Abstracts
PURPOSE: To study thermal variations obtained through infrared image in rats, and to evaluate the relationship between intestinal ischemic time and histopathological findings. METHODS: Thirty Wistar rats were operated after distribution in 5 groups with different times of ischemia. Thermograms were obtained by using a infrared camera. The surgical technique has been standardized for all groups: abdominal cavity opening by a 5cm length incision in the midline, abdominal wall plans section and cavity exposure, and exteriorization of the intestine. In group I (control), it was proceeded only laparotomy without superior mesenteric artery ligature. After first thermogram done, incision was closed with continuing suture. In each rat in groups II, III, IV and V, the superior mesenteric artery was located at its origin on abdominal aorta, dissected and occluded with a vascular microclamp, subjecting the intestine to ischemia in variable times. RESULTS: Rats submitted to a 30 minutes ischemia presented reactive hyperemia, thermal differential of 1.8°C and normal pathological examination. The 1 hour ischemia produced reactive hyperemia with ischemic areas, thermal differential of 1.0°C and injuries at villosities' tips. However, the 90 minutes ischemia had not shown reactive hyperemia with large ischemic areas, thermal differential of -1.0°C and injury in the upper third of the villosities. The 2 hours ischemia demonstrated a severe ischemia, thermal differential of -2.0°C and injury throughout the all villosities' extension. CONCLUSION: It has been possible studying thermal variations through infrared image in rats, showing correlation between thermal response in thermograms, ischemic time and histopathological findings.
Spectrophotometry, Infrared; Tissue Survival; Ischemia; Wound Healing; Rats
OBJETIVO: Estudar as variações térmicas obtidas por meio da imagem infravermelha em ratos, e avaliar sua correlação com o tempo de isquemia intestinal e os achados histopatológicos. MÉTODOS: Trinta ratos Wistar foram operados após distribuição em cinco grupos com diferentes tempos de isquemia. Os termogramas foram obtidos utilizando-se uma câmera infravermelha. A técnica operatória foi padronizada para todos os grupos, abertura da cavidade abdominal por uma incisão na linha média de 5 cm de comprimento com secção de todos os planos da parede abdominal e exposição da cavidade e as alças intestinais exteriorizadas. No grupo I (controle) foi feito apenas laparotomia sem ligadura da artéria mesentérica superior. Após realizado o primeiro termograma, foi fechada a incisão com sutura contínua. Em cada rato dos grupos II, III, IV e V a artéria mesentérica superior foi localizada em sua origem na aorta abdominal, dissecada com e ocluída por um microclampe vascular submetendo o intestino à isquemia em tempos variáveis. RESULTADOS: Os ratos submetidos à isquemia de 30 minutos apresentaram hiperemia reativa, diferencial térmico de 1,8°C e exame anatomopatológico normal. A isquemia de 1 hora produziu hiperemia reativa com áreas de isquemia, diferencial térmico de 1,0°C e lesões na ponta das vilosidades. Já a de 90 minutos não demonstrou hiperemia reativa com grandes áreas de isquemia, diferencial térmico de -1,0°C e lesão no terço superior das vilosidades. A isquemia de 2 horas mostrou isquemia grave, diferencial térmico de -2,0°C e lesão em toda a extensão das vilosidades. CONCLUSÃO: Foi possível estudar as variações térmicas por meio da imagem infravermelha em ratos, que mostrou haver correlação entre a resposta térmica dos termogramas, o tempo de isquemia e achados histopatológicos.
Espectrofotometria Infravermelho; Sobrevivência de Tecidos; Isquemia; Cicatrização de Feridas; Ratos
8 - ORIGINAL ARTICLE
WOUND HEALING
Infrared imaging contribution for intestinal ischemia detection in wound healing1 1 Research performed at Research Medical Institute of the University Evangelic Hospital/ Evangelic Paraná Faculty (FEPAR), Curitiba-PR, Brazil.
