Abstracts
PURPOSE: To study the effect of finasteride on the spermatogenesis of adult Mesocricetus auratus. METHODS: Twenty adult hamsters were evaluated. The animals were one year-older, and were randomly divided in 2 different groups: control group with ten animals (n=10) and experimental group also with ten animals (n=10). The animals in the experimental group were shot 7.14 ng/mL (0.5mL) of finasteride by 100mg/Kg, subcutaneously in the dorsal region three times per week during 90 days. This dose correspondes to 5mg of the drug used in adult men for the treatment of benign prostatic hyperplasia (BPH). After three months, the animals were anesthetized through association of 200mg/kg ketamine chloridrate and 2.5 mg/kg of diazepan and were dead through hypovolemia.. The testis removed along with the whole genitourinary apparel were fixed with 10% formalin and submitted to histological analisys by optical microscopy. The hematoxilin-eosin (HE) method was used to stain the slides. RESULTS: The mean weight of animals in the control group before death was 129.0±18.8gr. The mean weight of animals in experimental group was 145.0±15.25gr. The mean age of animals in control group before death was 15.2±1.13 months. The mean age of animals in experimental group before death was 17.16±0.82 months. The mean difference in weight between both groups was not statistical significant (p=0.0514). The totality of animals in control group (100%) presented no tubular alterations and showed no disturbancy in the spermatogenesis stages. Four animals (40%) in the experimental group showed hypotrophy of the seminiferous tubules and six (60%) showed normal spermatogenesis, however reduced compared to control group. There was statiscally significant difference (p=0.043) between the control and experimental group related to testicular alterations. CONCLUSION: The animals that were administered finasteride showed significant tubules atrophy and spermatogenesis reduction compared to control group.
Mesocricetus; Finasteride; Testis; Spermatogenesis
OBJETIVO: Estudar o impacto da finasterida na espermatogênese do Mesocricetus auratus, adulto. MÉTODOS: Foram avaliados 20 hamsters adultos, com idades superiores a 1 ano, distribuídos em dois grupos: grupo controle com dez animais (n=10) e grupo experimental também com dez animais (n=10). No grupo experimental foi aplicado 7,14ng/mL (0,5mL) de finasterida por 100mg/Kg/peso, subcutâneo (SC), na região dorsal do animal, três vezes por semana por 90 dias, dose correspondente a 5mg da droga usada em homens adultos, para tratamento da hiperplasia benigna da próstata (HBP). No final de três meses, esses Hamsters foram mortos por hipovolemia, após serem anestesiados com cloridrato de quetamina, na dosagem de 200 mg/kg juntamente com diazepam, na dosagem de 2,5 mg/kg. Os testículos foram retirados em monobloco juntamente com todo o aparelho geniturinário, fixados em formalina a 10% e encaminhados à histotécnica para posterior análise histológica em microscópico óptico. Foram usadas para coloração das lâminas a hematoxilina e eosina (HE). RESULTADOS: Quando foram mortos, os Hamsters do grupo de controle pesaram em média 129,0g e desvio padrão (DP) de 18,8g. O grupo de experimento apresentou média de peso de 145,0g e DP de 15,25g. A idade dos animas de controle quando foram mortos apresentou média de 15,2 meses e DP de 1,13 meses. Os animais de experimento apresentaram média de idade de 17,16 meses e DP de 0,82. A diferença das médias de peso entre os dois grupos não teve significado estatístico (p=0,0514). Os animais (100%) do grupo controle não tiveram alterações tubulares e apresentaram todas as etapas da espermatogênese normais. Quatro animais (40%) do grupo de experimento apresentaram hipotrofia dos túbulos seminíferos, e seis (60%) desses animais apresentaram espermatogênese normal, mas diminuída em relação ao grupo controle. Do ponto de vista estatístico houve significância (p=0,043) entre o grupo de experimento e controle em relação às alterações testiculares. CONCLUSÃO: Os animais em uso de finasterida apresentaram atrofia tubulares significativas e diminuição da espermatogênese em comparação com o grupo-controle.
