Abstracts
PURPOSE: To determine whether rosiglitazone-enriched diet offer protection in a classical model of liver ischemia-reperfusion injury in rats. METHODS: Two days before the experiment, rats were divided into 2 groups: Control Group (n=13) rats fed with standard diet; Rosi Group (n=13): rats fed with a powdered standard diet supplemented with rosiglitazone. The animals were submitted to liver ischemia-reperfusion by clamping the pedicle of median and left anterolateral lobes. After 1 hour of partial hepatic ischemia, the clamp was removed for reperfusion. After 2 or 24 hours (Control and Rosi Groups), blood was collected for enzymes and cytokines analysis. Ischemic and non-ischemic liver were collected for malondialdehyde analysis and histological assessment. Lungs were removed for tissue myeloperoxidase quantification. RESULTS: There were no statistical differences between groups for all analysed parameters. CONCLUSION: In this model, rosiglitazone-enriched diet did not protect liver against ischemia-reperfusion injury.
Ischemia; Reperfusion; Liver; Rats
OBJETIVO: Determinar se a dieta enriquecida com rosiglitazona oferece proteção em um modelo clássico de lesão de isquemia e reperfusão hepática em ratos. MÉTODOS: Dois dias antes do experimento, os ratos foram divididos em 2 grupos: Grupo Controle (n=13): ratos alimentados com dieta padrão; Grupo Rosi (n=13): ratos alimentados com dieta em pó padrão enriquecida com rosiglitazona. Os animais foram submetidos à isquemia e reperfusão hepática por clampeamento do pedículo dos lobos médio e anterolateral esquerdo. Após 1 hora de isquemia, o clampe foi removido para a reperfusão. Após 2 ou 24 horas (Grupos Controle e Rosi), o sangue foi coletado para análise de enzimas e citocinas. Os fígados isquêmico e não isquêmico foram coletados para análise de malondialdeído e avaliação histológica. Pulmões foram removidos para quantificação da mieloperoxidase tecidual. RESULTADOS: Não houve diferenças estatísticas entre grupos em todos os parâmetros analisados. CONCLUSÃO: Nesse modelo, a dieta enriquecida com rosiglitazona não protegeu contra a lesão de isquemia e reperfusão hepática.
Isquemia; Reperfusão; Fígado; Ratos
13 - ORIGINAL ARTICLE
ISCHEMIA-REPERFUSION
Rosiglitazone-enriched diet did not protect liver ischemia-reperfusion injury in a rat model1 Correspondence: Dr. Antônio Roberto Franchi-Teixeira Laboratório de Investigação Médica - LIM 37 Faculdade de Medicina - FMUSP Av. Dr. Arnaldo, 455/sala 3206 01246-903 São Paulo - SP Brazil Phone: (55 11)3061-8319 / 8558-8426 drrteixeira@uol.com.br
Dieta enriquecida com rosiglitazona não protege a lesão de isquemia e reperfusão hepática em modelo experimental no rato
Antonio Roberto Franchi TeixeiraI; Nilza Trindade MolanII; Marta Bellodi-PrivatoIII; Ana Maria CoelhoII; Kátia Ramos LeiteIV; Antônio Carlos SeguroV; Telésforo BacchellaVI; Marcel Cerqueira César MachadoVII
IAssistant Surgeon, Transplantation Division, Department of Gastroenterology, School of Medicine, USP, Brazil
IIPharmacist, Transplantation Division, Department of Gastroenterology, School of Medicine, USP, São Paulo, Brazil
IIIPost-Doctor Student, Transplantation Division, Department of Gastroenterology, School of Medicine, USP, São Paulo, Brazil
IVAssistant Pathologist, Department of Urology, School of Medicine, USP, São Paulo, Brazil
VAssociate Professor, Department of Nephrology, School of Medicine, USP, São Paulo, Brazil
VIAssociate Professor, Surgeon-in-Chief, Transplantation Division, Department of Gastroenterology, School of Medicine, USP, São Paulo, Brazil
VIIFormer Chairman, Transplantation Division, Department of Gastroenterology, School of Medicine, USP, São Paulo, Brazil
Correspondence Correspondence: Dr. Antônio Roberto Franchi-Teixeira Laboratório de Investigação Médica - LIM 37 Faculdade de Medicina - FMUSP Av. Dr. Arnaldo, 455/sala 3206 01246-903 São Paulo - SP Brazil Phone: (55 11)3061-8319 / 8558-8426 drrteixeira@uol.com.br
ABSTRACT
PURPOSE: To determine whether rosiglitazone-enriched diet offer protection in a classical model of liver ischemia-reperfusion injury in rats.