Contribuição da imagem infravermelha para detecção da isquemia intestinal na cicatrização das feridas
Osvaldo MalafaiaI; Marcos Leal BrioschiII; Sonia Maria Schneider AokiIII; Fernando Gallego DiasIV; Bruno Schneider GugelminV; Massao Schneider AokiVI; Yuki Schneider AokiVII
IPhD, Full Professor of Surgery, Coordinator of Principles of Surgery Post-Graduation Program, FEPAR, Curitiba-PR, Brazil
IIPhD, President of Brazilian Society of Thermology, Curitiba-PR, Brazil
IIIMaster in Sciences, Principles of Surgery Post-Graduation Program, FEPAR, Curitiba-PR, Brazil
IVMaster in Thermal Engineer, Federal University of Paraná (UFPR), Curitiba-PR, Brazil
VBiomedical Engineer, Duke-University, Durham, NC, USA
VIGraduate Student, FEPAR, Curitiba-PR, Brazil
VIIGraduate Student, UFPR, Curitiba- PR, Brazil
Correspondence Correspondence: IPEM Alameda Augusto Stellfeld, 1980 80730-150 Curitiba - PR Brazil ipem@evangelico.org.br
ABSTRACT
PURPOSE: To study thermal variations obtained through infrared image in rats, and to evaluate the relationship between intestinal ischemic time and histopathological findings.
METHODS: Thirty Wistar rats were operated after distribution in 5 groups with different times of ischemia. Thermograms were obtained by using a infrared camera. The surgical technique has been standardized for all groups: abdominal cavity opening by a 5cm length incision in the midline, abdominal wall plans section and cavity exposure, and exteriorization of the intestine. In group I (control), it was proceeded only laparotomy without superior mesenteric artery ligature. After first thermogram done, incision was closed with continuing suture. In each rat in groups II, III, IV and V, the superior mesenteric artery was located at its origin on abdominal aorta, dissected and occluded with a vascular microclamp, subjecting the intestine to ischemia in variable times.
RESULTS: Rats submitted to a 30 minutes ischemia presented reactive hyperemia, thermal differential of 1.8°C and normal pathological examination. The 1 hour ischemia produced reactive hyperemia with ischemic areas, thermal differential of 1.0°C and injuries at villosities' tips. However, the 90 minutes ischemia had not shown reactive hyperemia with large ischemic areas, thermal differential of -1.0°C and injury in the upper third of the villosities. The 2 hours ischemia demonstrated a severe ischemia, thermal differential of -2.0°C and injury throughout the all villosities' extension.
CONCLUSION: It has been possible studying thermal variations through infrared image in rats, showing correlation between thermal response in thermograms, ischemic time and histopathological findings.
Key words: Spectrophotometry, Infrared. Tissue Survival. Ischemia. Wound Healing. Rats.
RESUMO
OBJETIVO: Estudar as variações térmicas obtidas por meio da imagem infravermelha em ratos, e avaliar sua correlação com o tempo de isquemia intestinal e os achados histopatológicos.
MÉTODOS: Trinta ratos Wistar foram operados após distribuição em cinco grupos com diferentes tempos de isquemia. Os termogramas foram obtidos utilizando-se uma câmera infravermelha. A técnica operatória foi padronizada para todos os grupos, abertura da cavidade abdominal por uma incisão na linha média de 5 cm de comprimento com secção de todos os planos da parede abdominal e exposição da cavidade e as alças intestinais exteriorizadas. No grupo I (controle) foi feito apenas laparotomia sem ligadura da artéria mesentérica superior. Após realizado o primeiro termograma, foi fechada a incisão com sutura contínua. Em cada rato dos grupos II, III, IV e V a artéria mesentérica superior foi localizada em sua origem na aorta abdominal, dissecada com e ocluída por um microclampe vascular submetendo o intestino à isquemia em tempos variáveis.
RESULTADOS: Os ratos submetidos à isquemia de 30 minutos apresentaram hiperemia reativa, diferencial térmico de 1,8°C e exame anatomopatológico normal. A isquemia de 1 hora produziu hiperemia reativa com áreas de isquemia, diferencial térmico de 1,0°C e lesões na ponta das vilosidades. Já a de 90 minutos não demonstrou hiperemia reativa com grandes áreas de isquemia, diferencial térmico de -1,0°C e lesão no terço superior das vilosidades. A isquemia de 2 horas mostrou isquemia grave, diferencial térmico de -2,0°C e lesão em toda a extensão das vilosidades.
CONCLUSÃO: Foi possível estudar as variações térmicas por meio da imagem infravermelha em ratos, que mostrou haver correlação entre a resposta térmica dos termogramas, o tempo de isquemia e achados histopatológicos.
Descritores: Espectrofotometria Infravermelho. Sobrevivência de Tecidos. Isquemia. Cicatrização de Feridas. Ratos.