Mesocricetus; Finasterida; Testículo; Espermatogênese
12-ORIGINAL ARTICLE
GENITALIA, MALE
The effect of finasteride on spermatogenesis of Mesocricetus auratus1 1 Research performed at Post-Graduation Program in Surgery, Medicine School, Federal University of Minas Gerais (UFMG) and Laboratory of Apoptosis, Department of General Pathology, Institute of Biological Science, UFMG, Institute of Bio-Science, Presidente "Antônio Carlos" University (INBIO-UNIPAC).
Impacto da finasterida na espermatogênese do Mesocricetus auratus
Dimas José Araújo VidigalI; Alcino Lázaro da SilvaII; Luiz Mauro Andrade da FonsecaIII; Anilton Cesar VasconcelosIV; Dilermando Fazito de ResendeVI; Felipe Eduardo Costa VidigalVI
IFellow PhD degree in Surgery, Medicine School, Federal University of Minas Gerais (UFMG), Brazil
IIEmeritus Professor, UFMG, Brazil
IIIFull Professor, Department of Pathology, Medicine School of Barbacena, "José Bonifácio" Foundation (FAME-FUNJOB), Minas Gerais, Brazil
IVAssociate Professor, Laboratory of Apoptosis, Department of General Pathology, Institute of Biological Science, UFMG, Brazil
VFull Professor, Scientific Methodology, FAME-FUNJOB, Minas Gerais, Brazil
VIGraduate Student, SUPREMA, School of Medicine, Juiz de Fora, Minas Gerais, Brazil
Correspondence Correspondence: Dimas José Araújo Vidigal Rua Augusto Justi, 74 36201-613 Barbacena MG Brazil Phone/Fax: (55 32)3331-0124 dimas@barbacena.com.br
ABSTRACT
PURPOSE: To study the effect of finasteride on the spermatogenesis of adult Mesocricetus auratus.
METHODS: Twenty adult hamsters were evaluated. The animals were one year-older, and were randomly divided in 2 different groups: control group with ten animals (n=10) and experimental group also with ten animals (n=10). The animals in the experimental group were shot 7.14 ng/mL (0.5mL) of finasteride by 100mg/Kg, subcutaneously in the dorsal region three times per week during 90 days. This dose correspondes to 5mg of the drug used in adult men for the treatment of benign prostatic hyperplasia (BPH). After three months, the animals were anesthetized through association of 200mg/kg ketamine chloridrate and 2.5 mg/kg of diazepan and were dead through hypovolemia.. The testis removed along with the whole genitourinary apparel were fixed with 10% formalin and submitted to histological analisys by optical microscopy. The hematoxilin-eosin (HE) method was used to stain the slides.
RESULTS: The mean weight of animals in the control group before death was 129.0±18.8gr. The mean weight of animals in experimental group was 145.0±15.25gr. The mean age of animals in control group before death was 15.2±1.13 months. The mean age of animals in experimental group before death was 17.16±0.82 months. The mean difference in weight between both groups was not statistical significant (p=0.0514). The totality of animals in control group (100%) presented no tubular alterations and showed no disturbancy in the spermatogenesis stages. Four animals (40%) in the experimental group showed hypotrophy of the seminiferous tubules and six (60%) showed normal spermatogenesis, however reduced compared to control group. There was statiscally significant difference (p=0.043) between the control and experimental group related to testicular alterations.
CONCLUSION: The animals that were administered finasteride showed significant tubules atrophy and spermatogenesis reduction compared to control group.
Key words: Mesocricetus. Finasteride. Testis. Spermatogenesis.
RESUMO
OBJETIVO: Estudar o impacto da finasterida na espermatogênese do Mesocricetus auratus, adulto.
MÉTODOS: Foram avaliados 20 hamsters adultos, com idades superiores a 1 ano, distribuídos em dois grupos: grupo controle com dez animais (n=10) e grupo experimental também com dez animais (n=10). No grupo experimental foi aplicado 7,14ng/mL (0,5mL) de finasterida por 100mg/Kg/peso, subcutâneo (SC), na região dorsal do animal, três vezes por semana por 90 dias, dose correspondente a 5mg da droga usada em homens adultos, para tratamento da hiperplasia benigna da próstata (HBP). No final de três meses, esses Hamsters foram mortos por hipovolemia, após serem anestesiados com cloridrato de quetamina, na dosagem de 200 mg/kg juntamente com diazepam, na dosagem de 2,5 mg/kg. Os testículos foram retirados em monobloco juntamente com todo o aparelho geniturinário, fixados em formalina a 10% e encaminhados à histotécnica para posterior análise histológica em microscópico óptico. Foram usadas para coloração das lâminas a hematoxilina e eosina (HE).