METHODS: Two days before the experiment, rats were divided into 2 groups: Control Group (n=13) rats fed with standard diet; Rosi Group (n=13): rats fed with a powdered standard diet supplemented with rosiglitazone. The animals were submitted to liver ischemia-reperfusion by clamping the pedicle of median and left anterolateral lobes. After 1 hour of partial hepatic ischemia, the clamp was removed for reperfusion. After 2 or 24 hours (Control and Rosi Groups), blood was collected for enzymes and cytokines analysis. Ischemic and non-ischemic liver were collected for malondialdehyde analysis and histological assessment. Lungs were removed for tissue myeloperoxidase quantification.
RESULTS: There were no statistical differences between groups for all analysed parameters.
CONCLUSION: In this model, rosiglitazone-enriched diet did not protect liver against ischemia-reperfusion injury.
Key words: Ischemia. Reperfusion. Liver. Rats.
RESUMO
OBJETIVO: Determinar se a dieta enriquecida com rosiglitazona oferece proteção em um modelo clássico de lesão de isquemia e reperfusão hepática em ratos.
MÉTODOS: Dois dias antes do experimento, os ratos foram divididos em 2 grupos: Grupo Controle (n=13): ratos alimentados com dieta padrão; Grupo Rosi (n=13): ratos alimentados com dieta em pó padrão enriquecida com rosiglitazona. Os animais foram submetidos à isquemia e reperfusão hepática por clampeamento do pedículo dos lobos médio e anterolateral esquerdo. Após 1 hora de isquemia, o clampe foi removido para a reperfusão. Após 2 ou 24 horas (Grupos Controle e Rosi), o sangue foi coletado para análise de enzimas e citocinas. Os fígados isquêmico e não isquêmico foram coletados para análise de malondialdeído e avaliação histológica. Pulmões foram removidos para quantificação da mieloperoxidase tecidual.
RESULTADOS: Não houve diferenças estatísticas entre grupos em todos os parâmetros analisados.
CONCLUSÃO: Nesse modelo, a dieta enriquecida com rosiglitazona não protegeu contra a lesão de isquemia e reperfusão hepática.
Descritores: Isquemia. Reperfusão. Fígado. Ratos.
Introduction
Liver ischemia-reperfusion injury (LIRI) is a phenomenon inevitable after hepatic surgery, liver transplantation, shock and trauma1. Hepatic ischemia-reperfusion leads to an acute inflammatory response, causing significant hepatocellular damage and organ dysfunction. Mechanisms of LIRI involve complex and multiple pathways, including the direct ischemic cellular damage as well as the cell injury due to the activation of inflammatory response after reperfusion2. The accumulation of inflammatory cells contributes to the progression of parenchymal injury. Despite the recent improvements in liver preservation and surgical techniques, LIRI remains an important clinical complication.