Introduction
The intestine's viability evaluation is one of the most difficult situations through which passes the surgeon who works in emergences services1. When faced with cases of mesenteric ischemia, especially in doubtful ones, where it is feared to underestimate an intestinal segment with low blood perfusion preserving it into the abdominal cavity, there is a risk of progression to sepsis and death. In addition, also not desirable, ischemia's superestimation and extensive resections can cause intestinal failure, requiring total parenteral nutrition or intestinal transplantation.
A few centimeters preservation of the small intestine can determine difference between the patients be able to absorb adequate oral diet or rely on permanent parenteral nutrition2. Total parenteral nutrition has high costs and not always is available in public hospitals, as well as being an able treatment for infectious and metabolic complications.
Considering that clinical criteria such as coloring, peristalsis, pulse's presence in marginal arcades and sectioned edges' bleeding may be failure in up to 60%3, several methods more objective, have been used to predict intestinal viability as: injection of radioactive microspheres using technetium 994,5; coloring intravascular injection6,7,8,9,10,11,12; ultrasound Doppler13; ultrasound laser Doppler14,15,16,17,18; intestinal electromyography19,20,21,22,23; and surface oximetry3,24,25,26,27. These methods have been experimentally applied as intestinal viability indicators, however none has achieved widespread clinical use.
Thus, infrared image has its use highlighted by technique modernization and nowadays has become important tool for the study of human body temperature. Infrared image is a method that uses special camera, targeted to operative field, which detects infrared radiation emitted by the body to be examined.
This technique was experimentally used by Moss et al.28, who studied, in dogs, infrared image to evaluate the viability of revascularizated intestine, previously submitted to 2 ½ hours and 8 hours ischemia29. The thermograms showed reactive hyperemia and elevation of 1 to 4°C in intestinal surface temperature when compared to adjacent normal intestine30. In the intestinal segment subjected to an 8 hours ischemia there was no reactive hyperemia.
However, despite the great technological development applied to medicine in recent years, especially in image examination and in life support advances, mortality rates in cases of acute mesenteric ischemia remain high, around 60 to 80%, according to several authors31,32,33,34,35,36 experience, needing yet researches about more accurate techniques on fast and efficient assessment of mesenteric ischemia.
The aim of this research was to study thermal variations, obtained through infrared image, in Wistar rats subjected to different intestinal ischemia times, and to evaluate thermograms and its correlation with ischemia time and histopathological findings.
Methods
This is an experimental study, with sample subject's double-blind random selection. The experimental protocol was approved by the Research Ethics' Committee of Faculdade Evangélica do Paraná, and it was adopted the ethical principles for animal testing of the Animal Experimentation Brazilian College31, using Veterinary Anatomical Nomina (1983).
It was used 30 male rats (Rattus norvegicus, Rodentio, Mammalia, Wistar line) aged between 2.5 and 3 months, and weighing between 270 and 300 g, accommodated in cages with five animals in controlled temperature (22°C), with light/darkness cycle of 12/12 hours, receiving proper feed for the species and water ad libitum, three days before operation until the end of the experiment.
They were anesthetized with ketamine hydrochloride at dose of 15 mg/kg by intramuscular, on the inner face of the pelvis member. Intraperitonial additional doses were administered as needed. After reflexes' loss, animals were weighed. The experiments were conducted in laboratory during the morning due to animal's lower thermal variation in that period. The study was conducted in heated room (22°C) controlled by air conditioner, with humidity of 60% controlled by air dehumidifiers and without current air. Heat loss by forced convection was minimized avoiding moving around the animals. Doors and windows were maintained closed but with gaps to air circulation towards stabilizing environment's temperature by air conditioner.
After anesthesia, the 30 rats were randomly distributed into 5 groups (Table 1). It was considered intestinal ischemia the superior mesenteric artery occlusion in its origin at abdominal aorta artery.
Initial surgical technique has been standardized for all groups with abdominal cavity opening by a 5 cm length incision in the midline, all abdominal wall plans section and cavity exposure. Intestine was exteriorized by the right protected by saline humid gaze and disguised with plastic film (Royal-pack®), reducing drying caused by contact with the external environment. In group I (control), it was done only laparotomy without superior mesenteric artery ligature. After first thermogram was done, the incision was closed with continuing suture and mice were revived from anesthesia. In each rat of groups II, III, IV and V, the superior mesenteric artery (Figure 1) was found in its origin at abdominal aorta, dissected and occluded by a 5 mm vascular microclamp, subjecting intestine to ischemia.