RESULTADOS: Quando foram mortos, os Hamsters do grupo de controle pesaram em média 129,0g e desvio padrão (DP) de 18,8g. O grupo de experimento apresentou média de peso de 145,0g e DP de 15,25g. A idade dos animas de controle quando foram mortos apresentou média de 15,2 meses e DP de 1,13 meses. Os animais de experimento apresentaram média de idade de 17,16 meses e DP de 0,82. A diferença das médias de peso entre os dois grupos não teve significado estatístico (p=0,0514). Os animais (100%) do grupo controle não tiveram alterações tubulares e apresentaram todas as etapas da espermatogênese normais. Quatro animais (40%) do grupo de experimento apresentaram hipotrofia dos túbulos seminíferos, e seis (60%) desses animais apresentaram espermatogênese normal, mas diminuída em relação ao grupo controle. Do ponto de vista estatístico houve significância (p=0,043) entre o grupo de experimento e controle em relação às alterações testiculares.
CONCLUSÃO: Os animais em uso de finasterida apresentaram atrofia tubulares significativas e diminuição da espermatogênese em comparação com o grupo-controle.
Descritores: Mesocricetus. Finasterida, Testículo. Espermatogênese.
Introduction
Finasteride is a synthetic inhibitor of 5-a-reductase, an enzyme that changes testosterone into dihydrotestosterone (DHT). DHT is an active metabolite of testosterone, much more powerful than the last1.
Two different male steroid hormones secreted by the testis were isolated from the venous blood: testosterone and 4-androsterone-3,17-dione. However, the amount of testosterone is larger than the second hormone, so that testosterone can be considered as the only significant hormone responsible for the effects caused by male hormones. In the testis, both hormones are produced by the insterstitial cells of Leydig, which are located in the interstitial space between the seminiferous tubules1.
The testosterone that is released by the testis circulates in the blood for no longer than fifteen to thirty minutes, until it is fixed onto the tissues, or broken down into inactive substances to be excreted right after1. The share of testosterone fixed on the tissues is converted, inside the cells; into DHT by the 5-a-reductase1. This enzyme is responsible for the conversion of testosterone into DHT. As DHT, testosterone acts inside the cells1,2, it is assumed that DHT, after connecting with a plasmatic receptor, is transferred to the cellular nucleus, causing increased activity of the polymerase ribonucleic acid (polymerase RNA) and consequently, an increased synthesis of RNA and specific proteins2.
Spermatogenesis is a sequence of events that is dependent on testosterone, which leads the primitive germ cells to become spermatozoons. It starts in puberty when high levels of testosterone are released and it keeps occurring even in senescence1.
There are two types of 5-a-reductase: type 1 found in the skin and liver, and type 2 found in the genitourinary system, including the prostate3. The sexual impotence is the main side effect from using finasteride, followed by a reduction in the ejaculated volume4. However, studies have shown side effects from the use of finasteride similar to the patients who used placebo5.
Clinical studies have shown that the use of finasteride in a 5mg dose, once a day, decrease the volume of prostate and improve the symptoms of benign hyperplasia obstructiving this gland6. The 1mg/day dose used in the treatment of inherited androgenic alopecia, decreases the progress in hair loss and increase hair growth of7.
Because of its influence on the metabolism of androgens, there is some worry, regarding its use, especially in patients at fertile age8. The effects of finasteride on the spermatogenesis have been studied in depth when the quality of the ejaculated is evaluated and just a few studies have been published on the action of finasteride at cellular level on the testis.
The aim of this paper is to increase and improve the knowledge on this field of this subject. Therefore, this study is referred to the impact of finasteride on the spermatogenesis of adult Mesocricetus auratus.
Methods
This work began after its approval by the board of ethics in research of the Presidente "Antonio Carlos" University (CEP-UNIPAC).