An important pathway involved in inflamation and regeneration is the peroxisome proliferator-activated receptors (PPARs) family. PPARs are members of the nuclear hormone receptor superfamily of ligand-dependent transcription factors3. The PPAR subfamily comprises three members, PPAR-α, PPAR-β, and PPAR-γ4. Thiazolidinediones (pioglitazone, troglitazone, and rosiglitazone) are synthetic PPAR-γ agonists and they act to enhance insulin sensitivity and reduce serum glucose in diabetic patients5. Therefore, they are now widely used as antidiabetic agents. Besides the antidiabetic activity, it has been recently recognized to have other various physiological roles including inflammation. In particular, PPAR-γmay be a protective regulator against ischemic damage. In the liver, it has been suggested that PPAR-γ agonists are protective against liver injury in chronic disease conditions such as cirrhosis and fibrosis6. A recent study showed that PPAR-γ plays an inhibitory role in LIRI and the stimulation by selective agonist has a significant beneficial effect in mice. This protective effect of pioglitazone was associated with downregulation of the local expression of several potent proinflammatory cytokines, chemokines and adhesion molecules after reperfusion7. One speculate if PPAR-γ agonists may have a therapeutic role in liver ischemia-reperfusion injury.
Our aim was to determine whether rosiglitazone-enriched diet could offer protection in a classical model of liver ischemia-reperfusion injury in rats.
Methods
The study was designed in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health and the Guidelines of Animal Experimentation of the University of São Paulo School of Medicine, São Paulo, SP, Brazil, for the care and use of laboratory animals. Male Wistar rats weighing 250 ± 5 g were housed in a room with a temperature of 23 ± 1ºC and a controlled 12-h light/dark cycle. Animals were anestethized with ketamin 5% (30 mg/kg) and xylazine 2% (30 mg/kg) intraperitonially. During the experiment, animals were warmed by a halogen light (45W, 127V) and corporeal temperature was monitorized by a rectal digital thermometer (YSI Precision 4000A thermometer, USA) and kept around 37°C. Animals were allowed to spontaneous ventilation with an oxygen-enriched mixture (40%) during all the procedure.
The experimental protocol was pre-approved by Ethics Commission of the Hospital das Clínicas , University of São Paulo, Brazil.
Twenty six male Wistar rats, provided by the Medical Investigation Laboratory #37 (LIM-37) were used. Two days before the experiment, rats were divided into 2 groups: Control Group (n = 13): rats fed with standard diet; Rosi Group (n = 13): rats fed with a powdered standard diet supplemented with rosiglitazone to produce mixture with 92 mg/kg. All animals were allowed to have free access to diet and water. The food intake in milligrams was monitored daily (2.8 mg/rat/day).
Animals were than submitted to liver ischemia-reperfusion procedure. Briefly, a median laparotomy was performed and the pedicle of median and left anterolateral segments was dissected, exposed and clamped with an atraumatic microvascular bulldog clamp. In this technique, pedicle clamping is completely reversible, avoiding intestinal and caval stasis. After clamping, the abdominal wall was closed with ininterrupted 4-0 nylon suture in order to avoid dehydration. After 1 hour of partial hepatic ischemia, the clamp was removed to initiate hepatic reperfusion.
After 2 or 24 hours (Control and Rosi Groups), the animals were anestethized again and 5 ml of blood was collected by cardiac punction for biochemical analysis. Rats were than killed by aorta section. The liver was collected and identified as "ischemic liver" and "non-ischemic liver" for malondialdehyde analysis. For histological assessment liver was collected 24 hours after reperfusion.
Pulmonary artery was catheterized via right ventriculum with a plastic 2,0 mm diameter tube (Silastic, Dow Corning, no. 602.175) and 40 ml of saline 0.9% was infused, at a rate of 10ml/min. Lungs were removed for tissue myeloperoxidase quantification.
Liver enzymes
Hepatic enzymes [alanine aminotransferase (ALAT), aspartate aminotransferase (ASAT), alkaline phosphatasis (AP) and gamma-glutamyltransferase (GGT)] levels were quantified using a clinical chemistry kit (Testline - SG Tecnologia Clínica, São Paulo, Brazil) on Cobas Mira analyzer, according to the manufacturer's instructions.