Operation had begun by the 6 animals in group V whose ischemia time was of 2 hours. Before arterial clamping, it was obtained intestine's thermograms of all animals. During pinching period, in their respective groups, intestine was replaced in abdominal cavity and incision was temporarily closed by single plan continuous suture to prevent liquid loss through evaporation. Rats were revived from anesthesia and kept in cage with identification. Same procedure was performed in other groups. After each group's previewed time (Table 1) abdomen was reopened, extruding up the intestine and microclamp was withdrawn, freeing the artery occlusion. It was waited up for 10 minutes, allowing thermal equilibrium with the environment, and thermograms were performed by using infrared image in each group of six animals to monitor small intestine's revascularization or not, up to 15 minutes.
The infrared camera was positioned up to 60 cm of surgical incision where small intestine was exposed to be evaluated. In this study, it was used an infrared camera (ThermaCamTM S65 - FLIR Systems TM - Sweden) to capture electromagnetic waves spectral band emitted between 3.5 to 5µ m, infrared waves. The maximum spatial resolution obtained was 0.1mm. Infrared radiation naturally emitted from the examined surface was converted, through a platinum silicate detector (PtSi) cooled with liquid nitrogen (steerling cicle), in electrical signal. This signal was processed in a 76,000 points spreadsheet number of calibrated absolute temperature, seven frames per second, instantly represented in thermal image with a 320x240 pixels resolution and thermal sensitivity up to 0.08°C. Camera was directly focused on the rat's ventral surface, forming an angle of 80° (Figure 2).
The images were processed in a 7500 MHz Pentium III computer attached to the PCMCIA card. Through specific program for image obtained analysis, the external surface irradiated temperatures were recorded at seven frames per second throughout the experiment. All images were represented by infrared thermograms in a video display and recorded in hard disk for later statistical analysis done by the program.
The images were analyzed by using temperature range between 35.0°C and 25.0°C, average temperature of 28.5°C and continuous colorimetric scale rain (Figure 3), in which the colors white, red, yellow, green, blue and black, represented respectively a decreasing scale of temperature areas, also distributed on the scale, from warmer to cooler, maintained until the experiment's ending.
After experiment's ending, rats were sacrificed for deepening of the anesthetic dose. The small intestine was removed and a 3 cm segment from the jejunum was collected, at about 10 cm from duodenojejunal angle, put into vials containing formalin (formaldehyde 10%) and encrypted for histological examination. Material received histological processing by conventional technique. The preparations were cut in a paraffin microtome, with 4 micrometers thick, and stained by the hematoxylin and eosin (HE) method.
Calculating formula for dimensionless values
The dimensionless thermal differential (ΔT) was obtained by following this formula43:
Where:
* Δ T is the dimensionless thermal differential;
* T is the experimental acquired temperature by infrared image;
* T ∞ is the temperature within the laboratory;
* T Ь is the average central temperature.
Calculating formula for cooling speed
Where:
* S cool is the cooling speed;
* Δ T is the dimensionless thermal differential;
* t is the time established in this study that was of 15 minutes.
For statistical analysis, it was used specific program STATISTICA, Version 6 (Tulsa, OK, Stat Soft, Inc). For thermal variation analysis, it was used average and uncertainty of measurement (T±UT), and for heat loss rate, average and twice the standard deviation (x±2sd). Data were tabulated, and gaussians attributes, independence and consistency of variables, were tested. When data met the proposed criteria, they were subjected to tests of univariate analysis ANOVA and Tukey's parametric, to establish the results significance. In all tests, it was set up the interval 0.05 or 5% (P < 0.05) to exclude the nullity hypothesis.
Results
As in all printed papers in Acta Cirúrgica Brasileira, this article has colored images, which are interesting for reader's better understanding at www.scielo.br/acb.
No rat was deleted before the experiment and no deaths occurred in anesthesia. No complication occurred during laparotomies and clamping of superior mesenteric artery. There was no significant difference between animals' weight by the groups.