Twenty adult hamsters, older than 1 year, were evaluated. They were divided into two groups: the control group with ten animals (n=10) and the experimental group, also with ten animals (n=10). Those animals were obtained from the animal house of the Bio-science Institute at Presidente "Antonio Carlos" Magnus Campus University (INBIO-UNIPAC).
The animals were put in plastic cages with 40x60x20 cm. Each cage had two animals and were lined with appropriate sawdust, for the hamster's accommodation.
The hamsters were fed in an ad libitum alimentation: special food made for hamsters, seeds of sunflower, peanut and corn. It was also offered to the animals, ad libitum and drinkable water from a suction fountain. All the animals were exposed to indirect sunlight for twelve hours in a 21°C room.
In the experimental group, were shot 7.14 ng/mL (0.5mL) of finasteride by 100mg/Kg, subcutaneously (SC) in the dorsal region of the animal for three times per week during ninety days (Figure 1). This dose corresponds to 5mg of the drug used in adult men6 (70Kg) for the treatment of benign prostatic hyperplasia (BPH).
After three months the animals died due to hypovolemia, after being anesthetized with 200mg/kg of ketanine chloridrat and 2.5mg/kg of diazepam9.
The testis were removed along with all the genitourinary system and fixed with 10% formalin. After that, they were submitted to histological analysis by optical microscopy. The hematoxilin-eosin method was used to produce a slide stain.
Results
It was found that values of mean weight after death for the animals in the control group was 129.0±18.8g and for the animals in the experimental group was 145.0±15.25g. The difference between the means of weight of the two tested groups did not show statistical significance, p>0.05. The animals in the control group had a mean age of 15.2±1.13 months at the death, while this value was 17.6±0.82 months for the experimental group.
Tubular changes were not found in the animals in the control group, and all of them showed normal spermatogenesis stages (Figure 2).
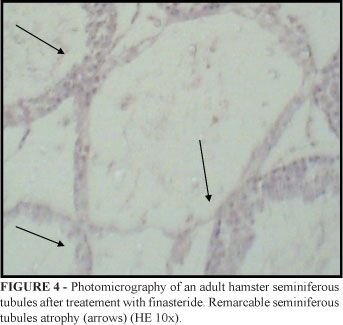
Four animals in the experimental group showed seminiferous tubules hypotrophy (Figures 3 to 4) and six presented normal spermatogenesis, although this spermatogenesis process was reduced compared to the animals in the control group (Figures 5 to 6).
There was statistically significant difference (p<0.05) in the testicular alterations between the two groups tested.
Discussion
Overstreet et al.10 investigating humans and Rhoden et al.11 investigating rats, did not show any alterations in the spermatogenesis after treatment with finasteride. Overstreet et al.10 evaluated the ejaculate from young men under use of finasteride 1mg and compared them to a control group. No histological assessment of the seminiferous tubules was performed. The Wistar rats were provided by Rhoden et al.11, and they received finasteride 2mg in a saline solution. It was difficult to accurately evaluate the absorption of the medication, fact that might have influenced the results. In this study, to certificate the adequate absorption of medication the finasteride was injected subcutaneously (SC) in the experimental group, thus, providing liability to results.
Studies have shown that only one dose of 0.5mg of finasteride causes a 65% reduction in plasma dihydrotestosterone (DHT). This effect can remain for five to seven days12. After 14 days of treatment discontinuity the levels of finasteride returned to baseline12,13. In this investigation the experimental animals were administered finasteride three times per week.
The histological analysis confirmed the drug action in the target organs, such as testis, suggesting that the therapeutic use of finasteride in longer intervals could provide the same therapeutic benefits to patients, with a lower cost. It could also reduce the adverse drug effects possibly caused by drug accumulation in the body.
Currently, finasteride is used both for the treatment of BPH and for the treatment of androgenic alopecia7. The results of the present study show deleterious effects in spermatogenesis in the testis of Hamsters that received a finasteride dose corresponding to a 5mg used in human treament. Therefore, further invastigation must be done for safe application of this medication, specially in young men seeking for better esthetic results, who are in fertile age. It must be clarified to the patient the possible effects of finasteride on spermatogenesis. When choosing this medication the risks and benefits must be highly evaluated.