TNF-α and interleukines
Serum TNF-α, IL-6 and IL-10 concentrations were measured in duplicate using a commercially available enzyme-linked immunosorbent assay kit (Biosource International Cytoscreen, Camarillo, CA) according to the manufacturer's instructions. The concentrations were expressed in pg/ml.
Pulmonary myeloperoxidase
Pulmonary myeloperoxidase (MPO) activity was used as an indicator of neutrophil accumulation. Samples of lung tissue homogenized with a Polytron® homogenizer using a buffer that contains 0.5% hexadecyltrimethyl ammonium bromide, 5mmol/L EDTA, and 50 mmol/L phosphate at pH 6.0. Homogenized samples were sonicated and centrifugated (3000g, 30 minutes) at 40C. MPO activity in the supernatant was assayed by measuring the change in A460 resulting from the decomposition of H2O2 in the presence O - dianiside. Results are expressed as optimal density (OD) at 490 nm.
Malondialdehyde measurement
Malondialdehyde (MDA) levels in the samples were determined to obtain quantitative estimation of the membrane lipid oxidative damage. MDA was assayed in terms of thiobarbituric acid reactive substrats. The thiobarbituric acid method was used to quantify lipid peroxidation in liver, measured as thiobarbituric acid-reactive substances (TBARS). Liver tissues (100 mg/mL) were homogeneized in 1.15% KCl buffer, and centrifuged at 14,000 g for 20 min. The supernatant was then stores at -70°C. an aliquot of the supernatant was added to a reaction mixture of 1.5 mL 0.8% thiobarbituric acid, 200 µL 8.1% (v/v) SDS, 1.5 mL 20% (v/v) acetic acid, pH 3.5, and 300 µL distilled H2O, and heated to 90°C for 45 min. After cooling to room temperature, the samples were cleared by centrifugation at 10,000 g for 10 min, and their absorbance was measured at 532 nm using malondialdehyde bis (dimethyl acetal) as an external standard. The quantity of lipid peroxides is reported in nmol malondialdehyde (MDA) equivalents/mg protein.
Histological analysis
Fragments of liver tissue previously fixed in 10% formalin solution were processed and stained with hematoxylin-eosin. The following histological variables were assessed: parenchymal detrabeculation, presence of apoptosis, cellular edema and congestion.
Statistical analysis
Comparisons between groups were statistically analyzed using a GraphPad Prism 2.01 software (San Diego, CA, USA). A nonparametric test (Mann-Whitney) was performed since the data distribution was considered as non-Gaussian. Mean values and SEM were displayed in graphics only as additional information. Differences were considered statistically significant when p < 0.05.
Results
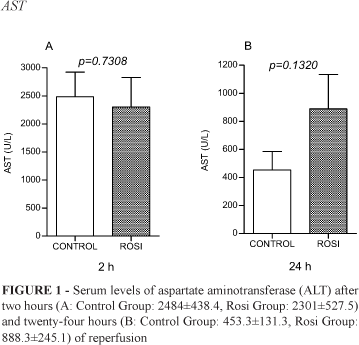
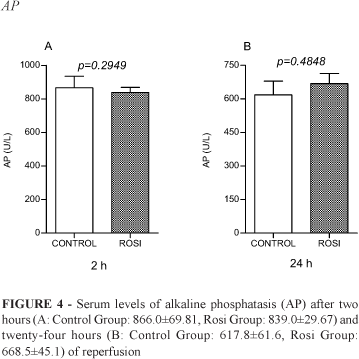
There were no statistical differences between Control Group and Rosi Group concerning liver enzymes after two and twenty-four hours of reperfusion (Figures 1-4).
The cytokines TNF-α, IL-6, IL-10 were assessed only after two hours of reperfusion and differences between Control and Rosi Groups were not significant as well (Figure 5).