Clinical criteria evaluation
Coloring and peristalsis (Table 2)
In groups I (control), II (30 minutes ischemia) and III (1 hour ischemia) the intestine were rose colored, with present peristalsis, and was evaluated as viable. In group IV (1 hour and 30 minutes ischemia) it showed rose colored areas alternated with cyanotic ones and reduced peristalsis, judged as uncertain. In group V (2 hours ischemia) the intestine was cyanotic, without peristaltic movements, and was deemed unviable.
Thermograms analysis
1. Thermogram in group I (control)
Thermograms of group I rats' intestine, submitted only to laparotomy without superior mesenteric artery's clamping, were used as reference for other groups analysis. The Figure 4 corresponds to a thermogram obtained by image's subtraction: the first and the last thermograms were stacked resulting in a picture that shows thermal differencial. The colors seen in the designated area, which are the exposed intestine at surgical incision, ranges from white to red (higher temperatures).
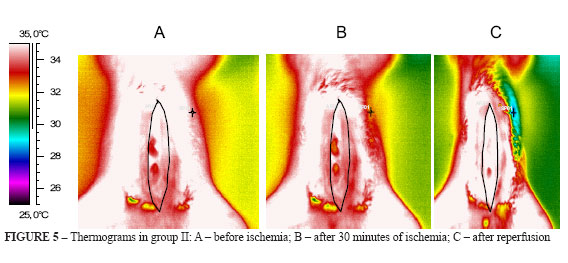
2. Thermograms in group II (Figure 5 A-C)
The exposed intestine at surgical incision, in group II rats, presented colors changing to yellow accusing mild heat loss (Figure 5B). After releasing of the arterial occlusion, thermogram showed temperature elevation corresponding to reactive hyperemia, observed by white predominant in the intestinal loops. The thermal differential was of 1.8°C (Figure 5C).
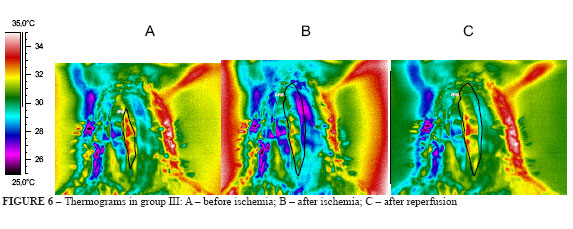
3. Thermograms in group III (Figure 6 A-C)
The rats' intestine in group III, submitted to 1 hour ischemia, showed heat loss that can be seen in Figure 6B, where the predominant color are yellow and green, lower temperatures than before arterial occlusion. After 15 minutes reperfusion, there was a reactive hyperemia alternated with small ischemic areas, but temperatures were raised with predominance of red on yellow (Figure 6C). The thermal differential was 1.0°C.
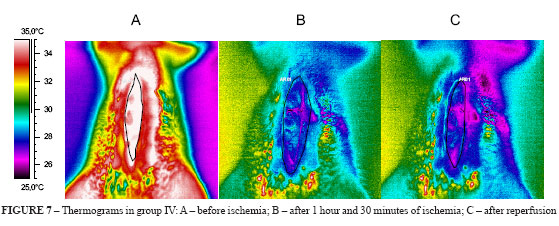
4. Thermograms in group IV (Figure 7 A-C)
The rats' intestine in group IV (Figure 7B) suffered heat loss as seen by predominance of blue and pink colors. After 15 minutes reperfusion there was no reactive hyperemia but large ischemic areas corresponding to pink and dark blue colors in the thermogram (Figure 7C). The thermal differential was -1.0°C.
5. Thermograms in group V (Figure 8 A-C)
The rats' intestine in group V had shown severe heat loss demonstrated by pink, dark blue and black colors (Figure 8B). After 15 minutes reperfusion there was no reactive hyperemia, the colors remained dark blue, pink and black accusing hard heat loss, the intestine was ischemic and thermal differential was -2.0°C (Figure 8C).
Analysis of thermal variations
1. Thermal differential (Δ T)
It was calculated intestine's initial temperature, as well as starting and ending temperature's differential by image subtraction. The program ThermaCAM TM Researcher 2001, FLIR Systems (Sweden) allows that first and last thermograms in procedure can be overlapped, resulting in a thermal differential's image of temperature increasing or not, in centigrade degrees (°C). The accuracy is 0.05°C (Table 3).
There were found the following values of thermal differential: group II of 1.8°C; group III of 1.0°C; group IV of -1.0°C; and group V of -2.0°C.