Further studies should be performed in order to clarify the safe dose of finasteride to be used, as well as the administration interval.
Conclusion
The animals treated with finasteride showed significant changes of the testicular seminiferous tubules as well as in the spermatogenesis when compared to the control group.
Received: November 27, 2007
Review: January 29, 2008
Accepted: February 25, 2008
Conflict of interest: none
Financial source: none
- 1. Guyton AC. Tratado de fisiologia médica. 4ed. Guanabara Koogan: Rio de Janeiro; 1973.
- 2. Silva P. Farmacologia. 4ed. Guanabara Koogan: Rio de Janeiro: 1994.
- 3. Thigpen AE, Silver RI, Guileyardo JM. Tissue distribuition and ontogeny of steroid 5-alpha-reductase isozyme expression. J Clin Invest. 1993; 92: 903-10.
- 4. Netto MR, Netto Junior NR. Avaliação e tratamento da hiperplasia de próstata benigna. J. Br. Urol. 1998; 24(3):184.
- 5. Gormley GJ, Stoner E, Bruskewitz RC, Imperato-McGinley J, Walsh PC, McConnell JD, Andriole GL, Geller J, Bracken BR, Tenover JS, Vaughan ED, Pappas F, Taylor A, Binkowitz B, Ng J; Finasteride Study Group. The effect of finasteride in men with benign prostatic hyperplasia. 1992; J Urol. 2002 Feb;167(2 Pt 2):1102-7.
- 6. McConnell JD, Bruskewitz R, Walsh P, Andriole G, Lieber M, Holtgrewe HL, Albertsen P, Roehrborn CG, Nickel JC, Wang DZ, Taylor AM, Waldstreicher J. The effect of finasteride on the risk of acute urinary retention and the need for surgical treatment among men with benign prostatic hyperplasia: finasteride long-term efficacy and safety study group. N Engl J Med. 1998; 338:557-63.
- 7. Netto Junior CC. Alopécia androgênica e finasterida. JBM. 1999; 76(5):39-40.
- 8. Glina S, Neves PA, Saade R, Netto NR, Soares JB, Galuppo AG. Infertilidade masculina associada ao uso de finasterida. Rev Hosp Clin. 2004; 59(4):203-5.
- 9. Vidigal DJA, Silva LA, Fonseca LMA, Resende DF. Técnica para obtenção do aparelho genitourinário e dosagem do PSA (Prostate Specific Antigen) no Hamster Sírio, Mesocricetus auratus. Acta Cir Bras. 2004; 19(6): 603-8.
- 10. Overstreet JW, Fuh VL, Gould J, Howards SS, Lieber MM, Hellstrom W, Shapiro S, Carrole P, Corfman RS, Petron S, Lewis R, Toth P, Shown T, Rov J, Jarow JP, Bonilla J, Jacobsen CA, Wang DZ, Kaufman KD. Chronic treatment with finasteride daily does not affect spermatogenesis or semen production in young men. J Urol. 2000; 164(4):1319-20.
- 11. Rhoden EL, Gobbi D, Menti E, Rhoden C, Talöken C. Effects of the chronic use of finasteride on testicular weight and spermatogenesis in Wistar rats. Braz J Urol. 2002 jun; 89 (9): 961-3.
- 12. Ohtawa M, Morikawa H, Shimazaki J. Pharmacokinetics and biochemical efficacy after single and multiple oral administration of N-(2-methyl-2 propyl)-3-oxo-4-aza-5alfha-androst-1-ene-17-beta-carboxamide, a new type of specific competitive anhibitor of testosterone 5alpha-reductase, in volunteers. Eur J Drug Metab Pharmacokinet. 1991;16(1): 15-21.
- 13. Stoner E. The clinical development of a 5-alpha-reductase inhibitor, finasteride. J Steroid Biochem Mol Biol. 1990; 37: 375-8.
Publication Dates
-
Publication in this collection
04 July 2008 -
Date of issue
June 2008
History
-
Accepted
25 Feb 2008 -
Reviewed
29 Jan 2008 -
Received
27 Nov 2007