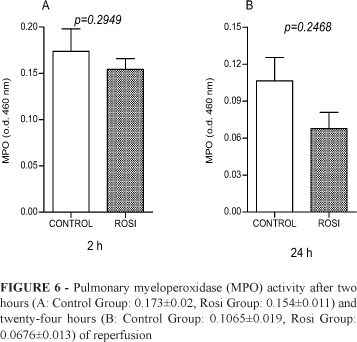
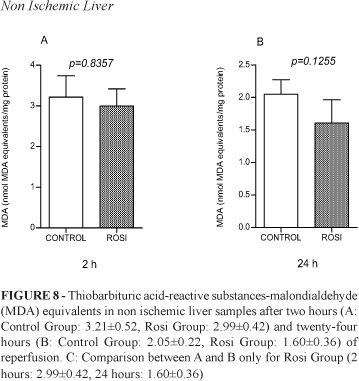
Pulmonary MPO (Figure 6) and hepatic MDA - in the ischemic (Figure 7) and non-ischemic liver (Figure 8) - were also analyzed after two and twenty-four hours of reperfusion in Control and Rosi Groups. Differences between the groups were not observed.
Histological assessment of ischemic and non-ischemic lobes (only after 24 hours of reperfusion) was performed by an experienced pathologist and showed a menagerie of mild alterations (detrabeculation, apoptotic bodies, congestion) not different between the groups (photographies not shown).
Discussion
Pro-inflammatory cytokines are up-regulated during acute liver injury. The increment of TNF-α and IL-6 has been associated with ischemic damage and lack of tissue protection8. They are responsible for the induction of neutrophil sequestration in the lungs9, analyzed by lung leukocyte recruitment (MPO). On the other hand, IL-10 appears to have an attenuating effect on organ damage, due to its anti-inflammatory properties10.
There are some evidences that PPAR-γ agonists may suppress the production of IL-1, IL-6 and TNF-α in stimulated human peripheral blood monocytes11.
The potential protective effect of thiazolidinediones in LIRI are under investigation. These drugs might have a role in clinical situations such as hepatectomies and liver transplantation. Theoretically, a patient could receive rosi or pioglitazone prior to a liver resection when ischemia is planned. Similarly, a liver donor could receive these drugs in intensive care unit before multiorgan retrieval, in order to protect tissues from ischemia reperfusion injury.
In this study, we tested the effects of a rosiglitazone-enriched diet in a classical model of LIRI in rats, focusing in the early (2hs) and delayed (24hs) effects of the drug.
We assessed classical markers of liver injury (AST, ALT, AP, GGT), pro- and anti-inflammatory cytokines (IL-6, TNF-α and IL-10) and lung leukocyte recruitment (MPO). We also evaluated lipid peroxidation (MDA) and liver histology in ischemic and non-ischemic liver in both groups.
Analyzing our results, we found that liver enzymes peaks (ALT, AST, AP and GGT) reached values that confirm clamping promoted effective hepatocyte injury. Statistical differences between Control and Rosi groups were not significant. It means that rosiglitazone did not modify the degree of injury inflicted to the hepatocytes.
Similarly, TNF-α, IL-6, IL-10 and lung myeloperoxidase (MPO) was not modulated by rosiglitazone administration.
Nevertheless, others researchers found beneficial effects of thiazolidinediones administration in a different model of LIRI in mice. Kuboki et al. demonstrated that PPAR-γ activation by rosiglitazone offers protection against hepatic ischemia (90 minutes) followed by eight hours of reperfusion. They showed that PPAR-γ is constitutively activated in normal liver and that hepatic ischemia causes a rapid decrease in PPAR-γ DNA binding. They suggest that PPAR-α is an important endogenous regulator of, and potential therapeutic target for, ischemic liver injury12. The differences between our and Kuboki's results may relay on the fact they applied a longer period of ischemia.
Lipid peroxidation refers to the oxidative degradation of lipids. It is the process where free radicals transfer electrons from the lipids to cell membranes, resulting in cell damage. The main final product of this reaction is malondialdehyde (MDA) and it is reactive with thiobarbituric acid (TBARS)13. To our knowledge, this is the first time that the potential capacity of rosiglitazone to ameliorate lipid peroxidation induced by LIRI was tested in an animal model. Our results did not show a significant difference between Rosi and Control groups concerning MDA analysis.