2. Dimensionless delta (Δ T Dimensionless)
The dimensionless delta was used to minimize errors due to thermal variations of the environment and the animal itself. The dimensionless differential thermal corrected values represent the variation in standard temperature related to the maximum variation found in several groups (which is the central temperature variation in relation to the environment's). The goal is to make any temperature ranged value to be found between 0 and 1 (Table 3).
3. Cooling speed of exposed intestine
It was calculated by the dimensionless delta values and the time, which in this study was of 15 minutes. In groups I and II, the cooling rate was 0.04°C/min; in group III, it was 0.06°C/min; in group IV of 0.10°C/min; and in group V of 0.13°C/min (Table 3).
Histopathological analysis
Group I (control): In this group, histological study demonstrated enteric mucosa with preserved villous architecture, absorptive epithelial cells with microvillus and chaliceform cells, and lamina propria with usual linfoplasmocitary cellularity.
Group II: Showed up an enteric mucosa with preserved villous architecture, absorptive epithelial cells with microvillus and chaliceform cells, and lamina propria with usual linfoplasmocitary cellularity.
Group III: Revealed a presence of serious and diffuse injury (grade 5, a destruction of all the mucosa layer, where it is not observed any glandular structure but only amorphous material deposited on the submucosa), committing only the villosities' tip.
Group IV: Presented serious and diffuse injury (grade 5, above) which undertook the upper third of the villosities; injury ahead from group III.
Group V: The injury was severe (grade 5) and diffuse compromising full extention of the villosities, leaving only the mucosa's lower third preserved; injury ahead from group IV.
Discussion
This is the first study that uses infrared image to record thermal variations occuring during the reactive hyperemia - in a revascularizated tissue - in the rats' small intestine when subjected to different times of experimentally induced ischemia. Therefore, it is difficult to carry out comparisons between this research and previous studies.
However, Moss et al.28 studied, in dogs, infrared imaging in the evaluation of revascularizated ischemic intestine viability. The small intestine of these animals were subjected to a 2 ½ hours and 8 hours ischemia by clamping nutritious artery at mesentery base and interruption of transmural collateral blood flow, and by distal segmentary intestine's margins yearling using elastic handle firmly attached. After these times of ischemia, the intestine was revascularizated by releasing the microclamp and the elastic loops. After 5 minutes of reperfusion, thermograms were recorded up to 30 minutes and the following aspects were found: thermograms of revascularizated segments, after 2 ½, showed reactive hyperemia and elevation of 1 to 4°C in intestinal surface temperature compared to adjacent normal intestine. This answer was higher between five and 15 minutes after revascularization, and persisted for 30 minutes. There was no reactive hyperemia in the intestinal segment subjected to an 8 hours ischemia.
In a second study, Moss et al.29 studied the infrared image to predict the viability of small intestine's segments of dogs, after several ischemia periods. Thermograms showed uniform reactive hyperemia in all segments undergo 2 to 3 hours ischemia periods, similarly to the result obtained in animals subjected 3 to 7 hours ischemia, but in this group it had been observed, in histological examination, areas with fibrosii. Finally, in all animals that were submitted to 8 hours ischemia, there was no reactive hyperemia; the dogs died and anatomic-pathological examination demonstrated intestinal infarction. The authors had noted that infrared image provides graphic demonstration of hyperemic response's regional variation following the revascularization, and successively from all regions that have suffered ischemic permanent damage. Thus, it appears that infrared image can detect intestine's areas that are enough committed to justify resection, suggesting its application in real clinical situations.
Similarly, Brooks et al.30, in swine's model, compared several methods of evaluating the intestinal viability (infrared imaging, visual inspection, Doppler ultrasound and fluorescence), and concluded that the more sensitive were Doppler ultrasound and infrared image, despite the last one overestimate intestinal necrosis when compared to the first one. Other methods had not provided satisfactory results when compared to Doppler or thermograph.
In this study, there was a significant increase in temperature of 1.8°C in group II and of 1.0°C in group III, demonstrating reactive hyperemia in both groups, but this was uniform in group II and not uniform in III. Moreover, in groups IV and V there was no reactive hyperemia and temperatures had significantly fallen in 1°C and 2°C, respectively37.
