats that were sacrificed after 2, 4 and 6 weeks for clinical-radiological and histological analyses. After two weeks, bone callus in control group animals was more stable than that in animals treated with diclofenac sodium. Radiological and histological analyses did not show any difference among groups in the subsequent periods. Yugoshi et al25 investigated the interference of diclofenac administration in the alveolar repair process. They used 42 Wistar mice, out of which 21 received diclofenac (10mg.Kg-1 a day) and the remaining received saline solution. The animals were sacrificed at 7, 14 and 21 days. The progressive formation of bone and the reduction of clot volume and connective tissue from 1 to 3 weeks after dental extraction were quantified through histometric method using the point counting technique. Treatment with diclofenac caused significant delay of bone neoformation, associated with the delay in the clot organization and remission.
Giordano Neto21 studied the effect of tenoxican, a non-hormonal anti-inflammatory inhibitor of COX-2, and observed that such drug presented inhibitory effect on the osteogenesis, which could be related to the treatment precocity and to the dose used. However, studies have demonstrated that meloxicam can decrease bone reabsorption by 43% in periapical lesions since they occur due to COX-2 production.
In biomedical literature, there are no studies about the effect of corticoids used for a short period on bone regeneration. The harmful effects of long-lasting use of corticoids on bone are well known and documented. Researchers carried out a biomechanical study of bone graft repair in rabbits treated with metilpredinisolone during six months and observed significant deterioration of the graft mechanical properties, when compared to the control group. The main role of corticoids in osteoporosis induction and bone formation decrease is to inhibit the enzyme phosphatase-kinase-128, which is essential to induce osteoblast proliferation and is time-dependent.
Control groups presented better results than the groups treated with NHAI drugs, which corroborate data from previous works that demonstrated the interference of those drugs in reparative osteogenesis.
The animals treated with dexamethasone and saline solution presented no statistically significant difference, which suggested that dexamethasone did not interfere in the physiological process of bone repair because such result was similar to that observed in the subgroup that was not treated with anti-inflammatory drugs.
The methodology used in the present experimental model (such as collagen and macrophage quantification) to study the effect of anti-inflammatory agents on bone repair was not found in literature. Even studies on inflammatory process and bone neoformation did not use the quantification of those parameters but employed a qualitative analysis, which hindered the confrontation of the findings of the present research with the ones from literature; however, the present results suggest many hypotheses for future investigation on bone repair under drug action.
The present study showed that NHAI agents (diclofenac sodium and meloxicam), despite their anti-inflammatory action, were not effective in reducing inflammatory reaction triggered by the implantation of devitalized bone matrix and, thus, did not contribute to a better integration of such graft. Dexamethasone did not interfere in the process as it led to results similar to those obtained in the control group.
Further studies are needed, therefore, to better explain the integration of inorganic bone matrix, which produces intense inflammatory reaction at the implantation site. It opens up the perspective of using other types of anti-inflammatory, chelant or cytostatic drugs.
Conclusions
The non-hormonal anti-inflammatory agents diclofenac sodium and meloxicam delayed the repair of autogenous bone graft and bovine bone devitalized matrix.
The anti-inflammatory steroid dexamethasone did not interfere in the repair of autogenous bone graft and bovine bone devitalized matrix.
Received: September 12, 2007
Review: November 14, 2007
Accepted: December 11, 2007
Conflict of interest: none
Financial source: none
- 1. Ameziane L, Daoudi A, Souhail MS. Coverage of tissue loss in the upper limb (ten cases). Chir Main. 2003;22:95-8.
- 2. Ahmad CS, Guiney WB, Drinkwater CJ. Evaluation of donor site intrinsic healing response in autologous osteochondral grafting of the knee. Arthroscopy. 2002; 18:95-8.
- 3. Figueiredo AS. Estudo morfológico comparativo entre implantes de osso bovino desvitalizado, hidroxiapatita porosa de coral, poliuretana de mamona e enxerto ósseo autógeno, em coelhos [Tese Doutorado]. Universidade Federal de São Paulo Escola Paulista de Medicina; 2001.
- 4. Finkemeier CG. Bone-grafting and bone-graft substitutes. J Bone Joint Surg [Am]. 2002;84:454-65.
- 5. Bostrom MPG, Yang X, Koutras IK. Biologics in bone healing. Curr Opin Orthop. 2000;11:403-12.
- 6. Schnürer SM, Gopp U, Kühn KD, Breusch SJ. Bone substitutes. Orthopade. 2003; 32:2-10.
- 7. Hirchowitz BI. Nonesteroidal anti-inflamatory drugs and the gut. South Med J. 1996; 89:259-63.