Despite these preliminary negative results, there are evidences that thiazolidinediones may have an important role in LIRI and further studies will be addressed in this direction.
Received: February 19, 2008
Review: April 23, 2008
Accepted: May 20, 2008
Conflict of interest: none
Financial source: none
1 Research performed of Transplantation Division, Laboratory of Medical Investigation #LIM37, Department of Gastroenterology, School of Medicine, University of São Paulo (USP), Brazil.
- 1. Lemasters JJ, Thurman RG. Reperfusion injury after liver preservation for transplantation. Ann Rev Pharmacol Toxicol. 1997;37:327-38.
- 2. Jaeschke H. Mechanisms of Liver Injury. II. Mechanisms of neutrophil-induced liver cell injury during hepatic ischemia-reperfusion and other acute in.ammatory conditions. Am J Physiol Gastrointest Liver Physiol. 2006;290(6):G1083-G88.
- 3. Kersten S, Desvergne B, Wahli W. Roles of PPARs in health and disease. Nature. 2000;405(6785):421-4.
- 4. Michalik L, Wahli W. Peroxisome proliferator-activated receptors: three isotypes for a multitude of functions. Curr Opin Biotechnol. 1999;10(6):564-70.
- 5. Plutzky J. The potential role of peroxisome proliferator-activated receptors on in.ammation in type 2 diabetes mellitus and atherosclerosis. Am J Cardiol. 2003;92(4A):34J-41J.
- 6. Galli A, Crabb DW, Ceni E, Salzano R, Mello T, Svegliati-Baroni G, Ridolfi F, Trozzi L, Surrenti C, Casini A. Antidiabetic thiazolidinediones inhibit collagen synthesis and hepatic stellate cell activation in vivo and in vitro.Gastroenterology. 2002;122(7):1924-40.
- 7. Akahori T, Sho M, Hamada K, Suzaki Y, Kuzumoto Y, Nomi T, Nakamura S, Enomoto K, Kanehiro H, Nakajima Y. Importance of peroxisome proliferator-activated receptor-gamma in hepatic ischemia/reperfusion injury in mice. J Hepatol. 2007;47(6):784-92.
- 8. Teoh N, Leclercq I, Pena AD, Farrell G. Low-dose TNF-alpha protects against hepatic ischemia-reperfusion injury in mice: implications for preconditioning. Hepatology. 2003;37(1):118-28.
- 9. Colletti LM, Remick DG, Burtch GD, Kunkel SL, Strieter RM, Campbell DA Jr. Role of tumor necrosis factor-alpha in the pathophysiologic alterations after hepatic ischemia/reperfusion injury in the rat. J Clin Invest. 1990;85(6):1936-43.
- 10. Le Moine O, Louis H, Demols A, Desalle F, Demoor F, Quertinmont E, Goldman M, Devière J. Cold liver ischemia-reperfusion injury critically depends on liver T cells and is improved by donor pretreatment with interleukin 10 in mice. Hepatology. 2000 ;31(6):1266-74.
- 11. Jiang C, Ting AT, Seed B. PPAR-gamma agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998;391(6662):82-6.
- 12. Kuboki S, Shin T, Huber N, Eismann T, Galloway E, Schuster R, Blanchard J, Zingarelli B, Lentsch AB. Peroxisome proliferator-activated receptor-gamma protects against hepatic ischemia/reperfusion injury in mice. Hepatology. 2008;47(1):215-24.
- 13. Marnett LJ. Lipid peroxidation-DNA damage by malondialdehyde. Mutat Res. 1999;424(1-2):83-95.
Publication Dates
-
Publication in this collection
15 July 2008 -
Date of issue
Aug 2008
History
-
Reviewed
23 Apr 2008 -
Received
19 Feb 2008 -
Accepted
20 Mar 2008