According to Morimoto et al.37, using of infrared image had proved to be useful in evaluating the viability of other gastrointestinal tract tissues, such as in dogs' pancreatic-duodenal graft. However, in humans, despite of the progress in the transplants field, the results of small intestine's transplantation are still below those obtained in other organs such as hearts, kidneys and liver. One of the reasons lies in the operation complications principally the exocrine fistula and the graft's thrombosis.
Considering that small intestine's transplantation is an alternative for the treatment of the complex intestinal failure, it is of great importance the improvement of methods which determine the graft's viability, especially after revascularization. Thus, infrared image is encouraged by results of this study. Moreover, despite the equipment used is specialized, it is portable, easily operable and its interpretation is imediate, allowing performing visual comparison between revascularizated and normal intestine30.
Conclusion
It was possible to study thermal variations through infrared image in rats showing correlation between thermograms' thermal response and intestinal time of ischemia plus histopathological findings of tissue aggression.
Received: May 14, 2008
Review: July 15, 2008
Accepted: August 18, 2008
Conflict of interest: none
Financial source: none
How to cite this article
Malafaia O, Brioschi ML, Aoki SMS, Dias FG, Gugelmin BS, Aoki MS, Aoki YS. Infrared imaging contribution for intestinal ischemia detection in wound healing. Acta Cir Bras. [serial on the Internet] 2008 Nov-Dec;23(6). Available from URL: http://www.scielo.br/acb
- 1. Esteves FP, Caravatto PPP, Yamakami LYS, Araujo SEA, Genzini T, Perosa M. Avaliação perioperatória da viabilidade intestinal. Rev Med São Paulo. 2003;82(1/4):46-57.
- 2. Horgan PG, Gorey TF. Operative assessment of intestinal viability. Surg Clin North Am. 1992;72(1)143-55.
- 3. Sheridan WG, Lownde RH, Young HL. Intraoperative tissue oximetry in the human gastrointestinal tract. Am J Surg. 1990;159:314-9.
- 4. Moossa AR, Skinner DB, Stark V, Hoffer P. Assesment of bowel viability using 99mtechnetiun-tagged albumin microspheres. J Surg Res. 1974;16:466-72.
- 5. Zarins CK, Skinner DB, Rhodes BA, James AE. Hyperemia as a measure of viability in revascularized ischemic intestine. Surg Forum. 1973;24:416-7.
- 6. Brito MVH, Araújo M, Acácio GJS, Acácio GJS, Reis JMC. Lesão intestinal após isquemia-reperfusão: estudo comparativo usando sal tetrazólico (MTT) e histologia. Acta Cir Bras. 2001;16(1):26-31.
- 7. Brolin RE, Bibbo C, Petschenik A, Reddell MT, Semmlow JL. Comparison of ischemic and reperfusion injury in canine bowel viability assessment. J Gastrointest Surg. 1997;1:511-6.
- 8. Bulkley GB, Zuidema GD, Hamilton SR, O'Mara CS, Klacsmann PG, Horn SD. Intraoperative determination of small intestinal viability following ischemic injury. Ann Surg. 1981;193:628-37.
- 9. Carter MS, Fantini GA, Sammartano RJ, Mitsudo S, Silverman DG, Boley SJ. Qualitative and quantitative fluorescein fluorescense in determining intestinal viability. Am J Surg. 1984;147(1):117-23.
- 10. Mann A, Fazio VW, Lucas FV. A comparative study of the use of fluorescein and the Doppler device in the determination of intestinal viability. Surg Gynecol Obstet. 1982;154:53-5.
- 11. Silverman DG, Hurford WE, Cooper HS, Robinson M, Brousseau DA. Quantification of fluorescein distribution to strangulated rat ileum. J Surg Res. 19833;34:179-86.
- 12. Stolar CJH, Randolph JG. Evaluation of ischemic bowel viability with a fluorescent technique. J Pediatr Surg. 1978;13:221-5.
- 13. Right CB, Hobson RW. Prediction of intestinal viability using Doppler ultrasound technique. Am J Surg. 1975;129:642-5.
- 14. Ahn H, et al. Assessment of blood flow in the small intestine with laser Doppler flowmetry. Acta Chir Scand. 1989;155(6-7):341-6.
- 15. Johansson K, Ahn H, Lindhagen J. Assessment of small bowel ischemia by laser Doppler flowmetry- some case reports. Scand J Gastroenterol. 1986;21:1147-52.