- 8. Sell S, Teschner M, Gaissmaier C. Effect of diclofenac on human osteoblasts and their stromal precursors in vitro in relation to arthroplasty. Z Rheumatol. 1999; 58:13-20.
- 9. Akman S, Gögüs A, Sener N. Effect of diclofenac sodium on union of tibial fractures in rats. Adv Ther. 2002;19:119-25.
- 10. Giordano Neto V. Influência do tenoxicam no processo de consolidação de fratura. Estudo experimental em tíbia de ratos [Tese Mestrado]. Universidade Federal do Rio de Janeiro; 1999.
- 11. Van Staa TP, Leufkens HG, Cooper C. Use of nonsteroidal anti-inflammatory drugs and risk of fractures. Bone. 2000;27:563-8.
- 12. Sanches MG, Okamoto T, Carvalho ACP. Efeitos da prednisolona no processo de reparo em feridas de extração dental: estudo histológico em ratos. Rev Fac Odont. 1975;4:195-200.
- 13. Ogston N, Harrison AJ, Cheung HF. Dexamethasone and retinoic acid differentially regulate growth and differentiation in an immortalised human clonal bone marrow stromal cell line with osteoblastic characteristics. Steroids. 2002;67: 895-906.
- 14. Mizuno H, Liang RF, Kawabata A. Effects of oral administration of various anti-inflammatory drugs on bone growth and bone wound healing in mice. Meikai Daigaku Shigaku Zasshi. 1990;19:234-50.
- 15. Latta K, Krieg RJ, Carbajo-Pérez E. Effects of deflazacort and cortisone on cellular proliferation in the rat thymus. Life Sci. 2002;71:1951-60.
- 16. Sokal RR, Rohlf FJ. Biometry. San Francisco: WH Freeman; 1969.
- 17. Siegel S, Castellan Jr NJ. Nonparametric statistic. New York: McGraw-Hill; 1988.
- 18. Busch U, Roth W, Schmaus H. Pharmacokinetics of meloxicam in animals. Scand J Rheumatol. 1994; 98:111.
- 19. Figueiredo AS, Takita LC, Goldenberg S. Comparação entre a hidroxiapatita porosa de coral e o enxerto ósseo autólogo em coelhos. Acta Cir Bras. 1998;12:84-8.
- 20. Jacques JW. Estudo comparativo entre enxerto ósseo autógeno e polímero vegetal, em coelhos [Tese Mestrado]. Universidade Federal de São Paulo Escola Paulista de Medicina; 2000.
- 21. Sassioto MCP, Inouye CM, Aydos RD. Estudo morfológico do reparo de defeito ósseo preenchido com enxerto ósseo autógeno ou matriz óssea bovina desvitalizada, em ratos. Ensaios e ciência, Campo Grande-MS. 2004;7:543-50,
- 22. Martinez SA, Walker T. Bone grafts. Vet Clin North Am Small Anim Pract. 1999; 29:1207-19.
- 23. Holmes RE, Bucholz RW, Mooney V. Porous hydroxyapatite as a bone-graft substitute in metaphyseal defects. J Bone Joint Surg [Am]. 1986; 68: 904-11.
- 24. Risto O, Wahlström O, Abdiu A. The effect of low dose diclofenac sodium administered locally on heterotopic bone formation in rats. Int Orthop. 1995;19: 392-5.
- 25. Engelbrecht Y, de Wet H, Horsch K. Glucocorticoids induce rapid up-regulation of mitogen-activated protein kinase phosphatase-1 and dephosphorylation of extracellular signal-regulated kinase and impair proliferation in human and mouse osteoblast cell lines. Endocrinology. 2003;144: 412-22.
- 26. Mast BA. Cicatrização em outros tecidos. In: Clínicas Cirúrgicas da América do Norte. Rio de Janeiro: Interlivros; 1997. p529-46.
- 27. Braga FJC, Silva GM, König Jr B. Obtenção de Matriz mineral de osso bovino e a comprovação de sua biocompatibilidade. Rev Bras Cir Protese Implant. 1999; 6:43-9.
- 28. Srinivas GR, Chichester CO, Barrach HJ, Matoney AL. Effects of certain antiarthritic agents on the synthesis of type II collagen and glycosaminoglycans in rat chondrosarcoma cultures. Agents Actions. 1994; 41:193-9.
Publication Dates
-
Publication in this collection
26 Mar 2008 -
Date of issue
Apr 2008
History
-
Received
12 Sept 2007 -
Reviewed
14 Nov 2007 -
Accepted
11 Dec 2007