- 16. Kreyer I, Lehmann C, Kékesi V, Dóbi I, Luther B. Motility, haemodynamics and responsibility to vasoactive agents after revascularization of autotransplanted small intestine segments in the dog. Acta Chir Hung. 1989;30(4):261-71.
- 17. Redaelli CA, Schilling MK, Büchler MW. Intraoperative Laser Doppler flowmetry: a predictor of ischemic injury in acute mesenteric infarction. Dig Surg. 1998;15:55-9.
- 18. Shepherd AP, Riedel GL. Continuous measurement of intestinal mucosal blood flow by laser Doppler velocimetry. Am J Physiol. 1982;242(6):668-72.
- 19. Brolin RE, Semmlow JL, Mackenzie JW, Reddell MT. Quantitative myoletric determination of bowel viability. J Surg Res. 1986;41:557-62.
- 20. Hegde SS, Seidel SA, Ladipo JK, Bradshaw LA, Halter S, Richards WO. Effects of mesenteric ischemia and reperfusion on small bowel electrical activity. J Surg Res. 1998;1:86-95.
- 21. Orland PJ, Cazi GA, Semmlow JL, Reddell MT, Brolin RE. Determination of small bowel viability using quantitative myoeletric and color analysis. J Surg Res. 1993;55:581-7.
- 22. Pawlik WW, Thor P, Sendur R, Biernat J, Koziol R, Wasowicz P. Myoeletric bowel activity in ischemia/reperfusion damage: role of sensory neurons. J Physiol Pharmacol. 1998;49:543-51.
- 23. Semmlow JL, Brolin RE. Instrumentation for quantitative assessment of intestinal viability. IEEE Trans Biomed Eng. 1988;35:888-92.
- 24. La Hei ER, Shun A. Intra-operative pulse oximmetry can help determine intestinal viability. Pediatr Surg Int. 2001;17:120-1.
- 25. Locke R, Hanser CJ, Shoemaker WC. The use of surface oximetry to assess bowel viability. Arch Surg. 1984;119:1252-6.
- 26. Piasecki C. A new method for the assessment of gut viability. Br J Surg. 1981;68:319-22.
- 27. Von Bahten LC, Mantovani M, Nicoluzzi JEL, Silveira F, Von BahtenV AC. Perda de calor determinada pela exposição das alças intestinais em ratos. Rev Col Bras Cir. 2006;33(5):265-71.
- 28. Moss AA, Kressel HY, Brito AC. Thermographic Assessment of intestinal viability following ischemic damage. Invest Radiol. 1978;13(1):16-20.
- 29. Moss AA, Kressel WY, Brito AC. Use of thermography to predict intestinal viability and survival after ischemic injury: a blind experimental study. Invest Radiol. 1981;16(1):24-9.
- 30. Brooks JP, Perry WB, Putnam AT, Karulf RE. Thermal imaging in the detection of bowel ischemia. Dis Colon Rectum. 2000;43(9):1319-21.
- 31. Boley SJ, Brandt LJ, Sammartano RJ. History of mesenteric ischemia. the evolution and management. Surg Clin North Am. 1997;77(2)275-88.
- 32. Lock G. Acute intestinal ischemia: best practice res. Clin Gastroenterol. 2001;15(1):83-98.
- 33. Navarro AR. Isquemia mesentérica. Gastroenterologia. 2005;15:101-5.
- 34. Oldenburg WA, Lau LL, Rodenberg TJ, Edmonds HJ, Burger CD. Acute mesenteric ischemia. Arch Intern Med. 2004;164:1054-64.
- 35. Schneider TA, Longo WE, Ure T, Vernava AM 3rd. Mesenteric ischemia: acute arterial syndromes. Dis Colon Rectum. 1995;38(7):778-9.
- 36. Yasuhara H. Acute mesenteric ischemia: the challenge of gastroenterology. Surg Today. 2005;35:185-95.
- 37. Morimoto T, Chikashige T, Yamada F, Sakamoto J, Yasui K, Yasue M, Miyaishi S, Kido C, Takagi H. Thermographic study of the canine pancreaticoduodenal graft. Transplant Proc. 1988;20(Supl. 1):900-3.
Publication Dates
-
Publication in this collection
12 Nov 2008 -
Date of issue
Dec 2008
History
-
Accepted
18 Aug 2008 -
Reviewed
15 July 2008 -
Received
14 May 2008